Abstract
Glioblastoma multiforme (GBM) is the most aggressive and the commonest primary brain tumor with a tendency for local invasiveness. The pathways of neoplasia, invasion and inflammation are inextricably linked in cancer and aberrations in several regulatory pathways for these processes have been identified. Here we have studied the FAT1 (Homo sapiens FAT tumor-suppressor homolog 1 (Drosophila)) gene to identify its role in the tumorigenecity of the gliomas. The expression of FAT1 was found to be high in grade IV glioma cell lines (U87MG, A172, U373MG and T98G) but low in grade III glioma cell lines (GOS3 and SW1088). Two cell lines (U87MG and A172) with high FAT1 expression were chosen for in vitro FAT1-knockdown studies. FAT1 knockdown by small interfering RNA resulted in decreased migration and invasion of both the cell lines along with increased expression of the tumor-suppressor gene programmed cell death 4 (PDCD4). Increased PDCD4 expression led to the attenuation of activator protein-1 (AP-1) transcription by inhibiting c-Jun phosphorylation and resulted in concomitant decrease in the expression of AP-1-target genes like MMP3, VEGF-C and PLAU, the pro-inflammatory regulator COX-2 and cytokines IL1β and IL-6. Conversely, simultaneous silencing of PDCD4 and FAT1 in these cells significantly enhanced AP-1 activity and expression of its target genes, resulting in increase in mediators of inflammation and in enhanced migratory and invasive properties of the cells. We also observed a negative correlation between the expression of FAT1 and PDCD4 (P=0.0145), a positive correlation between the expression of FAT1 and COX-2 (P=0.048) and a similar positive trend between FAT1 and IL-6 expression in 35 primary human GBM samples studied. Taken together, this study identifies a novel signaling mechanism mediated by FAT1 in regulating the activity of PDCD4 and thereby the key transcription factor AP-1, which then affects known mediators of neoplasia and inflammation.
Similar content being viewed by others
Introduction
The signaling pathways propagated from the cell surface, through transmembrane receptors, to intracellular regulatory molecules are critical for maintaining cellular homeostasis. Knowledge of how these transmembrane proteins regulate the terminal components of the signaling pathway is crucial for understanding normal as well as aberrant cellular function. The link between cancer and inflammation is well established and a set of common molecules (for example, COX-2, IL-1β, IL-6 and so on) may mediate both the processes.1, 2, 3, 4, 5 Various molecular pathways that promote tumor invasion and expression of pro-inflammatory molecules in cancer have been identified6, 7 but many more are yet to be explored.
Glioblastoma multiformes (GBMs) are the most frequent and most malignant form of brain tumors.8, 9 Tumor angiogenesis, invasiveness and rapid growth go in concert in GBM.10, 11, 12 The tumor microenvironment exhibits expression of pro-inflammatory molecules that promote migration and invasion of tumor cells.13, 14 There is increasing evidence of the role of the pro-inflammatory molecules in making glioma and other tumors more aggressive and resistant to chemo- and/or radio-therapy.15, 16, 17
The common pathways may promote tumor invasiveness and expression of pro-inflammatory molecules in GBM.13, 18 Of these, COX-2 and cytokines like IL-1β and IL-6 are the known mediators of both the processes.19, 20 However, the therapeutic translation of this knowledge has been limited.21 Here we have studied the role of FAT1, a transmembrane protein, in linking the neoplastic phenotype and inflammatory mediators in glial cells and have corroborated some of the results in primary human tumors.
FAT1 is a member of cadherin superfamily22, 23 and was first identified as a tumor suppressor in Drosophila melanogaster, acting via the Salvador–Warts–Hippo signaling pathway.24, 25, 26, 27, 28, 29, 30 There are contrasting studies about the role of FAT1 in human cancers, and its precise role in development and progression of human cancer is still being investigated.
Available literature on FAT1 in human cancers points toward its dual role, both as an oncogene as well as tumor suppressor. Overexpression of FAT1 has been reported in invasive breast carcinoma31 and leukemia,32 and loss of FAT1 has been reported to inhibit migration and invasion in OSCC,33 indicating the oncogenic role of FAT1 in these tumors. However, tumors like oral cancer34 and Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome35 have shown deletion/loss-of-heterozygosity of the chromosomal region 4q35 harboring FAT1 gene, suggesting its tumor-suppressor role. An initial report from our laboratory on FAT1 in glioma36 had shown high loss-of-heterozygosity and low mRNA expression in 33% of the tumors studied and suggested that it may have a tumor-suppressor role. However, the signaling cascades and cellular processes through which FAT1 acts in different contexts are still being elucidated and very few functional studies are available on the role of FAT1 in human cancers, including GBM.
Programmed cell death 4 (PDCD4) is a known tumor-suppressor gene and it has an essential role in many biological processes like regulating cap-dependent translation, apoptosis, modulating various signal transduction pathways and so on.37, 38, 39 The expression of PDCD4 is often decreased in human glioma40 and many other progressive tumors like lung, breast41, 42 and so on, leading to increased invasiveness and metastasis.43, 44, 45 In addition, PDCD4 is also reported to suppress induction of inflammatory mediators.46, 47 PDCD4 is known to inhibit activator protein-1 (AP-1)-mediated transcription,48, 49, 50 which has a central role in multiple processes involved in tumorigenesis, including proliferation, migration and invasion,51, 52, 53, 54, 55, 56, 57 and AP-1 inhibition had been shown to have anti-invasive and anti-growth effect.58, 59
In order to elucidate the role of FAT1 in human glioma, we studied its expression in several glioma cell lines followed by knockdown in two GBM cell lines (U87MG and A172) with high FAT1 expression. There was a marked reduction in cell motility and invasiveness, as well as upregulation of PDCD4 expression. This in turn reduced AP-1 transcriptional activity, thus affecting transcription of downstream genes, including extra cellular matrix (ECM)-remodeling molecules (MMP3, PLAU and VEGF-C) and pro-inflammatory markers (COX-2, IL1β and IL-6). This process was reversed by simultaneous knockdown of FAT1 and PDCD4, thus validating the link between FAT1 and PDCD4 in regulating cellular motility, invasiveness and inflammatory microenvironment in glioma. These in vitro findings were further supported by the inverse relationship observed between mRNA levels of FAT1 and PDCD4 and a positive correlation between the transcript levels of FAT1 and COX-2, as well as of FAT1 and IL-6 in primary human GBM. Thus, this study identifies a novel function of FAT1 in regulating AP-1-mediated transcription via PDCD4.
Results
FAT1 knockdown reduces migration and invasion of glioma cells
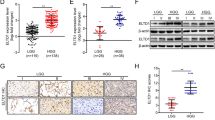
FAT1 mRNA expression was checked in a panel of glioma cell lines and high FAT1 expression was observed in grade IV glioma (GBM) cell lines (U87MG, A172, U373MG and T98G) as compared with grade III glioma cell lines (GOS3 and SW1088) (Figure 1a). The cell lines U87MG and A172 were studied further to analyze the effect of FAT1 knockdown. Knockdown efficiency of FAT1 in cells was checked by using a set of three FAT1-specific small interfering RNA (siRNA) (details in Materials and methods section) from Invitrogen (Carlsbad, CA, USA), and FAT1 siRNA I (HSS176716) was found to have maximum knockdown efficiency (Supplementary Figure S1). FAT1 siRNA I was used for further experiments. We found ⩾90% knockdown of FAT1 mRNA expression in FAT1 siRNA-treated cells (U87MGsiFAT1 and A172siFAT1) as compared with control siRNA-treated cells (siControl; Invitrogen), 72 h post transfection (Figure 1b). There were significant morphological alterations (cells were more spindly) in siFAT1-treated cells (Figure 1c).
