Key Points
-
To date, >40 genetic variants have been identified that influence the risk of developing ankylosing spondylitis (AS), including HLA alleles such as HLA-B27, among other, non-HLA alleles
-
Genetic studies have also provided strong evidence of a role for the IL-23 pathway in AS pathogenesis
-
Other pathways associated with AS include peptide handling prior to HLA presentation, innate and adaptive immune cell differentiation and activation, and bacterial sensing in the gut
-
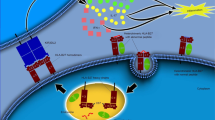
The demonstration that patients with AS have a distinct gut microbiome is consistent with theories that AS is caused by IL-23-dependent interactions between the gut microbiome and the host immune system
-
Data on AS genetics have provided preliminary support for the use of therapies targeting the IL-23 pathway and identified other potential targets, including aminopeptidases and the gut microbiome
-
Studies performed to date have uncovered less than one-third of the overall genetic risk in AS; new, larger studies should help define the immunopathogenesis of AS and related diseases
Abstract
Ankylosing spondylitis (AS), an immune-mediated arthritis, is the prototypic member of a group of conditions known as spondyloarthropathies that also includes reactive arthritis, psoriatic arthritis and enteropathic arthritis. Patients with these conditions share a clinical predisposition for spinal and pelvic joint dysfunction, as well as genetic associations, notably with HLA-B*27. Spondyloarthropathies are characterized by histopathological inflammation in entheses (regions of high mechanical stress where tendons and ligaments insert into bone) and in the subchondral bone marrow, and by abnormal osteoproliferation at involved sites. The association of AS with HLA-B*27, first described >40 years ago, led to hope that the cause of the disease would be rapidly established. However, even though many theories have been advanced to explain how HLA-B*27 is involved in AS, no consensus about the answers to this question has been reached, and no successful treatments have yet been developed that target HLA-B27 or its functional pathways. Over the past decade, rapid progress has been made in discovering further genetic associations with AS that have shed new light on the aetiopathogenesis of the disease. Some of these discoveries have driven translational ideas, such as the repurposing of therapeutics targeting the cytokines IL-12 and IL-23 and other factors downstream of this pathway. AS provides an excellent example of how hypothesis-free research can lead to major advances in understanding pathogenesis and to the development of innovative therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
de Blecourt, J., Polman, A. & de Blecourt-Meindersma, T. Hereditary factors in rheumatoid arthritis and ankylosing spondylitis. Ann. Rheum. Dis. 20, 215–223 (1961).
Brown, M. A., Laval, S. H., Brophy, S. & Calin, A. Recurrence risk modelling of the genetic susceptibility to ankylosing spondylitis. Ann. Rheum. Dis. 59, 883–886 (2000).
Thjodleifsson, B., Geirsson, A. J., Bjornsson, S. & Bjarnason, I. A common genetic background for inflammatory bowel disease and ankylosing spondylitis: a genealogic study in Iceland. Arthritis Rheum. 56, 2633–2639 (2007).
Geirsson, A. J., Kristjansson, K. & Gudbjornsson, B. A strong familiality of ankylosing spondylitis through several generations. Ann. Rheum. Dis. 69, 1346–1348 (2010).
Calin, A., Marder, A., Becks, E. & Burns, T. Genetic differences between B27 positive patients with ankylosing spondylitis and B27 positive healthy controls. Arthritis Rheum. 26, 1460–1464 (1983).
van der Linden, S., Valkenburg, H. & Cats, A. The risk of developing ankylosing spondylitis in HLA-B27 positive individuals: a family and population study. Br. J. Rheumatol. 22, 18–19 (1983).
Brown, M. A. et al. Susceptibility to ankylosing spondylitis in twins: the role of genes, HLA, and the environment. Arthritis Rheum. 40, 1823–1828 (1997).
Pedersen, O. B. et al. Ankylosing spondylitis in Danish and Norwegian twins: occurrence and the relative importance of genetic vs. environmental effectors in disease causation. Scand. J. Rheumatol. 37, 120–126 (2008).
