Abstract
Zebrafish are making big waves in the field of cancer research. The effect has been widespread and continues to gain speed as more and more cancer researchers ride the wave of zebrafish biology. This has been largely due to the development of transgenic and xenograft models of cancer, which recapitulate many aspects of different human cancers including lymphoblastic T-cell leukemia, pancreatic cancer, melanoma and rhabdomyosarcoma. These models are already being utilized by academia and industry to search for genetic and chemical modifiers of cancer with success. The attention has been further stimulated by the amenability of zebrafish to pharmacological testing and the superior imaging properties of fish tissues that allow visualization of cancer progression and angiogenesis in live animals. This review summarizes the current zebrafish models of cancer and discusses their utility in human cancer research and future directions in the field.
Similar content being viewed by others
Introduction
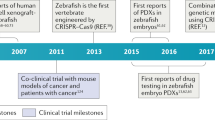
Zebrafish (Danio rerio) recently entered the stage as a promising model system to study human cancer (Table 1). While it has been known for more than a century that teleost fish can develop tumors spontaneously or in response to various chemical carcinogens, it has not been until recently that this small creature has received so much attention by cancer researchers (Amatruda et al., 2002; Smolowitz et al., 2002; Stern and Zon, 2003; Berghmans et al., 2005a; Goessling et al., 2007a). This is largely due to the development of fish lines harboring oncogenic transgenes and their amenability to genetic and pharmacological testing. Cancer progression in these animals recapitulates many aspects of human disease and opens the door for studies to identify genetic and chemical modifiers of cancer (Amatruda et al., 2002; Smolowitz et al., 2002; Stern and Zon, 2003; Berghmans et al., 2005a; Goessling et al., 2007a; Kari et al., 2007). The attention has been further fueled by the development of xenograft models that allow the propagation and visualization of human cancer cells engrafted in optically transparent zebrafish (Haldi et al., 2005; Lee et al., 2005; Topczewska et al., 2006; Nicoli et al., 2007; Stoletov et al., 2007). The integration of zebrafish genetics with the large tool chest of reagents available to study human cancer cells provides a powerful new vertebrate model to visualize and dissect the mechanisms that drive cancer formation, angiogenesis and metastasis. Finally, zebrafish have many attributes that cancer researchers find attractive. Compared to mice, zebrafish require minimal care and are cost effective to maintain in the laboratory (Amatruda et al., 2002; Smolowitz et al., 2002; Stern and Zon, 2003; Berghmans et al., 2005a; Goessling et al., 2007a; Kari et al., 2007). A pair of adult fish produces several hundred fertilized eggs a week. The embryos develop externally and are transparent up to 1 month of age (Spitsbergen, 2007; Stoletov et al., 2007). Adult, casper mutant (roy−/−;nacre−/−) animals are also available that remain transparent throughout life and are amenable to in vivo transplantation studies (Figure 1) (White et al., 2008). The remarkable transparency of zebrafish tissues allows direct imaging of cancer progression including cell invasion, intravasation, extravasation and angiogenesis (Stoletov et al., 2007; White et al., 2008). A complete sequence of the zebrafish genome is available and many human cancer genes are conserved structurally and functionally in zebrafish (http://www.ncbi.nlm.nih.gov/genome/guide/zebrafish). Zebrafish genetics is easily manipulated with numerous mutant or tissue-specific transgenic fish lines available (Amatruda et al., 2002; Smolowitz et al., 2002; Stern and Zon, 2003; Berghmans et al., 2005a; Beis and Stainier, 2006; Goessling et al., 2007a; Kari et al., 2007). Zebrafish can also efficiently absorb small molecular weight compounds directly from water which makes this organism especially appealing for screening anticancer agents (Parng et al., 2002; Kari et al., 2007).
Comparison of tissue transparency of wild-type and pigmentation mutant zebrafish. (a) Brightfield image of adult casper mutant (roy−/−;nacre−/−) and AB wild-type (b) fully pigmented zebrafish are shown (courtesy of Dr Richard White, White et al., 2008). Scale bar=1 cm.
Chemical mutagenesis
Chemical carcinogenesis was the first approach used to induce tumor formation in zebrafish. Water-soluble carcinogens can be added directly to the fish water and a number of chemical compounds that are carcinogenic in mammals induce tumor formation in zebrafish (Amatruda et al., 2002; Berghmans et al., 2005a; Goessling et al., 2007a, 2007b). Exposure to dimethylbenzanthracene, ethylnitrosourea, N-methyl-N-nitro-N-nitrosoguanidine, diethylnitrosamine results in a variety of tumors in zebrafish or medaka (Oryzias latipes) (Okihiro and Hinton, 1999; Beckwith et al., 2000; Spitsbergen et al., 2000a, 2000b; Mizgireuv et al., 2004; Lam and Gong, 2006; Lam et al., 2006; Mizgireuv and Revskoy, 2006). Most of the tumors that arise are hepatic but can also include other organs and tissues such as skin (papillomas), muscle (rhabdomyosarcomas and leiomyosarcomas), vasculature (hemangiosarcomas), testis (seminomas) and pancreas (pancreatic carcinomas) (Okihiro and Hinton, 1999; Beckwith et al., 2000; Spitsbergen et al., 2000a, 2000b; Mizgireuv et al., 2004; Lam and Gong, 2006; Lam et al., 2006; Mizgireuv and Revskoy, 2006). The basic histological and molecular characteristics of chemically induced neoplasia in zebrafish show significant similarity to human cancer including increased cell proliferation, atypical nuclear morphology and low degree of cell differentiation. Comparison of fish and human cancer gene signatures has also revealed significant resemblance between these species including genes involved in regulating the cell cycle, apoptosis and DNA repair (Lam and Gong, 2006; Lam et al., 2006). Altogether, these findings suggest that the mechanisms driving carcinogenesis are highly conserved between mammals and zebrafish.
Chemical carcinogenesis has obvious advantages in studying tumor biology due to its technical simplicity and low cost. Tumor growth under these conditions represents a spontaneous tumor initiation within the proper tissue microenvironment. Disadvantages include low incidence of tumor development (∼10%), late tumor onset and the tumors that develop are heterogenic in their genetic background and location (Table 1). Also, tumor detection requires gross visible appearance of the tumor or histological examination of fixed zebrafish tissues. Recently, these obstacles were partially overcome by the generation of chemically induced zebrafish tumor cell lines that can be propagated and transplanted into syngenic animals (Mizgireuv and Revskoy, 2006). These cell lines can be transplanted multiple times and maintain their relative growth and invasive properties in vivo. This area of zebrafish cancer biology needs to be further developed as establishment of zebrafish cancer cell lines would enable genetic profiling studies and imaging-based approaches to visualize tumor formation and invasion after labeling with fluorescent dyes or proteins. Fish cancer cell lines would also be instrumental in determining whether human oncogenes or treatment with anticancer agents directed against human cancer proteins impact fish cancer in a similar manner.