Knockdown of FAT1 inhibited glioma cell migration and invasion. (a) FAT1 expression was checked in six glioma cell lines by q–PCR, out of which grade IV glioma cell lines U87MG, A172, U373MG and T98G were found to have high FAT1 expression, whereas grade III glioma cell lines GOS3 and SW1088 had low FAT1 expression. 18S was used as internal control. (b) Knockdown of FAT1 in U87MG and A172 cell lines (72 h post transfection) was confirmed by q–PCR using FAT1-specific primers. We found ⩾90% downregulation of FAT1 mRNA in siFAT1 cells as compared with siControl-treated cells. 18S was used as internal control; all experiments were done in triplicates and repeated thrice. (c) Distinct phenotypic changes (72 h post transfection) were observed in U87MGsiFAT1 and A172siFAT1 cells as compared with siControl cells. siFAT1-treated cells were spindle shaped or rounded, scattered and had reduced cell–cell interaction. (d, e) FAT1 knockdown inhibited cell migration and invasion in U87MGsiFAT1 as well as A172siFAT1 cells as compared with siControl cells. Modified Boyden chamber assay/matrigel assay was performed for 24 h (72 h post transfection) to assess cell migration and invasion. Cells that migrated across the membrane or invaded through matrigel were fixed, stained and were counted in five different fields and means were calculated. The number of cells that migrated or invaded in siFAT1 cells were normalized against number of cells that migrated in siControl cells. Each value is expressed as mean±s.d. Each experiment was done in triplicate and repeated twice. Significant differences were observed in siFAT1-treated cells as compared with siControl cells as determined by Student’s t-test. Mock: lipofactamin-treated cells; siControl: Invitrogen universal medium GC control siRNA-treated cells and siFAT1: FAT1-specific siRNA-treated (Invirogen) cells. A full colour version of this figure is available at the Oncogene journal online.
We observed significant reduction in migration (about 50% decrease in U87MGsiFAT1 (P<0.01) and about 75% decrease in A172siFAT1 cells (P<0.001)) (Figure 1d) as well as invasion (about 60% decrease in U87MGsiFAT1 and about 40% decrease in A172siFAT1 cells (P<0.01)) (Figure 1e) upon FAT1 knockdown as compared with siControl cells. There was no variation in the distribution of cell population in different phases of cell cycle, as assessed by fluorescence-activated cell sorting analysis (Supplementary Figure S2a), as well as in cell viability as assessed by MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Supplementary Figure S2b), after FAT1 knockdown. Further, there was no DNA fragmentation (4',6-diamidino-2-phenylindole staining) and no cleaved caspase-3 (western blot) after 72 h of transfection (Supplementary Figures S2c and d), indicating that FAT1 knockdown did not affect cell viability and apoptosis.
FAT1 knockdown enhances PDCD4 expression
In initial screening by microarray (unpublished data) to study altered gene expression after FAT1 knockdown in U87MG cell line, we identified PDCD4 as one of the upregulated genes. This was further confirmed by quantitative PCR (q–PCR) and western blot analysis in four GBM cell lines, U87MG, A172, U373MG and T98G. There was significant (P<0.05) upregulation of about 2.4, 2.8, 3.2 and 3.5 fold in PDCD4 mRNA expression (Figure 2a) as well as in PDCD4 protein expression (Figure 2b) in siFAT1-treated U87MG, A172, U373MG and T98G cells, respectively, as compared with their respective siControl-treated cells. The grade III glioma cell line, GOS3, with low endogenous FAT1 expression (Figure 1a) had high PDCD4 mRNA expression (Figure 2c), corroborating the inverse relationship between FAT1 and PDCD4.
Knockdown of FAT1 upregulated PDCD4 expression. (a) PDCD4 mRNA expression as assessed by q–PCR (72 h post FAT1 siRNA transfection) was found to be increased by more than two fold in all the GBM cell lines (U87MG, A172, U373 and T98) analyzed after FAT1 knockdown as compared with their respective siControl cells. 18 S was used as internal control (b) Similarly PDCD4 protein level was also found to be increased in the above GBM cell lines after FAT1 knockdown as compared with respective siControl cells as assessed by western blot analysis. β-actin was used as loading control. (c) GOS3 (World Health Organization grade III glioma) cell line with low endogenous FAT1 was found to have high PDCD4 mRNA expression, which was comparable with U87MGsiFAT1-treated cells. A full colour version of this figure is available at the Oncogene journal online.
In literature, phospho-Akt and PDCD4 are known to negatively regulate each others expression.37, 60, 61 However, we observed increased phospho-Akt levels (Supplementary Figure S3) along with increased PDCD4 expression (Figures 2a, b) after FAT1 knockdown in U87MG cells, suggesting that increased PDCD4 expression after FAT1 knockdown was independent of the p-Akt pathway.
FAT1 knockdown diminishes AP-1-mediated transcription
PDCD4 is reported to inhibit AP-1-dependent transcription48, 62 via suppression of c-Jun phosphorylation.49, 50 Because c-Jun phosphorylation is required for AP-1 activity, we investigated the effect of FAT1 knockdown on c-Jun phosphorylation status by western blotting and found to be significantly decreased along with reduction in the total c-Jun protein level in U87MGsiFAT1 cells as compared with siControl cells (Figure 3a). The c-jun mRNA level, as checked by q–PCR, was also found to be markedly decreased (0.51±0.12, P<0.01) in U87MGsiFAT1 cells as compared with U87MGsiControl cells (Figure 3b). This could be due to decreased positive autoregulatory loop of c-jun transcription by AP-1.63, 64 Hence, the reduction in the total c-Jun protein level in U87MGsiFAT1 cells may be due to both reduction in the c-jun mRNA level and ubiquitination and fast degradation of unphosphorylated c-Jun.50, 65, 66 However, the exact mechanisms leading to decrease in p-c-Jun levels need to be elucidated further.
Inhibition of AP-1 transcriptional activity after FAT1 knockdown. (a) FAT1 knockdown in U87MG cells upregulated PDCD4 expression, which in turn inhibits phosphorylation of c-Jun. Western blot was performed on cell lysates from U87MGsiFAT1 and siControl cells with phospho-c-Jun (Ser63) and c-Jun antibodies. There was significant reduction in both the c-Jun and p-c-Jun level. β-actin antibody was used as loading control. (b) The mRNA level of c-jun was also found to be markedly reduced after FAT1 knockdown. A reduction in the total c-Jun protein observed after FAT1 knockdown could be due to decreased positive autoregulation of c-Jun expression by AP-1, as well as the ubiquitination and rapid degradation of the unphosphorylated. (c, d) AP-1 luciferase assay was done to assess AP-1 transcriptional activity. U87MG cells were independently transfected with siFAT1 or siPDCD4 and siControl, respectively. After 24 h, cells were transfected with 1 μg of AP-1 reporter plasmid, along with 50 ng of pRL-TK (Renilla luciferase) control plasmid. The luciferase activity was measured after 48 h. The luciferase activity with control siRNA was designated as 100% and the difference calculated, we observed significant difference in siFAT1- and siPDCD4-treated cells (P<0.01 and P<0.05, respectively) compared with siControl cells. A full colour version of this figure is available at the Oncogene journal online.
Further, we performed AP-1 luciferase assay to examine whether the upregulation of PDCD4 and diminished c-Jun phosphorylation in siFAT1 cells has any effect on AP-1 activity. We observed about 60% reduction (40.6±1.2, P<0.01) in AP-1 luciferase activity in U87MGsiFAT1 cells as compared with U87siControl cells (Figure 3c). Moreover the mRNA expression of AP-1-target genes like MMP3, VEGF-C and PLAU (urokinase) were found to be decreased by >90% (Supplementary Figure S4a), as well as ECM protease activity was found to be decreased in U87MGsiFAT1 cells as compared with siControl cells (Supplementary Figure S4b). To further corroborate that PDCD4 regulates the AP-1-dependent transcription, PDCD4 expression was knocked down by using siPDCD4 in U87MG cells, and we observed about 1.5-fold increase (157±5.4, P<0.05) in AP-1 luciferase activity (Figure 3d). Thus, confirming that increased PDCD4 expression after FAT1 knockdown attenuates AP-1 transcriptional activity via reduction in the level of phosphorylated c-Jun.