Hamersma, J. et al. Is disease severity in ankylosing spondylitis genetically determined? Arthritis Rheum. 44, 1396–1400 (2001).
Brophy, S. et al. Concordance of disease severity among family members with ankylosing spondylitis? J. Rheumatol. 31, 1775–1778 (2004).
Duncan, E. L., Cardon, L. R., Sinsheimer, J. S., Wass, J. A. & Brown, M. A. Site and gender specificity of inheritance of bone mineral density. J. Bone Miner. Res. 18, 1531–1538 (2003).
Kwoh, C. K. et al. Age, sex, and the familial risk of rheumatoid arthritis. Am. J. Epidemiol. 144, 15–24 (1996).
Edmunds, L., Elswood, J., Kennedy, L. G. & Calin, A. Primary ankylosing spondylitis, psoriatic and enteropathic spondyloarthropathy: a controlled analysis. J. Rheumatol. 18, 696–698 (1991).
Parkes, M., Cortes, A., van Heel, D. A. & Brown, M. A. Genetic insights into common pathways and complex relationships among immune-mediated diseases. Nat. Rev. Genet. 14, 661–673 (2013).
Caffrey, M. F. & James, D. C. Human lymphocyte antigen association with ankylosing spondylitis. Nature 242, 121 (1973).
Brewerton, D. A. et al. Ankylosing spondylitis and HL-A 27. Lancet 301, 904–907 (1973).
Schlosstein, L., Terasaki, P. I., Bluestone, R. & Pearson, C. M. High association of an HL-A antigen, W27, with ankylosing spondylitis. N. Engl. J. Med. 288, 704–706 (1973).
Renwick, J. H. & Lawler, S. D. Genetical linkage between the ABO and nail–patella loci. Ann. Hum. Genet. 19, 312–331 (1955).
Stokes, P. L., Asquith, P., Holmes, G. K., Mackintosh, P. & Cooke, W. T. Histocompatibility antigens associated with adult coeliac disease. Lancet 2, 162–164 (1972).
Russell, T. J., Schultes, L. M. & Kuban, D. J. Histocompatibility (HL-A) antigens associated with psoriasis. N. Engl. J. Med. 287, 738–740 (1972).
White, S. H., Newcomer, V. D., Mickey, M. R. & Terasaki, P. I. Disturbance of HL-A antigen frequency in psoriasis. N. Engl. J. Med. 287, 740–743 (1972).
Baum, J. & Ziff, M. The rarity of ankylosing spondylitis in the black race. Arthritis Rheum. 14, 12–18 (1971).
Brown, M. A. et al. Ankylosing spondylitis in West Africans—evidence for a non-HLA-B27 protective effect. Ann. Rheum. Dis. 56, 68–70 (1997).
Concannon, P., Rich, S. S. & Nepom, G. T. Genetics of type 1A diabetes. N. Engl. J. Med. 360, 1646–1654 (2009).
Penrose, L. S. The importance of statistics in psychiatry. Proc. R. Soc. Med. 40, 863–870 (1947).
Edwards, J. H. Penrose and sib-pairs. Ann. Hum. Genet. 62, 365–377 (1998).
Brown, M. A. et al. A genome-wide screen for susceptibility loci in ankylosing spondylitis. Arthritis Rheum. 41, 588–595 (1998).
Laval, S. H. et al. Whole-genome screening in ankylosing spondylitis: evidence of non-MHC genetic-susceptibility loci. Am. J. Hum. Genet. 68, 918–926 (2001).
Carter, K. W. et al. Combined analysis of three whole genome linkage scans for ankylosing spondylitis. Rheumatology (Oxford) 46, 763–771 (2007).
Burton, P. R. et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 39, 1329–1337 (2007).
Hill, A. V. et al. HLA class I typing by PCR: HLA-B27 and an African B27 subtype. Lancet 337, 640–642 (1991).
D'Amato, M. et al. Frequency of the new HLA-B*2709 allele in ankylosing spondylitis patients and healthy individuals. Dis. Markers 12, 215–217 (1995).
