Abstract
Signal transducer and activator of transcription 3 (STAT3) overactivation is a common event in many cancers, including head and neck squamous cell carcinoma (HNSCC), where STAT3 represents a promising therapeutic target. HNSCC is not characterized by frequent kinase mutations, in contrast to some malignancies where mutational activation of kinases upstream of STAT3 is common. Instead, STAT3 may be activated by loss-of-function of negative regulators of STAT3, including by promoter hypermethylation of PTPRT. Here we first analyzed The Cancer Genome Atlas data and determined that the PTPRT promoter is frequently hypermethylated in several cancers, including HNSCC (60.1% of tumors analyzed) in association with downregulation of PTPRT mRNA expression and upregulation of pSTAT3 expression. These findings were confirmed in an independent cohort of HNSCC tumors by methylation-specific PCR and immunohistochemistry. We demonstrate that PTPRT promoter methylation and gene silencing is reversible in HNSCC cells, leading to PTPRT-specific downregulation of pSTAT3 expression. We further show that PTPRT promoter methylation is significantly associated with sensitivity to STAT3 inhibition in HNSCC cells, suggesting that PTPRT promoter methylation may serve as a predictive biomarker for responsiveness to STAT3 inhibitors in clinical development.
Similar content being viewed by others
Introduction
Head and neck squamous cell carcinoma (HNSCC) is an invasive malignancy with more than 40 000 expected diagnoses and more than 8000 expected deaths in 2014 in the United States alone.1 Most cancers, including HNSCC, are characterized by constitutive activation of signal transducer and activator of transcription 3 (STAT3) via phosphorylation of tyrosine 705 (Y705). STAT3 is a transcription factor and potent oncoprotein that activates or maintains many cancer phenotypes including abnormal growth, proliferation, angiogenesis and invasion, as well as evasion of apoptosis and the anti-tumor immune response.2 Although aberrant activation of kinases upstream of STAT3 likely contributes to constitutive activation of STAT3 in cancer, the role of loss-of-function of downstream regulators, especially protein tyrosine phosphatases (PTPs), remains incompletely understood.
The PTP superfamily is a large group of enzymes that, in close coordination with tyrosine kinases, tightly regulates diverse signaling pathways by catalyzing the removal of a phosphate group from specific signaling proteins. The largest class of PTPs in the human genome is comprised of the receptor-like PTP (PTPR) family, which contains 21 distinct members. PTPR family members span the membrane once and contain one or two intracellular catalytic domains, as well as a modular extracellular region that typically contains several protein–protein interaction domains. A role for PTPRs in the context of cancer is increasingly apparent, with many members implicated as oncogenes or tumor suppressor genes.3 We recently reported that members of the PTPR family are frequently somatically mutated across all solid tumors analyzed, including HNSCC, with PTPR type T (PTPRT) representing the single most commonly mutated PTP in HNSCC and across all cancers.4 Importantly, pSTAT3 is a validated direct substrate of PTPRT, and loss-of-function mutations of PTPRT lead to increased pSTAT3 expression and enhanced HNSCC cell growth or survival.4, 5 As these loss-of-function PTPRT mutations are found in relatively few HNSCC tumors (5.6% of tumors analyzed), we sought to determine if loss of PTPRT expression by aberrant promoter methylation contributes to pSTAT3 overexpression and sensitivity to STAT3 inhibition in HNSCC.
Herein, we report a high frequency of aberrant PTPRT promoter hypermethylation in HNSCC and other cancers. We demonstrate that PTPRT promoter methylation significantly downregulates PTPRT expression, with an associated increase in expression of the PTPRT substrate pSTAT3 in HNSCC. We show that this methylation is reversible, leading to specific downregulation of pSTAT3 in HNSCC cells. Further, we demonstrate a correlation between PTPRT promoter methylation and sensitivity to STAT3 inhibition in HNSCC cell lines, suggesting that PTPRT methylation may serve as a predictive biomarker of responsiveness to STAT3 inhibitors currently in clinical development.