Mutant lines
The ability to perform large-scale genetic screens in zebrafish resulted in identification of several mutant zebrafish lines that have increased rates of spontaneous neoplasia and higher sensitivity to carcinogen treatment (Amsterdam et al., 2004; Berghmans et al., 2005b; Shepard et al., 2005, 2007; Wallace et al., 2005; Haramis et al., 2006; Faucherre et al., 2008) (Table 1). These mutants can be divided in two groups. Group 1 animals display mutations in known mammalian tumor suppressor genes (p53, apc, pten; Berghmans et al., 2005b; Haramis et al., 2006; Faucherre et al., 2008). Group 2 animals have mutations in genes that have not yet been linked to mammalian cancer (bmyb, separase, multiple ribosomal proteins, myosin; Amsterdam et al., 2004; Shepard et al., 2005; Wallace et al., 2005). Many of these mutants are homozygous lethal (apc, pten, separase) and therefore, require maintenance as a heterozygous population. The main features that distinguish these models from classic carcinogen-induced mutagenesis are higher rates of cancer incidence (for example, 28% for p53, ∼17% for apc, up to 100% for ribosomal proteins mutants) and that each of these mutant lines shows predisposition for a defined set of cancer types. For example, the p53 mutant line develops peripheral nerve sheath tumors while vascular and testicular cancers are common in bmyb mutants (Berghmans et al., 2005b; Shepard et al., 2005). Since the cancer gene is known (such as p53 or bmyb), secondary screens for genetic or chemical modifiers of the neoplastic phenotype can easily be performed. For example, a screen for chemical modifiers of bmyb-induced hyperplasia/cell proliferation defects resulted in the identification of a novel small molecular weight compound with anticancer properties (Stern et al., 2005). Similar to chemical-induced carcinogenesis, documentation of tumor progression and metastasis in mutant lines has been mainly limited to histological analysis of fixed tissue and organ samples that precludes monitoring these dynamic processes in vivo over time. However, Goessling et al. (2007b) have recently utilized ultrasound to image progression of chemically induced tumors in wild-type or p53-deficient zebrafish. Using this technique the authors were able to detect tumors as small as 2 mm. They were also able to monitor the response to anticancer treatment over time. This technique could provide a low-cost and noninvasive method to monitor tumor growth and therapeutic effects in live fish. It should also be possible to cross these mutant fish with the casper mutant line that remains transparent throughout adulthood or with transgenic zebrafish that express tissue-specific fluorescent marker proteins in various organs (Her et al., 2003; Beis and Stainier, 2006; Wan et al., 2006; Kari et al., 2007; White et al., 2008). This would allow direct visualization of tumor progression and distant metastases using standard brightfield and fluorescence microscopy.
Transgenic lines
Myc and TEL-AML1 leukemia models
The power of zebrafish genetics allows one to express a gene of interest (for example, oncogene or mutant form of tumor suppressor) in a particular organ or tissue through the use of tissue-specific promoters. Additionally, the gene can be engineered to coexpress various forms of fluorescent proteins. This allows for direct selection of stable transgenic lines and in vivo monitoring of tumor progression by fluorescence microscopy. This strategy was pioneered by Langenau et al. (2003) when they overexpressed human c-myc under the control of the zebrafish rag2 promoter that drives T-cell expression. The resulting transgenic zRag2-EGFP-cMyc fish line showed rapid (21–42 days) onset of T-cell acute lymphoblastic leukemia. The GFP-c-myc cells were observed to rapidly infiltrate multiple organs and tissues that eventually led to animal lethality. In fact, these animals obtained cancer so rapidly that it precluded efficient maintenance of the fish line. Subsequently, the researchers improved their model by rendering c-myc expression conditionally using a Cre/lox and heat shock promoter systems (Langenau et al., 2005a, 2005b; Feng et al., 2007). Additionally, tumor cells transplanted into irradiated recipient animals fully recapitulated the original cancer (Langenau et al., 2005a, 2005b; Feng et al., 2007). The examination of molecular pathways activated in response to c-myc overexpression closely resembled the most common and most aggressive subclass of human leukemia, which is characterized by the coexpression of tal2 and lmo2 markers (Langenau et al., 2005a). In a separate study, the same group showed that irradiation or dexamethasone-induced apoptosis of fish leukemic cells is dependent on bcl-2 gene function that also operates in mammalian forms of leukemia (Langenau et al., 2005b).
Two independent studies used a similar approach to induce different subtypes of leukemia mediated by overexpression of the aberrant TEL–AML1 fusion protein using the ubiquitous β-actin promoter or overexpression of Notch using the rag2 promoter (Sabaawy et al., 2006; Chen et al., 2007). Overexpression of TEL-AML1 led to the development of infiltrating B-lineage leukemia while rag2-mediated Notch overexpression resulted in T-cell leukemia. Both cancers also displayed similar properties with respect to mammals (Sabaawy et al., 2006; Chen et al., 2007).
MYCN and kRASG12D solid tumors models
Recently, tissue-specific oncogene overexpression was utilized in zebrafish for inducing not only blood borne neoplasia, but also various types of solid tissue cancers. MYCN overexpression, under the control of the myoD promoter, resulted in formation of pancreatic neuroendocrine tumors that originated from insulin-producing islet cells (Yang et al., 2004). In another study, conditional overexpression of activated human Ras (kRASG12D), under the control of ubiquitous β-actin promoter, resulted in several tumor types including rhabdomyosarcoma, intestinal hyperplasia and malignant peripheral nerve sheath tumors (Patton et al., 2005). These fish tumors displayed similar molecular characteristics to that of mammals and were able to infiltrate various tissues and were transplantable into syngenic animals. Interestingly, since conditional expression of the kRASG12D gene was under the control of the heat shock promoter, the authors showed that the gene could be activated ex vivo by heat shock. The cells could then be transplanted into immunosuppressed animals where they induced tumors (Le et al., 2007).