López-Larrea C. et al. HLA-B27 subtypes in Asian patients with ankylosing spondylitis. Evidence for new associations. Tissue Antigens 3, 169–176 (1995).
Khan, M. A. Polymorphism of HLA-B27: 105 subtypes currently known. Curr. Rheumatol. Rep. 15, 362 (2013).
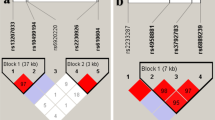
Cortes, A. et al. Major histocompatibility complex associations of ankylosing spondylitis are complex and involve further epistasis with ERAP1. Nat. Commun. http://dx.doi.org/10.1038/ncomms8146.
Strange, A. et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat. Genet. 42, 985–990 (2010).
Kirino, Y. et al. Genome-wide association analysis identifies new susceptibility loci for Behçet's disease and epistasis between HLA-B*51 and ERAP1. Nat. Genet. 45, 202–207 (2013).
Goyette, P. et al. High-density mapping of the MHC identifies a shared role for HLA-DRB1*01:03 in inflammatory bowel diseases and heterozygous advantage in ulcerative colitis. Nat. Genet. 47, 172–179 (2015).
Mielants, H. et al. Intestinal mucosal permeability in inflammatory rheumatic diseases. II. Role of disease. J. Rheumatol. 18, 394–400 (1991).
Danoy, P. et al. Association of variants at 1q32 and STAT3 with ankylosing spondylitis suggests genetic overlap with Crohn's disease. PLoS Genet. 6, e1001195 (2010).
Cortes, A. & Brown, M. A. Promise and pitfalls of the Immunochip. Arthritis Res. Ther. 13, 101 (2011).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Cortes, A. et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat. Genet. 45, 730–738 (2013).
Crane, A. M. et al. Role of NOD2 variants in spondylarthritis. Arthritis Rheum. 46, 1629–1633 (2002).
Ferreiros-Vidal, I. et al. Lack of association of ankylosing spondylitis with the most common NOD2 susceptibility alleles to Crohn's disease. J. Rheumatol. 30, 102–104 (2003).
D'Amato, M. The Crohn's associated NOD2 3020InsC frameshift mutation does not confer susceptibility to ankylosing spondylitis. J. Rheumatol. 29, 2470–2471 (2002).
Miceli-Richard, C. et al. CARD15/NOD2 analyses in spondylarthropathy. Arthritis Rheum. 46, 1405–1406 (2002).
Hugot, J. P. et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411, 599–603 (2001).
Becker, C. et al. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J. Clin. Invest. 112, 693–706 (2003).
Ciccia, F. et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 60, 955–965 (2009).
Awasthi, A. et al. Cutting edge: IL-23 receptor GFP reporter mice reveal distinct populations of IL-17-producing cells. J. Immunol. 182, 5904–5908 (2009).
Kenna, T. J. & Brown, M. A. The role of IL-17-secreting mast cells in inflammatory joint disease. Nat. Rev. Rheumatol. 45, 730–738 (2012).
Baeten, D. et al. The anti-IL17A monoclonal antibody secukinumab (AIN457) showed good safety and efficacy in the treatment of active ankylosing spondylitis [abstract L7]. In Late-breaking abstracts: American College of Rheumatology 2010 annual scientific meeting. Arthritis Rheum. 62, 3837–3845 (2010).
Poddubnyy, D., Hermann, K. G., Callhoff, J., Listing, J. & Sieper, J. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann. Rheum. Dis. 73, 817–823 (2014).
Baeten, D. et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 382, 1705–1713 (2013).
Cua, D. J. & Sherlock, J. P. Autoimmunity's collateral damage: gut microbiota strikes 'back'. Nat. Med. 17, 1055–1056 (2011).
Kenna, T. J. & Brown, M. A. Immunopathogenesis of ankylosing spondylitis. Int. J. Clin. Rheumatol. 8, 265–274 (2013).
Uotila, T. et al. Reactive arthritis in a population exposed to an extensive waterborne gastroenteritis outbreak after sewage contamination in Pirkanmaa, Finland. Scand. J. Rheumatol. 40, 358–362 (2011).