Knockdown of FAT1 decreases the expression of COX-2 and other cytokines
COX-2 is known to be negatively regulated by PDCD4,62, 67 and aberrant induction of COX-2 with upregulation of prostaglandin synthesis is reported to have a pivotal role in carcinogenesis.68 COX-2 expression at mRNA and protein level was analyzed by q–PCR and western blotting. We found significant reduction of COX-2 expression at both mRNA (0.04±0.002, P<0.05) (Figure 4a) and at protein level (Figure 4b) in U87MGsiFAT1 cells as compared with siControl cells. The enzymatic product of COX-2, prostaglandin E2 (PGE2), has a key role in influencing tumor progression.69 The concentration of PGE2 secreted into the medium was found to be reduced by about 60% in U87MGsiFAT1 cells as compared with siControl cells (42±2.6, P<0.01) (Figure 4c). Thus, this result depicts inhibition of COX-2 function leading to diminished PGE2 synthesis after FAT1 knockdown.
Decrease in COX-2 expression and cytokines synthesis after FAT1 knockdown. (a) Expression of COX-2 and cytokines was found to be decreased after FAT1 knockdown. q–PCR was done to measure the mRNA expression of COX-2, IL-6 and IL-1β and about 90% reduction was observed in U87MGsiFAT1 cells as compared with siControl cells. 18S was used as internal control. (b) FAT1 knockdown was found to inhibit COX-2 protein expression. Western blot showed decreased COX-2 expression in siFAT1 cells as compared with siControl cells. β-actin was used as loading control. (c) PGE2 assay was done to quantify the production of PGE2 after FAT1 knockdown. Synthesis of enzymatic product of COX-2, PGE2, was decreased significantly by about 60% in U87MGsiFAT1 cells as compared with siControl cells. Experiment was done in triplicate and repeated twice. Each value is expressed as mean±s.d. Significant difference (P<0.01) was observed as determined by Student’s t-test. (d) COX-2 luciferase assay was done in U87MG cells after treatment with siFAT1, SR11302 or both and a significant (P<0.05) reduction in COX-2 luciferase activity was observed as compared with siControl+DMSO-treated cells. (e) q–PCR was done to evaluate the mRNA expression of the AP-1-regulated transcripts COX-2, IL-6 and IL1β after treatment of U87MG cells with 10 μM SR11302. There was a marked reduction in the mRNA expression of COX-2, IL-6 and IL1β after treatment with SR11302 as compared with DMSO control. A full colour version of this figure is available at the Oncogene journal online.
COX-2 has also been reported to regulate endogenous cytokine production, and its inhibition markedly reduces the release of pro-inflammatory cytokines like IL-1β and IL-6.70 We also observed significant reduction in mRNA expression of COX-2 along with IL-1β (0.12±0.09, P<0.05) and IL-6 (0.13±0.11, P 0.01) after FAT1 knockdown in U87MG cells (Figure 4a), thus identifying a novel function of FAT1 in regulating the expression of pro-inflammatory molecules.
AP-1-mediated regulation of COX-2 transcription has been reported earlier.71, 72 To investigate whether the regulation of COX-2 expression by FAT1 in U87MG cells is mediated via AP-1, we inhibited AP-1 activity by treating cells with specific small-molecule inhibitor of AP-1, SR11302. On treating U87MG cells with increasing concentration of SR11302 (0.1–10 μM), a dose-dependent decrease in COX-2 luciferase activity was observed, with a maximum inhibition of about 80% at the dose of 5 and 10 μM concentrations (Supplementary Figure S5). We observed marked decrease in COX-2 luciferase activity after treatment of U87MG cells with FAT1 siRNA (P<0.01) or 10 μM SR11302 (P<0.05), as well as combined treatment with FAT1 siRNA and SR11302 (P<0.001), as compared with siControl+DMSO-treated U87MG cells (Figure 4d). We also observed significant reduction in mRNA expression of COX-2 (0.37±0.09, P<0.01), IL1β (0.42±0.08, P<0.01) and IL-6 (0.29±0.1, P<0.05) after 48 h of SR11302 treatment (Figure 4e). Our results thus provide further supportive evidence that AP-1 is a key link in the regulation of expression of COX-2 and other cytokines.
Inhibition of PDCD4 reverses the effects of FAT1 knockdown in FAT1-attenuated cells
To confirm that the demonstrated effects of FAT1 knockdown were indeed due to the upregulation of PDCD4, we cotransfected FAT1 and PDCD4 siRNA in U87MG cells and could satisfactorily knock down levels of both the genes simultaneously (Figure 5a). Migration and invasion were found to be significantly restored (P<0.001) in U87MG cells after simultaneous knockdown of FAT1 and PDCD4 (Figures 5b, c). Moreover, AP-1 luciferase activity (P<0.01), as well as the mRNA expression of AP-1-target genes, was found to be significantly regained with simultaneous knockdown of FAT1 and PDCD4 as compared with U87MG cells treated with siFAT1 alone (Figure 5d). At protein level, high PDCD4 expression observed after FAT1 knockdown was found to be decreased after simultaneous knockdown of FAT1 and PDCD4. However, the expression of p-c-Jun, VEGF-C and COX-2 was regained after simultaneous knockdown of PDCD4 and FAT1 as compared with cells treated with siFAT1 alone (Figure 5f). We also observed low expression of AP-1-regulated transcripts in GOS3 cell line (Supplementary Figure S6), which has low FAT1 and high PDCD4 expression (Figure 2c). Together, these results confirm the role of PDCD4 in mediating the observed effects of FAT1.
Simultaneous knockdown of FAT1 and PDCD4 reverses the effects of FAT1 knockdown. (a) FAT1 and PDCD4 mRNA expression was analyzed by q–PCR in U87MG cells treated with siFAT1 and siPDCD4 alone, as well as both the siRNAs treated simultaneously. Treatment with siFAT1+siControl was found to upregulate PDCD4 expression, whereas treatment with siFAT1+siPDCD4 downregulated the PDCD4 expression to the level of siControl-treated cells alone. (b, c) Simultaneous knockdown of FAT1 and PDCD4 in U87MG cells restored their migratory and invasive properties comparable to that of siControl-treated cells. There was significant increase in cell migration and invasion in U87MG cells treated with siFAT1+siPDCD4 as compared with cells treated with siFAT1+siControl. Cells were counted in five different fields. Each value is mean±s.d. Experiment was put up in triplicate and repeated twice. (d) AP-1 luciferase activity significantly increased after PDCD4 knockdown in U87MGsiFAT1 cells. The luciferase activity with siControl is designated as 100%. There was a significant increase in AP-1 luciferase activity in U87MG cells treated with siFAT1+siPDCD4 as compared with siFAT1+siControl. And the luciferase activity in cells treated with siFAT1+siPDCD4 is comparable to siControl-treated cells. The experiment was repeated thrice following each of three independent transfections and representative data are shown. Results are expressed as mean±s.d. (e) The mRNA expression of AP-1-target genes increased in U87MG cells treated with siFAT1+siPDCD4 as compared with siFAT1+siControl. 18S was used as internal control and experiments were done in triplicate. (f) PDCD4 knockdown in U87MGsiFAT1 cells revert back the protein expression of p-c-Jun, VEGF-C and COX-2 comparable to siControl-treated cells alone. Lysates from indicated cells were probed with respective antibodies. β-actin was used as control antibody. A full colour version of this figure is available at the Oncogene journal online.
FAT1 and PDCD4 expression is inversely correlated in primary GBM samples
PDCD4 expression is reported to be lost in human glioma and may contribute to the development of tumor.40, 73 To study the relationship between FAT1 and PDCD4 expression in tumors, we checked the mRNA expression of both FAT1 and PDCD4 in 35 GBM samples by q–PCR (Table 1). The 35 GBM samples were arranged according to decreasing FAT1 expression and divided into quartiles.74 The PDCD4 expression (mean value±s.d.) in the first quartile (group A) with the highest FAT1 expression (0.586±0.998) was significantly less than that in the fourth quartile (group D) with the lowest FAT1 expression (54.108±51.18), with P=0.0145. The inverse relationship observed between the expression of FAT1 and PDCD4 in tumor samples supports the in vitro results.