BRAFV600E melanoma model
The mutation of the serine threonine kinase B-Raf (BRAFV600E) is involved in development of human melanoma. Patton et al. (2005) showed that melanocyte-specific expression of activated human BRAFV600E in zebrafish under the control of mitfa promoter leads to multiple nevi formation. When crossed with the p53-deficient zebrafish line, the overexpression of BRAFV600E led to formation of highly invasive melanomas that had molecular characteristics similar to those of humans including mitogen-activated protein kinase activation (Patton et al., 2005). Recently, White et al. (2008) transplanted BRAFV600E; p53 melanoma cells into irradiated transparent adult casper mutant fish. The authors were able to monitor tumor progression and invasion into the distant tissues in the same animal for up to 1 month after transplantation using brightfield or fluorescent microscopy. These animals will greatly aid in the visualization of tumor progression and invasion in adult zebrafish transplanted with cancer cells or animals that acquired endogenous cancers.
kRASG12D embryonic rhabdomyosarcoma model
Langenau et al. (2007) developed a zebrafish model of a very aggressive childhood cancer, embryonic rhabdomyosarcoma (ERMS. Expression of activated kRASG12D under rag2 control led to the rapid (∼50% of mosaic transgenic fish within 3 months of age) development of ERMS. The rag2 promoter drives the gene expression not only in T- and B-cell lineages but also in olfactory rosettes, sperm and skeletal musculature. Developing tumors in skeletal muscle tissue showed a high degree of local and distant invasion with tumor cells being found in various distant organs including the intestine, kidney and testes. Remarkably, the molecular pathways activated in fish RMS showed significant overlap with human RMS (Langenau et al., 2007).
A current belief postulates that within the primary tumor only limited subsets of cells are responsible for tumor cell renewal and metastasis (cancer stem or initiating cells). Interestingly, Langenau et al. (2007) were also able to selectively label differentiated and progenitor tumor cells within the RMS tumor cell mass. They demonstrated that only the undifferentiated early muscle cell progenitors were capable of inducing tumor formation when transplanted into a recipient animal. Using this approach they were able to identify the cancer stem cell in zebrafish RMS.
Xenotransplantant models
Xenotransplantation of human cancer cells has been a major tool utilized by cancer biologists for many years. Recently, zebrafish has emerged as a new xenograft animal model for investigating human tumor cell biology. The ability to propagate human cancer cells in fish has three major advantages compared to the traditional rodent and chick xenograft models (Table 1). First, it integrates the superior imaging qualities and power of zebrafish genetics with the extensive knowledgebase of human cancer biology and the deep tool chest of reagents developed through decades of research. Second, zebrafish harboring human cells are highly amenable to pharmacological testing, which combined with their cost effectiveness makes this animal attractive for developing new cancer therapeutics. Third, zebrafish provide a relatively simple, whole vertebrate system, to decipher the mechanism(s) of drug action in vivo (Amatruda et al., 2002; Smolowitz et al., 2002; Stern and Zon, 2003; Berghmans et al., 2005a; Parng et al., 2002; Goessling et al., 2007a; Kari et al., 2007). These unique properties of zebrafish open the door to explore the underlying mechanisms that drive cancer progression in vertebrates and provide the means to develop promising preclinical agents and determine their mechanism of action.
Cancer cells derived from various species (mouse and human) and tissues (adenocarcinoma, fibrosarcoma, melanoma) have been introduced into both young embryos (2–5-days old post fertilization; Haldi et al., 2005; Lee et al., 2005; Topczewska et al., 2006; Nicoli et al., 2007) and juvenile 30-day-old zebrafish (Stoletov et al., 2007). Both transplantation models have their specific benefits and limitations when it comes to understanding cancer cell behavior in a living organism (Table 1).
A 30-day-old xenotransplantant model
The transplantation of human cancer cells in 30-day-old transparent fish was developed in our laboratory to investigate the dynamics of microtumor formation and angiogenesis in live animals using multicolor high-resolution confocal microscopy (Stoletov et al., 2007). For these experiments, we used the Tg(fli1:egfp) transgenic vascular fish and a standard Nikon confocal microscope equipped with × 10–60 long working distance objectives. This allowed us to view deep inside (200 μm) tissues the dynamics of microtumor formation and angiogenesis with unprecedented clarity. Under these conditions, detailed analysis of remodeling vessels could be observed and subcellular structures of cancer cells can be resolved including membrane protrusions and the nucleolus (Figure 2).
Human tumor cell extravasation in 2-day-old zebrafish. (a) MDA-435-DsRed tumor cells (arrows) exiting the blood vessels in the head region of a 3-day-old Tg(fli1:egfp) zebrafish embryo. Cells were injected into the heart and extravasating cells imaged 24 h post-injection with a confocal microscope. (b) Single confocal optical section of the same specimen as in (a). Arrows point to extravasating cells exiting through the blood vessel wall. Also, see Supplementary Movie 1 for the complete 3D rotation of panel (a). Image in (a) is a 3D reconstruction that was rendered using Imaris software. Colors: green, fish blood vessels; red, human tumor cells. Scale bars=20 μm.
This model system is advantageous in that it provides a clear window to visualize mechanisms of microtumor formation, cell invasion and tumor-induced angiogenesis in a mature animal. Another advantage to using juvenile fish compared to using developing embryos is that all the major organs including the vasculature have completed development and have reached their mature pattern that is the same from animal to animal (Isogai et al., 2003). This makes interpretation of tumor-induced vascular effects uncomplicated and eliminates concerns with the perturbation of developmental processes. Also, using juvenile fish as a host for human cancer cells more closely recapitulates the events associated with tumor-induced remodeling of the adult vasculature in cancer patients. However, a limitation is that 30-day-old fish have a functional immune system that must be chemically suppressed with dexamethasone for successful engraftment, whereas young embryos do not.
Most of our understanding of tumor-induced vascular remodeling and tumor cell vessel interactions has been obtained from static images captured from mature late-stage tumors. This is mostly because small microtumors and micrometastatic lesions have proven quite difficult to detect in patients and animal models of cancer (Sahai, 2007). Consequently, the early events that initiate vessel remodeling and angiogenesis during microtumor formation remain poorly defined. This is especially troublesome in regards to micrometastatic disease, which leads to death of most cancer patients. Currently, there are no therapeutic agents available to treat this fatal component of cancer.