Costello, M. E. et al. Intestinal dysbiosis in ankylosing spondylitis. Arthritis Rheumatol. http://dx.doi.org/10.1002/art.38967.
Rosenbaum, J. T. et al. Does the microbiome play a causal role in spondyloarthritis? Clin. Rheumatol. 33, 763–767 (2014).
Costello, M. E., Elewaut, D., Kenna, T. J. & Brown, M. A. Microbes, the gut and ankylosing spondylitis. Arthritis Res. Ther. 15, 214 (2013).
Taurog, J. D. et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 180, 2359–2364 (1994).
Rehaume, L. M. et al. ZAP-70 genotype disrupts the relationship between microbiota and host, leading to spondyloarthritis and ileitis in SKG mice. Arthritis Rheumatol. 66, 2780–2792 (2014).
Robinson, P. C. et al. ERAP2 is associated with ankylosing spondylitis in HLA-B27-positive and HLA-B27-negative patients. Ann. Rheum. Dis. (2015).
Tsoi, L. C. et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat. Genet. 44, 1341–1348 (2012).
Hinks, A. et al. Subtype specific genetic associations for juvenile idiopathic arthritis: ERAP1 with the enthesitis related arthritis subtype and IL23R with juvenile psoriatic arthritis. Arthritis Res. Ther. 13, R12 (2011).
Franke, A. et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat. Genet. 42, 1118–1125 (2010).
Kuiper, J. J. et al. A genome-wide association study identifies a functional ERAP2 haplotype associated with birdshot chorioretinopathy. Hum. Mol. Genet. 23, 6081–6087 (2014).
Robinson, P. C. et al. Genetic dissection of acute anterior uveitis reveals similarities and differences in associations observed with ankylosing spondylitis. Arthritis Rheumatol. 67, 140–151 (2015).
Evans, D. M. et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat. Genet. 43, 761–767 (2011).
Harvey, D. et al. Investigating the genetic association between ERAP1 and ankylosing spondylitis. Hum. Mol. Genet. 18, 4204–4212 (2009).
Costantino, F. et al. ERAP1 gene expression is influenced by non-synonymous polymorphisms associated with predisposition to spondyloarthritis. Arthritis Rheumatol. 67, 1525–1534 (2015).
Andrés, A. M. et al. Balancing selection maintains a form of ERAP2 that undergoes nonsense-mediated decay and affects antigen presentation. PLoS Genet. 6, e1001157 (2010).
Haroon, N., Tsui, F. W., Chiu, B., Tsui, H. W. & Inman, R. D. Serum cytokine receptors in ankylosing spondylitis: relationship to inflammatory markers and endoplasmic reticulum aminopeptidase polymorphisms. J. Rheumatol. 37, 1907–1910 (2010).
Kochan, G. et al. Crystal structures of the endoplasmic reticulum aminopeptidase-1 (ERAP1) reveal the molecular basis for N-terminal peptide trimming. Proc. Natl Acad. Sci. USA 108, 7745–7750 (2011).
Evnouchidou, I., Weimershaus, M., Saveanu, L. & van Endert, P. ERAP1–ERAP2 dimerization increases peptide-trimming efficiency. J. Immunol. 193, 901–908 (2014).
Mear, J. P. et al. Misfolding of HLA-B27 as a result of its B pocket suggests a novel mechanism for its role in susceptibility to spondyloarthropathies. J. Immunol. 163, 6665–6670 (1999).
Kenna, T. J. et al. Disease-associated polymorphisms in ERAP1 do not alter endoplasmic reticulum stress in patients with ankylosing spondylitis. Genes Immun. 16, 35–42 (2015).
Ciccia, F. et al. Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation. Ann. Rheum. Dis. 73, 1566–1574 (2014).
Campbell, E. C., Fettke, F., Bhat, S., Morley, K. D. & Powis, S. J. Expression of MHC class I dimers and ERAP1 in an ankylosing spondylitis patient cohort. Immunology 133, 379–385 (2011).