Results
Frequent PTPRT promoter hypermethylation leads to decreased PTPRT mRNA expression
To assess aberrant PTPRT promoter methylation in HNSCC, we analyzed The Cancer Genome Atlas (TCGA) data derived from the Illumina HumanMethylation450 platform (San Diego, CA, USA). We first determined which CG dinucleotide methylation event was most negatively correlated with PTPRT mRNA expression (Figure 1a). We then defined aberrant hypermethylation as a fractional methylation level (beta value) at least three s.d. above the mean methylation level of the same genetic locus in organ-matched normal tissue samples and found that 60.1% (256/426 tumors analyzed) of HNSCC tumors were hypermethylated (Figure 1b). By this stringent measure, hypermethylated tumors exhibit significantly decreased PTPRT mRNA expression levels as determined by RNA-Seq (Figure 1c), suggesting the validity of the above definition and that PTPRT hypermethylation has the expected biologic effect. In contrast, copy number alterations of the PTPRT gene are relatively infrequent and are not significantly associated with altered PTPRT mRNA expression (Supplementary Figure 1). As human papillomavirus (HPV) infection is an etiologic and prognostic factor in a subset of HNSCC, we sought to determine if PTPRT promoter hypermethylation is associated with HPV status and observed no significant association (P=1.00, Fisher’s exact test; PTPRT promoter hypermethylation in 21/36 (58.3%) HPV-positive tumors versus 145/243 (59.7%) HPV-negative tumors), suggesting that HPV infection is not a driver of PTPRT promoter methylation.6
Frequent PTPRT promoter hypermethylation is associated with downregulation of PTPRT mRNA in HNSCC tumors. (a) Methylation at the CG dinucleotide denoted cg04541293 significantly correlates with decreased PTPRT mRNA expression (n=279, Pearson r=−0.2670, P<0.0001, R2=0.07131, 95% confidence interval depicted). (b) PTPRT promoter hypermethylation (defined as a methylation level greater than three s.d. above the mean methylation level of the same genetic locus in organ-matched normal tissue samples) was assessed in 426 tumors from TCGA. (c) PTPRT hypermethylation is significantly associated with downregulation of PTPRT mRNA (two-tailed unpaired t-test). Whiskers represent minimal or maximal values.
In order to validate TCGA findings in an independent HNSCC human cohort, we performed methylation-specific PCR (MSP) on 45 formalin-fixed paraffin-embedded oral squamous cell cancers with primers directed at the promoter region of PTPRT (representative analysis in Figure 2a). Using this semi-quantitative analysis, a similar high frequency of methylation was observed in this cohort (71.1%, 32/45 tumors analyzed; Figure 2b), further suggesting that PTPRT promoter methylation represents a common mechanism of PTPRT downregulation in HNSCC.
The PTPRT promoter is frequently methylated in an independent cohort of HNSCC tumors. (a) Representative MSP analysis of the PTPRT promoter from four HNSCC tumors. M denotes primers amplifying methylated sequences and U denotes primers amplifying unmethylated sequences. (b) Summary of MSP analysis of 45 HNSCC tumors. A tumor is considered methylated when the methylation level is >50% of the total signal.
The PTPRT promoter is frequently hypermethylated across human cancers
Further analysis of TCGA data reveals that the PTPRT promoter is frequently hypermethylated across a broad array of cancer types when hypermethylation is defined as a fractional methylation level (beta value) at least three s.d. above the mean methylation level of the same genetic locus in organ-matched normal tissue samples. The highest incidence of PTPRT promoter hypermethylation occurs in colon adenocarcinoma (78.7%, 289/367 tumors analyzed), whereas HNSCC exhibits the second highest incidence (60.1%; Figure 3a). Of the cancers analyzed, four exhibit significant downregulation of PTPRT mRNA in hypermethylated tumors (HNSCC, colon adenocarcinoma, lung adenocarcinoma and breast invasive carcinoma; P<0.05), suggesting a functional role for aberrant PTPRT promoter methylation across several cancer types (Figure 3b).