Zebrafish (30-day-old) as a model to study microtumor formation and metastasis
Zebrafish provide a promising new model system to study microtumor formation and to develop therapeutic agents to combat the spread of cancer. In live anesthetized Tg(fli1:egfp) transgenic vascular zebrafish, we demonstrated that small tumor cell aggregates expressing DsRed and/or CFP can be directly visualized over many days (10–14) forming small microtumors, actively invading solid tissues, and remodeling the host vasculature in the body wall surrounding the peritoneal cavity. Interestingly, in these animals, microtumor formation always occurred in the body wall tissue in close association with the intersegmental vessels. While the mechanism is not known, it may involve direct homing of the cells to the vessels and/or the microenvironment of this particular tissue may provide a growth advantage. In any case, these intravital observations demonstrate that there is significant level of interaction between the human cancer cells and the microenvironment of the host. Indeed, in most cases, we found that human cancer cell lines (HT1080, fibrosarcoma; Hep3, lung adenocarcinoma) reported to be highly invasive in other vertebrate models were also highly invasive in zebrafish, whereas low invasive cells (MDA-435, breast adenocarcinoma) did not significantly disseminate in the host tissue (Zijlstra et al., 2002). Furthermore, human cancer cells harboring known metastatic genes like RhoC or src kinase display increased cell dissemination when transplanted into the peritoneal cavity of zebrafish. The dynamic interaction between host tissues and the tumor cell was clearly evident from time-lapse experiments and computer-assisted 3D morphological measurements of invading tumor cells. This high-resolution, real-time analysis revealed extensive invadopodia formation, membrane blebbing, shedding of membrane particles and cell movement. The extensive interaction of the cancer cells with the host tissues is not that surprising as many extracellular matix proteins including collagen and fibronectin are highly conserved as are the integrin adhesion receptors that mediate these interactions (Sun et al., 2005; Mould et al., 2006; Ablooglu et al., 2007).
Role of RhoC in tumor cell invasion
An interesting finding in this study was that cells expressing the metastatic gene RhoC invade tissues by utilizing a primitive amoeboid-type of invasion characterized by a rounded morphology and extensive membrane blebbing, whereas in the absence of RhoC these cells use a mesenchymal mode of invasion characterized by the formation of long extended invadopodia (Clark et al., 2000). Recent work indicates that mesenchymal migration involves the release of matrix-degrading proteases and matrix digestion, whereas amoeboid migration utilizes dynamic membrane blebbing and cell contractile processes to traverse through existing openings in the surrounding matrix independent of proteases (Friedl and Bröcker, 2000; Wolf et al., 2003). The bad news is that metastatic cells may be armed with both modes of invasion and that blocking one mode may not be sufficient to prevent cell dissemination since they can switch to the other mode. This suggests that to prevent cell invasion and metastasis in cancer patients it will be necessary to devise a strategy to knockout both invasive modes. In this regards, zebrafish seem ideal for unraveling mechanisms of human cancer cell invasion in vivo, and provide a novel system to develop therapeutic agents designed to block cell dissemination and microtumor formation.
Role of RhoC in tumor cell intravasation
While it is clear that cancer cells must gain access to the vasculature (intravasation) and lymphatic systems to metastasize, little is known about how this process occurs. Transparent 30-day-old Tg(fli1:egfp) transgenic zebrafish and confocal microscopy provide a unique opportunity to view this process in high-resolution detail. In our study, we specifically investigated how the metastatic gene RhoC mediates vascular remodeling and cell intravasation with the assumption that RhoC amplification would increase vessel remodeling and intravasation. Surprisingly, the RhoC MDA-435 (MDA-435, breast adenocarcinoma that overexpress RhoC) cells did not show increased ability to remodel vessels or intravasate, even though they show increased invasiveness in tissues and metastasis in mice (Hakem et al., 2005). In fact, the RhoC cells randomly scattered throughout the fish tissues, but did not tightly cluster around and remodel the intersegmental vessels, which is in contrast to the low metastatic control cells. This was surprising as many highly aggressive tumors display increased vascular remodeling and angiogenesis that contributes to the metastatic process (Naumov et al., 2006). How then does RhoC facilitate cancer cell metastasis?
Role of VEGF in RhoC-induced tumor cell intravasation
Since RhoC is not involved in tumorigenesis per se, we reasoned that RhoC may be a late-activating gene that facilitates intravasation after the angiogenic switch has been triggered in advanced-stage tumors (Hakem et al., 2005). To mimic the angiogenic switch in zebrafish, we engineered the MDA-435-RhoC cells to secrete human vascular endothelial growth factor (VEGF) and injected these cells into the peritoneal cavity of 30-day-old animals. Intravital imaging of the tumor cell vasculature interface in these animals revealed the following important information. First, human VEGF induced a classic angiogenic response by the host characterized by a tortuous, disorganized network of newly formed blood vessels with enlarged vessel lumens, irregular vessel wall thickness and increased leakiness due to vessel permeabilization (Jain, 2002; Fukumura and Jain, 2007). These vascular changes could be reversed by treatment of animals with the vascular endothelial growth factor receptor (VEGFR) kinase inhibitor SU5416 (Stoletov et al., 2007). Second, new vessels appeared within 3 days making this the most rapid and robust tumor-induced angiogenesis model to date. Third, optical sectioning and computer assisted 3D modeling of the neovasculature revealed small regions of the vessel wall that were extremely thin or not present at all. Upon 3D reconstruction, these areas appeared as 1–3 μm vascular openings.
Finally and most importantly, in many cases MDA-RhoC cells were no longer observed to scatter throughout the tissue, but rather were found to localize at the injection site and to directly dock onto the vascular openings where they protruded large invadopodia well into the vessel lumen. The low metastatic control cells without RhoC also docked onto the vessel openings, but they did not protrude large membrane structures into the lumen. These findings suggested that RhoC increases the ability of human cancer cells to intravasate through formation of specialized invadopodia capable of protruding through vascular openings. These findings were also corroborated in the chick CAM model where RhoC expression was also found to significantly increase cell intravasation in a VEGF-dependent manner. Together the findings indicate that RhoC and VEGF work cooperatively to facilitate tumor cell intravasation through regulation of the actin-myosin cytoskeleton and vascular remodeling. This work highlights the use of translucent zebrafish as model system to uncover mechanisms of tumor progression and cancer cell dissemination in vivo. In the future, it will be important to identify the molecular mechanisms that facilitate vascular pore formation and tumor cell docking on the vessel surface, and whether the cooperative interactions between metastatic gene expression and vascular remodeling are specific to VEGF and RhoC or whether these events also occur in response to other angiogenic agents and metastatic genes.