Further, we also analyzed the mRNA expression of COX-2 and IL-6 in these GBM samples and observed positive correlation with FAT1 expression. The COX-2 expression in group A (4.99±4.074) was significantly higher as compared with group D (0.248±0.174) with P=0.048. The IL-6 expression in groups A (13.6±25.69) and D (1.21±2.97) was found to follow a similar trend, however, it was not statistically significant (P=0.146).
Discussion
The role of FAT1 as an apical regulator of the Salvador–Warts–Hippo pathway and its function as a tumor-suppressor gene is well documented in Drosophila24, 28 but there are limited reports regarding the role of FAT1 in human cancers. A study from our laboratory36 had shown extensive variation in FAT1 expression with 33% of glioma samples having low FAT1 mRNA expression and loss-of-heterozygosity at FAT1 locus in a significant number of the glioma samples studied. Another study34 had showed homozygous deletion of FAT1 gene and low expression in oral carcinoma. Role of FAT1 had also been suggested in development of fallopian tube cancer in patients of MRKH syndrome.35 In contrast, high FAT1 expression has been reported in breast cancer31 and leukemia where it is considered as an independent prognostic marker.32 The role of FAT1 in cell migration is also well studied75, 76, 77, 78 and its knockdown has been reported to inhibit migration of VSMC79 and OSCC cells.33
The signaling cascade being regulated by FAT1 gene and the function of FAT1 gene in human cancers including GBM is not known. On the basis of the loss-of-heterozygosity analysis and the expression analysis in various tumors, FAT1 gene may have a dual role, both as tumor suppressor and oncogene, but no functional studies are available to support these notions. Genes like TGFβ,80 STAT381 and so on have also been reported to have a dual role in tumorigenesis, depending upon varying cellular contexts. Therefore, proper functional studies need to be done to support and identify the precise role of FAT1 gene in tumors including GBM.
Here we investigated the functional role of FAT1 by analyzing its expression in glioma cell lines followed by siRNA knockdown of FAT1 in cells with high FAT1 expression. We observed high FAT1 mRNA expression in grade IV glioma cell lines like U87MG, A172, U373MG and T98G and decreased expression in grade III glioma cell lines (GOS3 and SW1088). Knockdown of FAT1 in U87MG and A172 led to marked reduction in migratory and invasive properties of the cells along with high expression of PDCD4. Increased PDCD4 expression was also observed upon FAT1 knockdown in U373MG and T98 cell lines. PDCD4 is known to inhibit migration and invasion in various cancers like breast, ovarian, colon and so on.49, 82 Loss of PDCD4 expression has been linked with cell proliferation and unfavorable prognosis in gliomas.40, 73, 83 Decreased migration and invasion observed in U87MG and A172 cell lines after FAT1 knockdown in this study might be attributed to increased PDCD4 expression. High PDCD4 expression after FAT1 knockdown was not found to induce apoptosis or alter cell-cycle distribution in both U87MG and A172 cell lines, which is in accordance to a similar study in NIH3T3 cells by Shibahara et al.84 but in contrast to report on breast cancer cell lines.85 This difference can be due to molecular cross-talk between different signaling cascades in cell lines affecting the functioning of the cells in a context-dependent manner. In our study, we observed that high PDCD4 levels after FAT1 knockdown mainly regulate migration and invasion in glioma cells. Decreased PDCD4 is known to have an important role in tumor progression86, 87, 88 and in remodeling ECM during invasion.89, 90 To further confirm the link between FAT1 and PDCD4, we simultaneously knocked down PDCD4 and FAT1 expression and found reversal of migratory and invasive properties of the cells, which further confirmed that the observed effects of FAT1 knockdown are mainly mediated by PDCD4.
Because PDCD4 is known to attenuate AP-1-mediated transcription by inhibiting c-Jun phosphorylation,49, 50 we checked the effect of FAT1 knockdown on the AP-1 activity by luciferase assay and observed diminished AP-1 transcriptional activity and decreased expression of AP-1-regulated transcripts like ECM-remodeling molecules MMP3, VEGF-C, PLAU, inflammatory mediator COX-2 and cytokines IL1β and IL-6. Overexpression of pro-inflammatory molecules in GBM and their role in glioma progression is well known.13 Moreover, the role of various signaling molecules in promoting inflammatory response in gliomas has also been convincingly demonstrated.18, 20, 91, 92 The reduced AP-1 activity observed in glioma cell lines after FAT1 knockdown could be attributed to inhibition of c-jun transcription and c-Jun phosphorylation by increased PDCD4 expression. These effects were further confirmed by dual knockdown of FAT1 and PDCD4 in both U87MG and A172 cell lines. Also, the increased PDCD4 expression after FAT1 knockdown in glioma cells was found to be independent of the Akt pathway. The observation of FAT1 in regulating PDCD4 expression and its downstream effect on tumor migration and invasion is a novel finding with implications for glioma biology.
We have shown that FAT1 is linked to COX-2 via PDCD4 and AP-1. The downregulation of COX-2 expression and pro-inflammatory cytokines via PDCD4 upregulation after FAT1 knockdown has considerable significance in the regulation of inflammatory responses in glioma. Decreased COX-2 expression after FAT1 knockdown led to reduced PGE2 production and decreased mRNA expression of IL-6 and IL-1β, thus providing a crucial link between tumor progression and inflammation. COX-2 expression is known to be regulated by AP-1 binding to cyclic adenosine monophosphate response element in the COX-2 promoter.71 We have shown that inhibition of AP-1 by SR11302 led to significant reduction in COX-2 promoter luciferase activity as well as mRNA expression of the AP-1-regulated genes COX-2, IL-6 and IL-1β. The role of PDCD4 in downregulating COX-2 is known62, 67 and suppression of PDCD4 is found to increase invasive activity of COX-2 in breast cancer cells.82 COX-2 overexpression is also reported in high-grade gliomas93, 94, 95 and many other tumors with potential for targeting it as anti-cancer modality.69, 70, 96, 97, 98, 99, 100 Our finding of reduced COX-2 expression and decreased synthesis of prostanoids (PGE2) and cytokines, IL1β and IL-6 after FAT1 knockdown points toward the role of FAT1 in regulating oncogenic and inflammatory properties in gliomas and this may also hold true for other tumors. Thus, targeting FAT1 would have strong advantages for managing both tumorigenicity and the inflammatory microenvironment of gliomas.
To further validate the in vitro findings about the relationship between FAT1 and PDCD4, we studied the expression of FAT1 and PDCD4 in 35 primary GBM samples by q–PCR and found a statistically significant inverse correlation between the two. Further, we also observed a positive correlation between FAT1 and COX-2 expression and a similar trend for IL-6 expression in GBM samples studied. These findings further support the in vitro results about the inverse relationship between FAT1 and PDCD4 and their influence on the expression of AP-1-mediated transcripts like COX-2, IL-6 and so on.
We therefore demonstrate for the first time that FAT1 has an important role in modulating PDCD4 expression, which in turn regulates AP-1-dependent transcription, controlling processes crucial for migration and invasion in glioma cells, as well as the induction of a pro-inflammatory microenvironment in glioma. The study illustrates a link between inflammation and cancer in glioma and raises the possibility that FAT1 might be a master regulator in this process. This work highlights the importance of FAT1 in the induction of the cellular pathways of migration and invasion, proteolysis of the ECM and the expression of pro-inflammatory molecules leading to a favorable microenvironment for tumor progression. The possible signaling pathway being regulated by FAT1 is shown in Figure 6 and has been illustrated in a set of glioma cell lines with corroborative evidence from primary human GBM. Till now, targeting pro-inflammatory molecules or the related pathways as therapeutic strategies to prevent or treat cancers has had a limited success and there is considerable interest in focusing on such molecules.101, 102 The transmembrane molecule, FAT1, thus promises to be one such novel target for therapeutic intervention in cancer and inflammation as well as the link between the two processes.