Haroon, N., Tsui, F. W., Uchanska-Ziegler, B., Ziegler, A. & Inman, R. D. Endoplasmic reticulum aminopeptidase 1 (ERAP1) exhibits functionally significant interaction with HLA-B27 and relates to subtype specificity in ankylosing spondylitis. Ann. Rheum. Dis. 71, 589–595 (2012).
Hill, A., Takiguchi, M. & McMichael, A. Different rates of HLA class I molecule assembly which are determined by amino acid sequence in the alpha 2 domain. Immunogenetics 37, 95–101 (1993).
Sanz-Bravo, A., Campos, J., Mazariegos, M. S. & Lopez de Castro, J. A. Dominant role of the ERAP1 polymorphism R528K in shaping the HLA-B27 peptidome through differential processing determined by multiple peptide residues. Arthritis Rheumatol. 67, 692–701 (2015).
Garcia-Medel, N. et al. Functional interaction of the ankylosing spondylitis-associated endoplasmic reticulum aminopeptidase 1 polymorphism and HLA-B27 in vivo. Mol. Cell. Proteomics 11, 1416–1429 (2012).
Chen, L. et al. Critical role of endoplasmic reticulum aminopeptidase 1 in determining the length and sequence of peptides bound and presented by HLA-B27. Arthritis Rheumatol. 66, 284–294 (2014).
Visscher, P. M., Brown, M. A., McCarthy, M. I. & Yang, J. Five years of GWAS discovery. Am. J. Hum. Genet. 90, 7–24 (2012).
Okada, Y. et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 506, 376–381 (2014).
International Multiple Sclerosis Genetics Consortium. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat. Genet. 45, 1353–1360 (2013).
Zervoudi, E. et al. Rationally designed inhibitor targeting antigen-trimming aminopeptidases enhances antigen presentation and cytotoxic T-cell responses. Proc. Natl Acad. Sci. USA 110, 19890–19895 (2013).
Rivas, M. A. et al. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat. Genet. 43, 1066–1073 (2011).
Craddock, N. et al. Genome-wide association study of CNVs in 16,000 cases of eight common diseases and 3,000 shared controls. Nature 464, 713–720 (2010).
Jung, S. H. et al. Genome-wide copy number variation analysis identifies deletion variants associated with ankylosing spondylitis. Arthritis Rheumatol. 66, 2103–2112 (2014).
Ulahannan, N. & Greally, J. M. Genome-wide assays that identify and quantify modified cytosines in human disease studies. Epigenetics Chromatin 8, 5 (2015).
Australo-Anglo-American Spondyloarthritis Consortium (TASC). Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat. Genet. 42, 123–127 (2010).
Karaderi, T. et al. Ankylosing spondylitis is associated with the anthrax toxin receptor 2 gene (ANTXR2). Ann. Rheum. Dis. 73, 2054–2058 (2014).
Author information
Authors and Affiliations
Contributions
M.A.B. and T.K. researched data for the article. All authors made substantial contributions to discussion of content and wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Brown, M., Kenna, T. & Wordsworth, B. Genetics of ankylosing spondylitis—insights into pathogenesis. Nat Rev Rheumatol 12, 81–91 (2016). https://doi.org/10.1038/nrrheum.2015.133
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2015.133
This article is cited by
-
Programmed cell death 10 can be used as a potential biomarker for ankylosing spondylitis diagnosis and treatment
Spinal Cord (2024)
-
Identification of potential biomarkers for ankylosing spondylitis based on bioinformatics analysis
BMC Musculoskeletal Disorders (2023)
-
Frontiers of ankylosing spondylitis research: an analysis from the top 100 most influential articles in the field
Clinical and Experimental Medicine (2023)
-
Immunglobulin-A-Vaskulitis (früher Schoenlein-Henoch-Purpura) – ein Update
Monatsschrift Kinderheilkunde (2023)
-
Immunglobulin-A-Vaskulitis (früher Schoenlein-Henoch-Purpura) – ein Update
Die Nephrologie (2023)