Early embryo xenotransplantant model
Transplantation of human cancer cells into early-stage (6–48 h post-fertilization) zebrafish embryos has also revealed important information about cancer biology as reported by several groups (Haldi et al., 2005; Lee et al., 2005; Topczewska et al., 2006; Nicoli et al., 2007). The major advantage of this model is that the immune system is still immature that permits human tumor cell engraftment. It is also possible to silence host genes using morpholinos and to perform immunostaining and in situ hybridization on whole embryos.
Early embryo model: tumor cell plasticity
Using young embryos, Lee et al. (2005) found that transplanted human melanoma cells were highly invasive and randomly scatter within the embryonic tissue, whereas primary melanocytes were often integrated normally with the developing skin. When examined for expression of melanocyte differentiation factors in vivo, aggressive melanoma cells were found to remain in a dedifferentiated state expressing both mesenchymal and epithelial markers. The authors concluded that aggressive human melanoma cells remain in a ‘plastic’ undifferentiated state, and are unable to properly respond to differentiation and migratory cues from the host environment, while differentiated melanocytes presumably can respond bi-directionally to these signals.
Interestingly, in a follow up study Lee et al. found that introduction of human melanoma cells into young zebrafish embryos could redirect normal embryonic development. Transplantation of aggressive human melanoma cells (C8161) into the animal pole promoted formation of a secondary embryonic axis while the less aggressive (C81-61) cells did not (Topczewska et al., 2006). The highly aggressive melanoma cells were found to secrete Nodal, a potent embryonic morphogen and member of the transforming growth factor-β superfamily. Consequently, the authors showed that Nodal expression correlates with human melanoma progression and inhibition of Nodal signaling impairs anchorage-independent growth and tumor formation in nude mice. Based on this work, Nodal has emerged as a promising diagnostic marker of disease progression and a possible target for development of an anticancer therapeutic. This powerful approach brings together the ability to investigate human cancer progression and developmental biology in a highly tractable animal system, which provides a promising mechanism to elucidate the complex developmental programs that control cancer cell renewal and differentiation and the epigenetic influences that modulate these cells.
Early embryo model: human tumor cell-induced angiogenesis and the role of VE-cadherin
Haldi et al. (2005) injected several human cell lines (WM-266-4 metastatic melanoma, FG pancreatic carcinoma and SW620 colon carcinoma) into the yolk sac of 2-day-old zebrafish embryos. Upon injection into the yolk sac these tumor cell lines survived, proliferated and migrated for up to a week, while normal human foreskin fibroblasts did not. In about half of the animals, the injected cells formed tumor-like masses primarily in the regions surrounding the intestine, pancreas and liver. Sectioning and immunostaining of the tumor cell masses using a specific antibody that recognizes activated zebrafish endothelial and angioblast cells revealed that endothelial cells selectively associate with tumor cell masses and form vessel-like structures. This early study demonstrated the feasibility of using young zebrafish embryos for human cancer research and laid the foundation for future work to develop efficient throughput methods for the identification of therapeutic agents that impact human cancer.
Nicoli et al. (2007) showed that when injected into 2-day-old zebrafish embryos human and murine tumorigenic cell lines (mouse aortic endothelial, MDA-MB-435 human adenocarcinoma and B16 human melanoma) induced a robust angiogenic response in association with the surrounding intestinal vascular plexus. This response was significantly increased when the injected cancer cells were genetically engineered to express human FGF2 or VEGF growth factors. The forming vessels expressed multiple markers previously associated with angiogenic vessels in mammals including Fli-1, VEGFR2/KDR and VE-cadherin. FGF2- and VEGF-induced vessel formation was specific to FGF2 or VEGF receptors since embryo exposure to chemical inhibitors that target these receptors (SU5402 and SU5416, respectively) potently abrogated the angiogenic response.
The researchers also investigate the role of VE-cadherin in mediating tumor cell-induced angiogenesis since it has been previously linked to tumor angiogenesis in mice (Wallez et al., 2006). VE-cadherin is a cell–cell adhesion molecule that mediates endothelial cell survival, proliferation and vessel tube formation. In zebrafish, the VE-cadhern/Cdh5 gene is widely expressed in angioblasts and the developing vascular system and into adulthood (Larson et al., 2004). Strikingly, this group showed that morpholino knockdown of VE-cadherin expression selectively prevented tumor cell-induced angiogenesis by FGF2, but not normal vessel development including formation of the intersegmental and subintestinal vessels. These findings are surprising in light of the fact that VE-cadherin null mice display several vascular defects in vessel assembly that cause embryonic lethality at day 9.5 (Carmeliet et al., 1999). The apparent discrepancy in these studies may be due to differences in the vascular programs utilized by fish and mammals. It is also possible that tumor-induced vessel formation in zebrafish may be uniquely sensitive to perturbation of VE-cadherin expression or that the same programs that drive tumor-induced angiogenesis are different from those that drive developmental vasculogenesis. Although the level of VE-cadherin expression was not examined in situ in this study, the morpholino knockdown of a gene product is rarely complete. The possibility remains that the residual VE-cadherin is sufficient to support developmental vasculogenesis, but not tumor-induced neovascularization. In any case, this work highlights the power of zebrafish embryos and morpholino technology to dissect the molecular differences that give rise to normal and tumor-associated vessels. The caveat is that it is not clear whether human cells can initiate new vessel growth or whether it results from the redirection of developing vessels already in the process of forming. The developing vessels may respond differently to tumor-induced angiogenic factors compared to mature, fully developed vessels. The 30-day-old fish seem better suited to address this type of question since the vasculature has obtained its final form.
Future directions
Within the past several years, zebrafish has shown great promise to become a powerful animal model to study cancer progression. Despite the significant progress additional work is necessary to fully explore how closely these processes parallel mammalian cancer mechanisms and how well they translate to human disease. This will require detailed comparative studies among transgenic and xenograft zebrafish and mouse models of cancer and their relevance to the disease. The experimental results then need to be carefully interrogated for their relevance to human disease. Only when zebrafish has undergone this degree of scrutiny will its full potential be realized and its acceptance widely embraced by the cancer research community.