The proposed signaling pathway downstream of FAT1 regulating AP-1-dependent transcription. Knockdown of FAT1 expression releases its inhibitory effect on PDCD4 and increase the expression of PDCD4. Increased PDCD4 expression in turn inhibits the phosphorylation of c-Jun, thus decreasing phospho-c-Jun levels. Because phospho-c-Jun is required for AP-1-dependent transcription, there was inhibition of AP-1 transcriptional activity and downregulation of target genes like COX-2, MMP3, VEGF-C, PLAU, IL-6 and IL-1β. A full colour version of this figure is available at the Oncogene journal online.
Materials and methods
Reagents and antibodies
Set of three siRNAs for FAT1 knockdown, FAT Stealth RNAi siRNA-I(HSS103567) FAT Stealth RNAi siRNA-II(HSS103568) and FAT Stealth RNAi siRNA-III(HSS176716) and universal medium GC control siRNA (cat. no.12935-112) were purchased from Invitrogen Life technologies (Grand Island, NY, USA). For simultaneous knockdown, on-target plus siRNA against FAT1(J-010513-07-0020) and PDCD4(J-004438-08-0020) and on-target plus non-targeting pool(D-001810-10-20) as control siRNA from Dharmacon (Lafayette, CO, USA). AP-1 luciferase reporter plasmid (AP-1 7 × ) was purchased from Stratagene, and COX-2 promoter luciferase construct was a kind gift from Dr Miguel A Iniguez,Centro de Biología Molecular Universidad Autónoma de Madrid, Spain. AP-1 inhibitor SR11302 was purchased from Tocris Biosciences (Bristol, UK).103 Normal brain RNA was procured from Ambion (Austin, TX, USA), Life Technologies (Carlsbad, CA, USA) and Clontech (Mountain view, CA, USA). Antibodies used for western blots, like PDCD4, COX-2, p-c-Jun, c-Jun and β-actin were from Cell Signaling Technology (Beverly, MA, USA), VEGF and Caspase-3 were from Abcam (Cambridge, UK). Primers were designed using Primer3 software and ordered from MWG Biotech (Ebersberg, Germany).
Cell culture and siRNA transfection
The human glioma cell lines U87MG, U373, A172, T98G, GOS3 and SW1088 were obtained from ATCC (Rockville, MD, USA) cultured in Dulbecco’s Modified Eagle Medium (Sigma-Aldrich, St Louis, MO, USA) supplemented with 10%(v/v) fetal calf serum (Sigma-Aldrich), 3.7 g/l sodium bicarbonate(Sigma-Aldrich), ciprofloxacin 10μg/ml and 5%CO2 at 37 °C. For transfection, 2 × 105 cells were seeded per 25-cm2 flask. After 24 h, cells were transfected with siFAT1 and siControl(Invitrogen) according to the manufacturer’s protocol with a final concentration of 20 nM of siRNA using Lipofectamine-2000 (Invitrogen) and Opti-Mem media (Invitrogen). After 4 h of siRNA transfection, the media was supplemented with Dulbecco’s Modified Eagle Medium containing 2 × fetal calf serum. The ability of each siRNA to knockdown FAT mRNA expression was confirmed using quantitative real-time PCR. Photomicrographs of the cells were taken using an inverted phase contrast microscope (Nikon TMS, Japan). For simultaneous knockdown of FAT1 and PDCD4, siRNA from Dharmacon were used. Transfection was performed as earlier with 50 nM of siFAT1 along with 50 nM of siPDCD4 or 50 nM of siControl.
cDNA synthesis and q–PCR
Total RNA was isolated from cells at appropriate time point using TRIzol reagent (Invitrogen), quantified using a Nanodrop ND-1000 spectrophotometer. DNase(Ambion) treatment was given and 1 μg of total RNA was used for cDNA synthesis, done by Fermentas RevertAid First Strand cDNA Synthesis Kit using random decamers. PCR reactions were carried in 10-μl reaction volumes (2.5 μl of 1:5 diluted cDNA, 0.5 μl of primer mix, 1 μl of 10 × Taq Buffer-A, 0.5 U Taq polymerase (Bangalore-Genei, Bangalore, India), 1 μl Syto9(Invitrogen), 0.25 μl of 10 mM dNTPs (MBI-Fermentas; Thermo Fisher Scientific, Rockford, IL, USA) and 4.6 μl of NFW (Ambion)) on a RotorGene6000 Real-Time PCR Corbett Life Science (Mortlake, NSW, Australia) in triplicates. The detail of the primers used is given in Supplementary Table S1. ΔCt values were defined as target gene Ct minus 18 S RNA Ct and averaged for each triplicate sample. Statistical significance was determined by Student’s t-test analysis (P<0.05).
Western blotting analyses
Cells were lysed in triple detergent buffer containing protease and phosphatase inhibitors (Sigma-Aldrich), lysates were quantified by BCA kit (Pierce, Thermo Fisher Scientific, Rockford, IL, USA) and electrophoresed in 10% SDS–PAGE and electroblotted on nitrocellulose membrane (Millipore, Billerica, MA, USA). Membrane was blocked with 5% bovine serum albumin in 1 × Tris–buffered saline at 4 °C O/N. Primary and secondary antibodies were diluted in 5% bovine serum albumin and 0.1% tween-20 in 1 × Tris-buffered saline. Blot was incubated in primary antibody overnight at 4 °C followed by secondary antibody incubation for 2 h at room temperature. Blot was developed by BCIP-NBT (Promega, Madison, WI, USA) and captured using Alpha Imager EP software (Alpha Innotech Corp, San Leandro, CA, USA).
Migration and invasion assays
The modified Boyden chamber/matrigel assay was performed according to the manufacturer’s directions (BD Biosciences, Bedford, MA, USA). For in vitro migration assays, we used chambers with control inserts that lacked the matrigel coating. Transfections were carried out in U87MG and A172 cells and 48 h later 3 × 104 U87MG cells per well and 7 × 104 A172 cells per well were resuspended in serum-free Dulbecco’s Modified Eagle Medium and seeded in triplicate in matrigel-coated (1 mg/ml; BD Biosciences) and uncoated control inserts (8-μm-pore-size, BD Biosciences). The remaining protocol was done as previously described.42
AP-1 and COX-2 promoter luciferase assay
U87MG cells (40 000 cells per well) were plated in six-well plates in triplicate. On day 1, cells were transfected with siFAT1/siPDCD4 and siControl. On day 2, cells were transiently transfected with 7 × AP-1 luciferase reporter plasmids as described previously.49, 62 Cells were transfected with 1 μg of AP-1 7 × luciferase reporter plasmid, along with 50 ng of pRL-TK (Renilla luciferase) control plasmid using Lipofectamine-2000 as the transfection reagent. After 48 h, the cells were lysed by 1 × passive lysis buffer (Promega) and assayed for luciferase activity as previously described.49 For COX-2 luciferase assay, U87MG cells were transfected with siFAT1 and siControl on day 1 and after 4 h incubation were treated with 10 μM SR11302. On day 2, cells were transiently transfected with COX-2 reporter plasmid104 and again treated with 10 μM SR11302. After 48 h, the cells were assayed for luciferase activity.
PGE2 enzyme-linked immunosorbent assay
U87MG cells (2.5 × 105cells per flask) were plated into 25-cm2 flask. Transfection was performed on day 1 and 72 h later, the conditioned medium was collected and concentrated using Centricon YM-30. The concentration of PGE2 secreted into the medium was measured with an EIA kit for human PGE2 (Cayman Chemical, Ann Arbor, MI, USA).
Human GBM samples
Tumor tissues were obtained at the time of surgery from 35 GBM (World Health Organization grade IV) patients from Department of Neurosurgery, AIIMS, with due consent from patients and ethical clearance obtained from the ethics committee of the institute. The tumor typing and grading was done by Prof Chitra Sarkar, Department of Pathology, AIIMS. Portions of the resected tumors were snap frozen in liquid nitrogen and stored at −80 °C until prior to RNA extraction. Of these 35 patients, 14 were female and 21 were male, age between 30–65 years with a mean age±s.d. of 43±12.2 years. q–PCR was done for genes FAT1, PDCD4, COX-2 and IL-6. Based on quantitative FAT1 expression, tumors were arranged in decreasing order of FAT1 mRNA expression and divided into four quartiles (A, B, C and D) with A having the highest FAT1 expression and D the lowest.
Statistical analysis
All in vitro experiments were performed in triplicate and repeated thrice. q–PCR for patient tumor samples were performed in triplicate. Differences were determined using Student’s t-test, and P<0.05 was considered significant. Data are shown as the mean±s.d.
References
Grivennikov SI, Karin M . Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev 2010; 20: 65–71.
Mantovani A, Allavena P, Sica A, Balkwill F . Cancer-related inflammation. Nature 2008; 454: 436–444.
Moore MM, Chua W, Charles KA, Clarke SJ . Inflammation and cancer: causes and consequences. Clin Pharmacol Ther 2010; 87: 504–508.
Mandal RK, Mittal RD . Polymorphisms in COX-2 gene influence prostate cancer susceptibility in a northern Indian cohort. Arch Med Res 42: 620–626.
Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB . From ancient medicine to modern medicine: ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol 2007; 5: 25–37.
Aggarwal BB, Gehlot P . Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol 2009; 9: 351–369.
Borrello MG, Degl'Innocenti D, Pierotti MA . Inflammation and cancer: the oncogene-driven connection. Cancer Lett 2008; 267: 262–270.
Lim SK, Llaguno SR, McKay RM, Parada LF . Glioblastoma multiforme: a perspective on recent findings in human cancer and mouse models. BMB Rep 2011; 44: 158–164.
Munshi A, Jalali R . Therapy for glioma: Indian perspective. Indian J Cancer 2009; 46: 127–131.
Alves TR, Lima FR, Kahn SA, Lobo D, Dubois LG, Soletti R et al. Glioblastoma cells: a heterogeneous and fatal tumor interacting with the parenchyma. Life Sci 2011; 89: 532–539.
Teodorczyk M, Martin-Villalba A . Sensing invasion: cell surface receptors driving spreading of glioblastoma. J Cell Physiol 2010; 222: 1–10.
Noble M, Mayer-Proschel M . Growth factors, glia and gliomas. J Neurooncol 1997; 35: 193–209.
Tafani M, Di Vito M, Frati A, Pellegrini L, De Santis E, Sette G et al. Pro-inflammatory gene expression in solid glioblastoma microenvironment and in hypoxic stem cells from human glioblastoma. J Neuroinflammation 2011; 8: 32.
Goldbrunner RH, Bernstein JJ, Tonn JC . ECM-mediated glioma cell invasion. Microsc Res Tech 1998; 43: 250–257.
Balkwill F, Mantovani A . Cancer and inflammation: implications for pharmacology and therapeutics. Clin Pharmacol Ther 2010; 87: 401–406.
Deorukhkar A, Krishnan S . Targeting inflammatory pathways for tumor radiosensitization. Biochem Pharmacol 2010; 80: 1904–1914.
de Visser KE, Jonkers J . Towards understanding the role of cancer-associated inflammation in chemoresistance. Curr Pharm Des 2009; 15: 1844–1853.
Tewari R, Choudhury SR, Ghosh S, Mehta VS, Sen E . Involvement of TNFalpha-induced TLR4-NF-kappaB and TLR4-HIF-1alpha feed-forward loops in the regulation of inflammatory responses in glioma. J Mol Med (Berl) 2012; 90: 67–80.
Chiu WT, Shen SC, Chow JM, Lin CW, Shia LT, Chen YC . Contribution of reactive oxygen species to migration/invasion of human glioblastoma cells U87 via ERK-dependent COX-2/PGE(2) activation. Neurobiol Dis 2009; 37: 118–129.
Sharma V, Dixit D, Ghosh S, Sen E . COX-2 regulates the proliferation of glioma stem like cells. Neurochem Int 2011; 59: 567–571.
Krakauer T . Molecular therapeutic targets in inflammation: cyclooxygenase and NF-kappaB. Curr Drug Targets Inflamm Allergy 2004; 3: 317–324.
Dunne J, Hanby AM, Poulsom R, Jones TA, Sheer D, Chin WG et al. Molecular cloning and tissue expression of FAT, the human homologue of the Drosophila fat gene that is located on chromosome 4q34-q35 and encodes a putative adhesion molecule. Genomics 1995; 30: 207–223.
Katoh Y, Katoh M . Comparative integromics on FAT1, FAT2, FAT3 and FAT4. Int J Mol Med 2006; 18: 523–528.
Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, Irvine KD . Delineation of a Fat tumor suppressor pathway. Nat Genet 2006; 38: 1142–1150.
Reddy BV, Irvine KD . The Fat and Warts signaling pathways: new insights into their regulation, mechanism and conservation. Development 2008; 135: 2827–2838.
Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H . The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr Biol 2006; 16: 2081–2089.
Willecke M, Hamaratoglu F, Kango-Singh M, Udan R, Chen CL, Tao C et al. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol 2006; 16: 2090–2100.
Bennett FC, Harvey KF . Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr Biol 2006; 16: 2101–2110.
Mao Y, Rauskolb C, Cho E, Hu WL, Hayter H, Minihan G et al. Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development 2006; 133: 2539–2551.
Mahoney PA, Weber U, Onofrechuk P, Biessmann H, Bryant PJ, Goodman CS . The fat tumor suppressor gene in Drosophila encodes a novel member of the cadherin gene superfamily. Cell 1991; 67: 853–868.
Kwaepila N, Burns G, Leong AS . Immunohistological localisation of human FAT1 (hFAT) protein in 326 breast cancers. Does this adhesion molecule have a role in pathogenesis? Pathology 2006; 38: 125–131.
de Bock CE, Ardjmand A, Molloy TJ, Bone SM, Johnstone D, Campbell DM et al. The Fat1 cadherin is overexpressed and an independent prognostic factor for survival in paired diagnosis-relapse samples of precursor B-cell acute lymphoblastic leukemia. Leukemia 2011; 26: 918–926.
Nishikawa Y, Miyazaki T, Nakashiro K, Yamagata H, Isokane M, Goda H et al. Human FAT1 cadherin controls cell migration and invasion of oral squamous cell carcinoma through the localization of beta-catenin. Oncol Rep 2011; 26: 587–592.
Nakaya K, Yamagata HD, Arita N, Nakashiro KI, Nose M, Miki T et al. Identification of homozygous deletions of tumor suppressor gene FAT in oral cancer using CGH-array. Oncogene 2007; 26: 5300–5308.
Bendavid C, Pasquier L, Watrin T, Morcel K, Lucas J, Gicquel I et al. Phenotypic variability of a 4q34-->qter inherited deletion: MRKH syndrome in the daughter, cardiac defect and Fallopian tube cancer in the mother. Eur J Med Genet 2007; 50: 66–72.
Chosdol K, Misra A, Puri S, Srivastava T, Chattopadhyay P, Sarkar C et al. Frequent loss of heterozygosity and altered expression of the candidate tumor suppressor gene 'FAT' in human astrocytic tumors. BMC Cancer 2009; 9: 5.
Lankat-Buttgereit B, Goke R . The tumour suppressor Pdcd4: recent advances in the elucidation of function and regulation. Biol Cell 2009; 101: 309–317.
Lankat-Buttgereit B, Goke R . Programmed cell death protein 4 (pdcd4): a novel target for antineoplastic therapy? Biol Cell 2003; 95: 515–519.
Waters LC, Strong SL, Ferlemann E, Oka O, Muskett FW, Veverka V et al. Structure of the tandem MA-3 region of Pdcd4 protein and characterization of its interactions with eIF4A and eIF4G: molecular mechanisms of a tumor suppressor. J Biol Chem 2011; 286: 17270–17280.