If zebrafish are going to contribute significantly to our understanding of cancer, then noninvasive and quantitative methodologies for imaging tumor formation and distant metastases will have to be developed, especially in regards to monitoring cancer progression in adult animals. It may be possible to reconfigure the various tumor detection methods established in mice for use on small zebrafish as was the case with ultrasound technology. The development of imaging technology should also include fluorescence protein-based approaches and quantitative software capable of measuring cancer progression over many days and months in live fish. The recent development of a transparent adult zebrafish line (casper) similar to the transparent Medaka TRA line will undoubtedly improve imaging of adult zebrafish tumors and their metastases (Wakamatsu et al., 2001; White et al., 2008). Continued effort needs to be put into developing environmentally controlled systems that facilitate prolonged time-lapse imaging of live animals for low- and high-resolution applications. Finally, the reengineering of existing high throughput imaging systems and liquid handling instrumentation for use with zebrafish embryos would be extremely valuable for gene and chemical screening studies (Parng et al., 2002).
While several groups have reported that zebrafish tumors can grow to a significant size and invade surrounding tissues, the ability of these cells to metastasize has not been formally demonstrated. In some cases invasive RMS or BRAFV600E melanoma cells were found at significant distances from the primary tumor site (Langenau et al., 2007; White et al., 2008). It remains to be determined whether the spread of cancer under these conditions was due to cell dissemination through the vasculature/lymphatics or resulted from cell invasion of the tissues. In our study, we showed that invasive human tumor cells can readily penetrate the remodeling blood vessel wall, which is a critical step in the metastatic process. We have also observed in preliminary studies that human cancer cells, when injected directly into the vasculature of 2-day-old zebrafish embryos, lodge in small vessels in various organs and extravasate into the surrounding tissue (Stoletov et al., 2007; Figure 3). Haldi et al. (2005) using human melanoma cells reported similar results. While these initial results are encouraging, additional work needs to be done to confirm that fish and human cancer cells metastasize using the typical vascular and lymphatic routes, and that similar mechanisms known to drive these processes in mammals also operate in zebrafish. If this turns out to be the case, then zebrafish could be a tractable model to address this troublesome problem that has faced cancer researchers for many years.
Human tumor cell-induced angiogenesis in 30-day-old zebrafish. (a, b) MDA-435-DsRed tumor cells secreting human vascular endothelial growth factor (VEGF) in the body wall of Tg(fli1:egfp) zebrafish at 4 (a), and 5 (b) days post-injection. Images were obtained from the same animal on consecutive days using a confocal microscope. (c, d) Single confocal optical sections of the tumors in (a) and (b). Note, that tumor cells nuclei can be seen. (e, f) Single blood vessel contacting tumor cells from the dotted squares in (a) and (b) that were digitally isolated using Imaris software. Insets show the interior vessel surface at sites of vessel openings and tumor cell membrane integration (dotted squares in e and f). Images in (a, b, e, f) are 3D reconstructions of confocal z-stacks that were rendered using Imaris software. Colors: green, fish blood vessels; red (or gray in c, d), human tumor cells. Scale bars=20 μm. Modified from Stoletov et al. (2007).
It is well recognized that the vasculature of the tumor differs significantly in molecular and morphological characteristics compared to the normal blood system (Fukumura and Jain, 2007). These abnormalities are being exploited as a potential means to direct antitumor therapies. While tumors growing in zebrafish have been reported to be vascularized, it is not yet clear if the vessels display similar morphological and biochemical markers as mammals. We found that human tumor cells secreting VEGF induce new vessels in zebrafish with a tortuous, irregular shape, altered thickness of the vessel wall and increased permeability, all of which typify the events observed in mammalian tumor development. However, additional work is necessary to determine if these vessels are expressing similar vascular markers established in mammals and whether the actual process of vessel formation is governed by the same mechanisms that drive tumor-induced angiogenesis in mammals. Nevertheless, this is a worthy pursuit since zebrafish is the only whole animal model with the potential for cost-effective, large-scale screens, to identify chemical or genetic modifiers that alter tumor dissemination and/or angiogenesis (Parng et al., 2002).
Xenotransplantation of human cells into immunocompromised mouse strains has greatly aided cancer researchers over the years. Currently, tumor cell transplantation in zebrafish requires chemical immunosuppression of the animals or tumor cell transplantation of syngenic zebrafish cell lines. The development of zebrafish with an impaired immune system similar to that of severe combined immunodeficiency or nude mice will allow efficient transplantation of mammalian and zebrafish cell lines into young and adult animals. However, while a large supply of mammalian cancer cell lines exists, there are no zebrafish cancer cell lines permanently established in culture. Consequently, significant effort needs to be put into developing fish cancer cell lines harboring known oncogenes relevant to human cancer. This will open the door for performing functional cell-based studies, prescreening genetic and chemical modifiers of cancer, and the development of antibodies to zebrafish proteins that are sorely needed.
Finally, there is a continued need to develop transgenic animals that develop cancer in response to tissue-specific expression of known oncogenes fused with marker proteins for imaging purposes and inducible promoter systems for controlled expression. These animals should then be crossed with transgenic animals that report changes in the blood vessel state and tumor–immune cell interactions. These animals would greatly facilitate real-time imaging of the tumor-induced vascular remodeling events and the dynamic immune cell movements that occur in the tumor microenvironment. Many transgenic lines have already been developed including T cell, macrophage and neutrophil reporter animals (Langenau et al., 2004; Mathias et al., 2006; Hall et al., 2007).
In summary, zebrafish is a major catch for cancer researchers offering a number of unique advantages for investigating the mechanisms that drive cancer formation and progression. At the top of the list are its optical clarity, genetic tractability and amenability to pharmacological testing. It is no wonder that cancer researchers are taking notice as these attributes provide new avenues for significant discoveries and an affordable whole animal system for therapeutic development. Now that zebrafish are clearly in the eyes of cancer researchers it will be fascinating to witness the evolution of zebrafish cancer research as it fulfills its many promises in the upcoming years.
References
Ablooglu AJ, Kang J, Handin RI, Traver D, Shattil SJ . (2007). The zebrafish vitronectin receptor: characterization of integrin alphaV and beta3 expression patterns in early vertebrate development. Dev Dyn 236: 2268–2276.
Amatruda JF, Shepard JL, Stern HM, Zon LI . (2002). Zebrafish as a cancer model system. Cancer Cell 3: 229–231.