Gao F, Zhang P, Zhou C, Li J, Wang Q, Zhu F et al. Frequent loss of PDCD4 expression in human glioma: possible role in the tumorigenesis of glioma. Oncol Rep 2007; 17: 123–128.
Chen Y, Knosel T, Kristiansen G, Pietas A, Garber ME, Matsuhashi S et al. Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. J Pathol 2003; 200: 640–646.
Santhanam AN, Baker AR, Hegamyer G, Kirschmann DA, Colburn NH . Pdcd4 repression of lysyl oxidase inhibits hypoxia-induced breast cancer cell invasion. Oncogene 2010; 29: 3921–3932.
Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH et al. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene 2008; 27: 4373–4379.
Wen YH, Shi X, Chiriboga L, Matsahashi S, Yee H, Afonja O . Alterations in the expression of PDCD4 in ductal carcinoma of the breast. Oncol Rep 2007; 18: 1387–1393.
Wang Q, Sun Z, Yang HS . Downregulation of tumor suppressor Pdcd4 promotes invasion and activates both beta-catenin/Tcf and AP-1-dependent transcription in colon carcinoma cells. Oncogene 2008; 27: 1527–1535.
Yasuda M, Schmid T, Rubsamen D, Colburn NH, Irie K, Murakami A . Downregulation of programmed cell death 4 by inflammatory conditions contributes to the generation of the tumor promoting microenvironment. Mol Carcinog 2010; 49: 837–848.
Schmid T, Bajer MM, Blees JS, Eifler LK, Milke L, Rubsamen D et al. Inflammation-induced loss of Pdcd4 is mediated by phosphorylation-dependent degradation. Carcinogenesis 2011; 32: 1427–1433.
Yang HS, AP Jansen, Nair R, Shibahara K, Verma AK, Cmarik JL et al. A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NF-kappaB or ODC transactivation. Oncogene 2001; 20: 669–676.
Yang HS, Matthews CP, Clair T, Wang Q, Baker AR, Li CC et al. Tumorigenesis suppressor Pdcd4 down-regulates mitogen-activated protein kinase kinase kinase kinase 1 expression to suppress colon carcinoma cell invasion. Mol Cell Biol 2006; 26: 1297–1306.
Bitomsky N, Bohm M, Klempnauer KH . Transformation suppressor protein Pdcd4 interferes with JNK-mediated phosphorylation of c-Jun and recruitment of the coactivator p300 by c-Jun. Oncogene 2004; 23: 7484–7493.
Fleenor DL, Pang IH, Clark AF . Involvement of AP-1 in interleukin-1alpha-stimulated MMP-3 expression in human trabecular meshwork cells. Invest Ophthalmol Vis Sci 2003; 44: 3494–3501.
Tsuji F, Seki I, Aono H, Odani N, Mizutani K, Okamoto M et al. Bucillamine mechanism inhibiting IL-1beta-induced VEGF production from fibroblast-like synoviocytes. Int Immunopharmacol 2007; 7: 1569–1576.
Bhattacharya A, Lakka SS, Mohanam S, Boyd D, Rao JS . Regulation of the urokinase-type plasminogen activator receptor gene in different grades of human glioma cell lines. Clin Cancer Res 2001; 7: 267–276.
Shen Q, Uray IP, Li Y, Krisko TI, Strecker TE, Kim HT et al. The AP-1 transcription factor regulates breast cancer cell growth via cyclins and E2F factors. Oncogene 2008; 27: 366–377.
Karamouzis MV, Konstantinopoulos PA, Papavassiliou AG . The activator protein-1 transcription factor in respiratory epithelium carcinogenesis. Mol Cancer Res 2007; 5: 109–120.
Ozanne BW, McGarry L, Spence HJ, Johnston I, Winnie J, Meagher L et al. Transcriptional regulation of cell invasion: AP-1 regulation of a multigenic invasion programme. Eur J Cancer 2000; 36: 1640–1648.
Leaner VD, Kinoshita I, Birrer MJ . AP-1 complexes containing cJun and JunB cause cellular transformation of Rat1a fibroblasts and share transcriptional targets. Oncogene 2003; 22: 5619–5629.
Leaner VD, Chick JF, Donninger H, Linniola I, Mendoza A, Khanna C et al. Inhibition of AP-1 transcriptional activity blocks the migration, invasion, and experimental metastasis of murine osteosarcoma. Am J Pathol 2009; 174: 265–275.
Liu Y, Ludes-Meyers J, Zhang Y, Munoz-Medellin D, Kim HT, Lu C et al. Inhibition of AP-1 transcription factor causes blockade of multiple signal transduction pathways and inhibits breast cancer growth. Oncogene 2002; 21: 7680–7689.
Palamarchuk A, Efanov A, Maximov V, Aqeilan RI, Croce CM, Pekarsky Y . Akt phosphorylates and regulates Pdcd4 tumor suppressor protein. Cancer Res 2005; 65: 11282–11286.
Wang WQ, Zhang H, Wang HB, Sun YG, Peng ZH, Zhou G et al. Programmed cell death 4 (PDCD4) enhances the sensitivity of gastric cancer cells to TRAIL-induced apoptosis by inhibiting the PI3K/Akt signaling pathway. Mol Diagn Ther 2010; 14: 155–161.
Yang HS, Knies JL, Stark C, Colburn NH . Pdcd4 suppresses tumor phenotype in JB6 cells by inhibiting AP-1 transactivation. Oncogene 2003; 22: 3712–3720.
Angel P, Hattori K, Smeal T, Karin M . The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell 1988; 55: 875–885.
Schonthal A, Srinivas S, Eckhart W . Induction of c-jun protooncogene expression and transcription factor AP-1 activity by the polyoma virus middle-sized tumor antigen. Proc Natl Acad Sci USA 1992; 89: 4972–4976.
Fuchs SY, Dolan L, Davis RJ, Ronai Z . Phosphorylation-dependent targeting of c-Jun ubiquitination by Jun N-kinase. Oncogene 1996; 13: 1531–1535.
Fuchs SY, Xie B, Adler V, Fried VA, Davis RJ, Ronai Z . c-Jun NH2-terminal kinases target the ubiquitination of their associated transcription factors. J Biol Chem 1997; 272: 32163–32168.
Zhang Z, DuBois RN . Detection of differentially expressed genes in human colon carcinoma cells treated with a selective COX-2 inhibitor. Oncogene 2001; 20: 4450–4456.
Dannenberg AJ, Subbaramaiah K . Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell 2003; 4: 431–436.
Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009; 30: 377–386.
Herseth JI, Refsnes M, Lag M, Schwarze PE . Role of IL-1 beta and COX2 in silica-induced IL-6 release and loss of pneumocytes in co-cultures. Toxicol In Vitro 2009; 23: 1342–1353.
Subbaramaiah K, Cole PA, Dannenberg AJ . Retinoids and carnosol suppress cyclooxygenase-2 transcription by CREB-binding protein/p300-dependent and -independent mechanisms. Cancer Res 2002; 62: 2522–2530.
Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ . Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer: evidence for involvement of AP-1 and PEA3. J Biol Chem 2002; 277: 18649–18657.
Gao F, Wang X, Zhu F, Wang Q, Zhang X, Guo C et al. PDCD4 gene silencing in gliomas is associated with 5′CpG island methylation and unfavourable prognosis. J Cell Mol Med 2009; 13: 4257–4267.
Fredlund E, Ringner M, Maris JM, Pahlman S . High Myc pathway activity and low stage of neuronal differentiation associate with poor outcome in neuroblastoma. Proc Natl Acad Sci USA 2008; 105: 14094–14099.
Moeller MJ, Soofi A, Braun GS, Li X, Watzl C, Kriz W et al. Protocadherin FAT1 binds Ena/VASP proteins and is necessary for actin dynamics and cell polarization. EMBO J 2004; 23: 3769–3779.
Tanoue T, Takeichi M . New insights into Fat cadherins. J Cell Sci 2005; 118: 2347–2353.
Braun GS, Kretzler M, Heider T, Floege J, Holzman LB, Kriz W et al. Differentially spliced isoforms of FAT1 are asymmetrically distributed within migrating cells. J Biol Chem 2007; 282: 22823–22833.
Tanoue T, Takeichi M . Mammalian Fat1 cadherin regulates actin dynamics and cell-cell contact. J Cell Biol 2004; 165: 517–528.
Hou R, Liu L, Anees S, Hiroyasu S, Sibinga NE . The Fat1 cadherin integrates vascular smooth muscle cell growth and migration signals. J Cell Biol 2006; 173: 417–429.
Muraoka-Cook RS, Dumont N, Arteaga CL . Dual role of transforming growth factor beta in mammary tumorigenesis and metastatic progression. Clin Cancer Res 2005; 11: 937s–943ss.
de la Iglesia N, Konopka G, Puram SV, Chan JA, Bachoo RM, You MJ et al. Identification of a PTEN-regulated STAT3 brain tumor suppressor pathway. Genes Dev 2008; 22: 449–462.
Nieves-Alicea R, Colburn NH, Simeone AM, Tari AM . Programmed cell death 4 inhibits breast cancer cell invasion by increasing tissue inhibitor of metalloproteinases-2 expression. Breast Cancer Res Treat 2009; 114: 203–209.
Gaur AB, Holbeck SL, Colburn NH, Israel MA . Downregulation of Pdcd4 by mir-21 facilitates glioblastoma proliferation in vivo. Neuro Oncol 2011; 13: 580–590.
Shibahara K, Asano M, Ishida Y, Aoki T, Koike T, Honjo T . Isolation of a novel mouse gene MA-3 that is induced upon programmed cell death. Gene 1995; 166: 297–301.
Afonja O, Juste D, Das S, Matsuhashi S, Samuels HH . Induction of PDCD4 tumor suppressor gene expression by RAR agonists, antiestrogen and HER-2/neu antagonist in breast cancer cells. Evidence for a role in apoptosis. Oncogene 2004; 23: 8135–8145.
Ding L, Zhang X, Zhao M, Qu Z, Huang S, Dong M et al. An essential role of PDCD4 in progression and malignant proliferation of gastrointestinal stromal tumors. Med Oncol 2012; 29: 1758–1764.
Wei ZT, Zhang X, Wang XY, Gao F, Zhou CJ, Zhu FL et al. PDCD4 inhibits the malignant phenotype of ovarian cancer cells. Cancer Sci 2009; 100: 1408–1413.
Wang X, Wei Z, Gao F, Zhang X, Zhou C, Zhu F et al. Expression and prognostic significance of PDCD4 in human epithelial ovarian carcinoma. Anticancer Res 2008; 28: 2991–2996.
Leupold JH, Yang HS, Colburn NH, Asangani I, Post S, Allgayer H . Tumor suppressor Pdcd4 inhibits invasion/intravasation and regulates urokinase receptor (u-PAR) gene expression via Sp-transcription factors. Oncogene 2007; 26: 4550–4562.
Mudduluru G, Medved F, Grobholz R, Jost C, Gruber A, Leupold JH et al. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer 2007; 110: 1697–1707.
Sen E . Targeting inflammation-induced transcription factor activation: an open frontier for glioma therapy. Drug Discov Today 2011; 16: 1044–1051.
Sinha S, Koul N, Dixit D, Sharma V, Sen E . IGF-1 induced HIF-1alpha-TLR9 cross talk regulates inflammatory responses in glioma. Cell Signal 2011; 23: 1869–1875.
Joki T, Heese O, Nikas DC, Bello L, Zhang J, Kraeft SK et al. Expression of cyclooxygenase 2 (COX-2) in human glioma and in vitro inhibition by a specific COX-2 inhibitor, NS-398. Cancer Res 2000; 60: 4926–4931.
Shono T, Tofilon PJ, Bruner JM, Owolabi O, Lang FF . Cyclooxygenase-2 expression in human gliomas: prognostic significance and molecular correlations. Cancer Res 2001; 61: 4375–4381.
Hara A, Okayasu I . Cyclooxygenase-2 and inducible nitric oxide synthase expression in human astrocytic gliomas: correlation with angiogenesis and prognostic significance. Acta Neuropathol 2004; 108: 43–48.
Eberstal S, Badn W, Fritzell S, Esbjornsson M, Darabi A, Visse E et al. Inhibition of cyclooxygenase-2 enhances immunotherapy against experimental brain tumors. Cancer Immunol Immunother 2012; 61: 1191–1199.
Sareddy GR, Geeviman K, Ramulu C, Babu PP . The nonsteroidal anti-inflammatory drug celecoxib suppresses the growth and induces apoptosis of human glioblastoma cells via the NF-kappaB pathway. J Neurooncol 2011; 106: 99–109.
New P . Cyclooxygenase in the treatment of glioma: its complex role in signal transduction. Cancer Control 2004; 11: 152–164.
Harris RE, Beebe-Donk J, Alshafie GA . Cancer chemoprevention by cyclooxygenase 2 (COX-2) blockade: results of case control studies. Subcell Biochem 2007; 42: 193–212.
Harris RE, Beebe-Donk J, Alshafie GA . Reduced risk of human lung cancer by selective cyclooxygenase 2 (COX-2) blockade: results of a case control study. Int J Biol Sci 2007; 3: 328–334.
Harris RE . Cyclooxygenase-2 (cox-2) and the inflammogenesis of cancer. Subcell Biochem 2007; 42: 93–126.
Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM et al. Cancer and inflammation: promise for biologic therapy. J Immunother 2010; 33: 335–351.
Huang C, Ma WY, Dawson MI, Rincon M, Flavell RA, Dong Z . Blocking activator protein-1 activity, but not activating retinoic acid response element, is required for the antitumor promotion effect of retinoic acid. Proc Natl Acad Sci USA 1997; 94: 5826–5830.
Iniguez MA, Martinez-Martinez S, Punzon C, Redondo JM, Fresno M . An essential role of the nuclear factor of activated T cells in the regulation of the expression of the cyclooxygenase-2 gene in human T lymphocytes. J Biol Chem 2000; 275: 23627–23635.
Acknowledgements
The work has been supported by grant from Defense Research and Development Organization (DRDO), India (no. LSRB167-2008) to KC and National Brain Research Center, India, core intramural grant to SS. RF (Research fellowship) to BD and EM from Council of Scientific and Industrial Research (CSIR, India) and RF to KI from Indian Council of Medical Research (ICMR, India). We would like to acknowledge Dr Miguel Iniguez for providing COX-2 promoter luciferase plasmid and Dr Shayamal Goswami, Dr Balaji and Dr Sandeep Saxena for their help during the progress of the work. We thank our Lab technician Ms Jyoti and Lab attendants Late Mathura Prasad, Mr Pappu, Mr Gopal and Mr Ajay for their assistance in routine lab work and cell-culture work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Dikshit, B., Irshad, K., Madan, E. et al. FAT1 acts as an upstream regulator of oncogenic and inflammatory pathways, via PDCD4, in glioma cells. Oncogene 32, 3798–3808 (2013). https://doi.org/10.1038/onc.2012.393
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.393
Keywords
This article is cited by
-
Favorable immune checkpoint inhibitor outcome of patients with melanoma and NSCLC harboring FAT1 mutations
npj Precision Oncology (2022)
-
Identification of prognostic genes in the pancreatic adenocarcinoma immune microenvironment by integrated bioinformatics analysis
Cancer Immunology, Immunotherapy (2022)
-
Identification of the atypical cadherin FAT1 as a novel glypican-3 interacting protein in liver cancer cells
Scientific Reports (2021)
-
Glioblastoma stem cells and Wnt signaling pathway: molecular mechanisms and therapeutic targets
Chinese Neurosurgical Journal (2020)
-
NFкB is a critical transcriptional regulator of atypical cadherin FAT1 in glioma
BMC Cancer (2020)