Amsterdam A, Sadler KC, Lai K, Farrington S, Bronson RT, Lees JA et al. (2004). Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biol 2: E139.
Beckwith LG, Moore JL, Tsao-Wu GS, Harshbarger JC, Cheng KC . (2000). Ethylnitrosourea induces neoplasia in zebrafish (Danio rerio). Lab Invest 80: 379–385.
Beis D, Stainier DY . (2006). In vivo cell biology: following the zebrafish trend. Trends Cell Biol 16: 105–112.
Berghmans S, Jette C, Langenau D, Hsu K, Stewart R, Look T et al. (2005a). Making waves in cancer research: new models in the zebrafish. Biotechniques 39: 227–237.
Berghmans S, Murphey RD, Wienholds E, Neuberg D, Kutok JL, Fletcher CD et al. (2005b). tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci USA 102: 407–412.
Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F et al. (1999). Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 198: 147–157.
Chen J, Jette C, Kanki JP, Aster JC, Look AT, Griffin JD . (2007). NOTCH1-induced T-cell leukemia in transgenic zebrafish. Leukemia 21: 462–471.
Clark EA, Golub TR, Lander ES, Hynes RO . (2000). Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406: 532–535.
Faucherre A, Taylor GS, Overvoorde J, Dixon JE, Hertog JD . (2008). Zebrafish pten genes have overlapping and non-redundant functions in tumorigenesis and embryonic development. Oncogene 27: 1079–1086.
Feng H, Langenau DM, Madge JA, Quinkertz A, Gutierrez A, Neuberg DS et al. (2007). Heat-shock induction of T-cell lymphoma/leukaemia in conditional Cre/lox-regulated transgenic zebrafish. Br J Haematol 138: 169–175.
Friedl P, Bröcker EB . (2000). The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci 57: 41–64.
Fukumura D, Jain RK . (2007). Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res 74: 72–84.
Goessling W, North TE, Zon LI . (2007a). New waves of discovery: modeling cancer in zebrafish. J Clin Oncol 25: 2473–2479.
Goessling W, North TE, Zon LI . (2007b). Ultrasound biomicroscopy permits in vivo characterization of zebrafish liver tumors. Nat Methods 4: 551–553.
Hakem A, Sanchez-Sweatman O, You-Ten A, Duncan G, Wakeham A, Khokha R et al. (2005). RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev 19: 1974–1979.
Haldi M, Ton C, Seng WL, McGrath P . (2005). Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis 9: 139–151.
Hall C, Flores MV, Storm T, Crosier K, Crosier P . (2007). The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev Biol 7: 42–59.
Haramis AP, Hurlstone A, van der Velden Y, Begthel H, van den Born M, Offerhaus GJ et al. (2006). Adenomatous polyposis coli-deficient zebrafish are susceptible to digestive tract neoplasia. EMBO Rep 7: 444–449.
Her GM, Chiang CC, Chen WY, Wu JL . (2003). In vivo studies of liver-type fatty acid binding protein (L-FABP) gene expression in liver of transgenic zebrafish (Danio rerio). FEBS Lett 538: 125–133.
Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM . (2003). Angiogenic network formation in the developing vertebrate trunk. Development 130: 5281–5290.
Jain RK . (2002). Tumor angiogenesis and accessibility: role of vascular endothelial growth factor. Semin Oncol 29: 3–9.
Kari G, Rodeck U, Dicker AP . (2007). Zebrafish: an emerging model system for human disease and drug discovery. Clin Pharmacol Ther 82: 70–80.
Lam SH, Gong Z . (2006). Modeling liver cancer using zebrafish: a comparative oncogenomics approach. Cell Cycle 5: 573–577.
Lam SH, Wu YL, Vega VB, Miller LD, Spitsbergen J, Tong Y et al. (2006). Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat Biotechnol 24: 73–75.
Langenau DM, Feng H, Berghmans S, Kanki JP, Kutok JL, Look AT . (2005a). Cre/lox-regulated transgenic zebrafish model with conditional myc-induced T cell acute lymphoblastic leukemia. Proc Natl Acad Sci USA 102: 6068–6073.
Langenau DM, Ferrando AA, Traver D, Kutok JL, Hezel JP, Kanki JP et al. (2004). In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc Natl Acad Sci USA 101: 7369–7374.
Langenau DM, Jette C, Berghmans S, Palomero T, Kanki JP, Kutok JL et al. (2005b). Suppression of apoptosis by bcl-2 overexpression in lymphoid cells of transgenic zebrafish. Blood 105: 3278–3285.
Langenau DM, Keefe MD, Storer NY, Guyon JR, Kutok JL, Le X et al. (2007). Effects of RAS on the genesis of embryonal rhabdomyosarcoma. Genes Dev 21: 1382–1395.
Langenau DM, Traver D, Ferrando AA, Kutok JL, Aster JC, Kanki JP et al. (2003). Myc-induced T cell leukemia in transgenic zebrafish. Science 299: 887–890.
Larson JD, Wadman SA, Chen E, Kerley L, Clark KJ, Eide M et al. (2004). Expression of VE-cadherin in zebrafish embryos: a new tool to evaluate vascular development. Dev Dyn 231: 204–213.
Le X, Langenau DM, Keefe MD, Kutok JL, Neuberg DS, Zon LI . (2007). Heat shock-inducible Cre/Lox approaches to induce diverse types of tumors and hyperplasia in transgenic zebrafish. Proc Natl Acad Sci USA 104: 9410–9415.
Lee LM, Seftor EA, Bonde G, Cornell RA, Hendrix MJ . (2005). The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev Dyn 233: 1560–1570.
Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A . (2006). Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol 80: 1281–1288.
Mizgireuv IV, Majorova IG, Gorodinskaya VM, Khudoley VV, Revskoy SY . (2004). Carcinogenic effect of N-nitrosodimethylamine on diploid and triploid zebrafish (Danio rerio). Toxicol Pathol 5: 514–518.
Mizgireuv IV, Revskoy SY . (2006). Transplantable tumor lines generated in clonal zebrafish. Cancer Res 66: 3120–3125.
Mould AP, McLeish JA, Huxley-Jones J, Goonesinghe AC, Hurlstone AF, Boot-Handford RP et al. (2006). Identification of multiple integrin beta1 homologs in zebrafish (Danio rerio). BMC Cell Biology 7: 24–39.
Naumov GN, Akslen LA, Folkman J . (2006). Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle 5: 1779–1787.
Nicoli S, Ribatti D, Cotelli F, Presta M . (2007). Mammalian tumor xenografts induce neovascularization in zebrafish embryos. Cancer Res 67: 2927–2931.
Okihiro MS, Hinton DE . (1999). Progression of hepatic neoplasia in medaka (Oryzias latipes) exposed to diethylnitrosamine. Carcinogenesis 20: 933–940.
Parng C, Seng WL, Semino C, McGrath P . (2002). Zebrafish: a preclinical model for drug screening. Assay Drug Dev Technol 1: 41–48.
Patton EE, Widlund HR, Kutok JL, Kopani KR, Amatruda JF, Murphey RD et al. (2005). BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol 15: 249–254.
Sabaawy HE, Azuma M, Embree LJ, Tsai HJ, Starost MF, Hickstein DD . (2006). TEL-AML1 transgenic zebrafish model of precursor B cell acute lymphoblastic leukemia. Proc Natl Acad Sci USA 103: 15166–15171.
Sahai E . (2007). Illuminating the metastatic process. Nat Rev Cancer 7: 737–749.
Shepard JL, Amatruda JF, Finkelstein D, Ziai J, Finley KR, Stern HM et al. (2007). A mutation in separase causes genome instability and increased susceptibility to epithelial cancer. Genes Dev 21: 55–59.
Shepard JL, Amatruda JF, Stern HM, Subramanian A, Finkelstein D, Ziai J et al. (2005). A zebrafish bmyb mutation causes genome instability and increased cancer susceptibility. Proc Natl Acad Sci USA 102: 13194–13199.
Smolowitz R, Hanley J, Richmond H . (2002). A three-year retrospective study of abdominal tumors in zebrafish maintained in an aquatic laboratory animal facility. Biol Bull 203: 265–266.
Spitsbergen J . (2007). Imaging neoplasia in zebrafish. Nat Methods 4: 548–549.
Spitsbergen JM, Tsai HW, Reddy A, Miller T, Arbogast D, Hendricks JD et al. (2000a). Neoplasia in zebrafish (Danio rerio) treated with N-methyl-N′-nitro-N-nitrosoguanidine by three exposure routes at different developmental stages. Toxicol Pathol 28: 716–725.
Spitsbergen JM, Tsai HW, Reddy A, Miller T, Arbogast D, Hendricks JD et al. (2000b). Neoplasia in zebrafish (Danio rerio) treated with 7,12-dimethylbenz[a]anthracene by two exposure routes at different developmental stages. Toxicol Pathol 28: 705–715.
Stern HM, Murphey RD, Shepard JL, Amatruda JF, Straub CT, Pfaff KL et al. (2005). Small molecules that delay S phase suppress a zebrafish bmyb mutant. Nat Chem Biol 1: 366–370.
Stern HM, Zon LI . (2003). Cancer genetics and drug discovery in the zebrafish. Nat Rev Cancer 3: 533–539.
Stoletov K, Montel V, Lester RD, Gonias SL, Klemke R . (2007). High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc Natl Acad Sci USA 104: 17406–17411.
Sun L, Zou Z, Collodi P, Xu F, Xu X, Zhao Q . (2005). Identification and characterization of a second fibronectin gene in zebrafish. Matrix Biol 24: 69–77.
Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW et al. (2006). Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med 12: 925–932.
Wakamatsu Y, Pristyazhnyuk S, Kinoshita M, Tanaka M, Ozato K . (2001). The see-through medaka: a fish model that is transparent throughout life. Proc Natl Acad Sci USA 98: 10046–10050.
Wallace KN, Dolan AC, Seiler C, Smith EM, Yusuff S, Chaille-Arnold L et al. (2005). Mutation of smooth muscle myosin causes epithelial invasion and cystic expansion of the zebrafish intestine. Dev Cell 8: 717–726.
Wallez Y, Vilgrain I, Huber P . (2006). Angiogenesis: the VE-cadherin switch. Trends Cardiovasc Med 16: 55–59.
Wan H, Korzh S, Li Z, Mudumana SP, Korzh V, Jiang YJ et al. (2006). Analyses of pancreas development by generation of gfp transgenic zebrafish using an exocrine pancreas-specific elastaseA gene promoter. Exp Cell Res 312: 1526–1539.
White RM, Zon LI, Sessa A, Burke CJ, Bowman T, Leblanc J et al. (2008). Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2: 183–189.
Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI et al. (2003). Compensation mechanism in tumor cell migration: mesenchymal–amoeboid transition after blocking of pericellular proteolysis. J Cell Biol 160: 267–277.
Yang HW, Kutok JL, Lee NH, Piao HY, Fletcher CD, Kanki JP et al. (2004). Targeted expression of human MYCN selectively causes pancreatic neuroendocrine tumors in transgenic zebrafish. Cancer Res 64: 7256–7562.
Zijlstra A, Mellor R, Panzarella G, Aimes RT, Hooper JD, Marchenko ND et al. (2002). A quantitative analysis of rate-limiting steps in the metastatic cascade using human-specific real-time polymerase chain reaction. Cancer Res 62: 7083–7092.
Acknowledgements
We thank Dr Mike Fried and Dr Tony Karnezis (UCSF), and Laurie Gay (UCSD) for critical reading of the paper. This work was supported by CBCRP postdoctoral fellowship no. 11FB-0088 (to KS) and NIH grants GM068487 and CA097022 (to RK). We also thank Dr Richard White for providing images of zebrafish casper mutant and discussion of its possible applications.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Stoletov, K., Klemke, R. Catch of the day: zebrafish as a human cancer model. Oncogene 27, 4509–4520 (2008). https://doi.org/10.1038/onc.2008.95
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.95
Keywords
This article is cited by
-
Anti-angiogenesis and anti-metastasis effects of Polyphyllin VII on Hepatocellular carcinoma cells in vitro and in vivo
Chinese Medicine (2021)
-
Edelfosine nanoemulsions inhibit tumor growth of triple negative breast cancer in zebrafish xenograft model
Scientific Reports (2021)
-
The use of zebrafish model in prostate cancer therapeutic development and discovery
Cancer Chemotherapy and Pharmacology (2021)
-
Long-term in vivo imaging reveals tumor-specific dissemination and captures host tumor interaction in zebrafish xenografts
Scientific Reports (2020)
-
Gelidiella acerosa inhibits lung cancer proliferation
BMC Complementary and Alternative Medicine (2018)