The PTPRT promoter is frequently hypermethylated across cancer types in association with downregulated PTPRT mRNA. (a) The PTPRT promoter is frequently hypermethylated in several cancers. (b) Table summarizing hypermethylation across cancers. Expression P-values represent the results of unpaired two-tailed t-tests between hypermethylated and non-hypermethylated tumors, with those in bold denoting significant (P<0.05) downregulation of PTPRT mRNA expression in hypermethylated tumors. Normal (n) and Tumor (n) denote the number of organ site-matched normal tissue or number of tumor samples used in this analysis, respectively. BRCA, breast invasive carcinoma; BLCA, bladder urothelial carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; COAD, colon adenocarcinoma; ESCA, esophageal carcinoma; HNSCC, head and neck squamous cell carcinoma; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma.
PTPRT expression is inversely associated with STAT3 activation in HNSCC
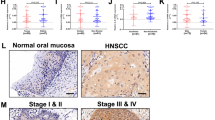
Additional analysis of TCGA data indicates that PTPRT mRNA expression is inversely correlated with pSTAT3 expression as determined by reverse-phase protein array (Figure 4a). Because pSTAT3 is known to be a direct substrate of PTPRT, we hypothesized that PTPRT promoter methylation may contribute to increased STAT3 activation.5 Indeed, representative immunohistochemical staining of an independent cohort of HNSCC tumors demonstrated that those with PTPRT promoter methylation, as determined by MSP, express elevated levels of pSTAT3 relative to tumors without PTPRT methylation (Figure 4b). Together, these findings suggest that PTPRT promoter methylation may be a frequent mechanism that contributes to STAT3 activation in HNSCC.
PTPRT promoter methylation and mRNA expression are associated with pSTAT3 expression. (a) PTPRT mRNA expression is significantly correlated with pSTAT3 expression (n=184, Pearson r=−0.1958, P<0.008, R2=0.03835, 95% confidence interval depicted). (b) Representative immunohistochemical analysis of pSTAT3 expression in six HNSCC tumors with the indicated PTPRT methylation status, performed as previously described.4
PTPRT promoter methylation is reversible, leading to PTPRT-specific pSTAT3 downregulation
To further investigate the association between PTPRT promoter methylation and STAT3 activation, we selected a HNSCC cell line (Cal27) that exhibits nearly complete methylation as determined by MSP. Treatment of Cal27 cells with 5-azacytidine (5-aza), a non-specific DNA methyltransferase inhibitor, led to an increase in the unmethylated signal as determined by MSP (Figure 5a), concurrent with overexpression of endogenous wild-type PTPRT and downregulation of pSTAT3 expression (Figures 5b and c). Transfection of Cal27 cells with shRNA directed against PTPRT following 5-aza treatment also resulted in an increase in the unmethylated signal (Figure 5a), but the expression of PTPRT was significantly downregulated relative to 5-aza treatment alone. Further, pSTAT3 expression remained unaffected under these conditions (Figures 5b and c), suggesting that 5-aza-mediated downregulation of pSTAT3 is dependent upon demethylation and re-expression of PTPRT in these cells. These results provide mechanistic evidence that PTPRT promoter methylation contributes directly to STAT3 activation in HNSCC by downregulation of PTPRT expression.
PTPRT promoter methylation is reduced by 5-azacytidine treatment, leading to PTPRT-specific pSTAT3 downregulation in HNSCC cells. (a) MSP analysis of the PTPRT promoter in HNSCC cells (Cal27) following treatment with 1 μM 5-azacytidine (or vehicle) and shRNA targeted toward PTPRT (shPTPRT) or scrambled non-targeting shRNA (shScr). (b) Western blot and RT-PCR analysis of Cal27 cells treated as indicated. (c) Graphical representation of pSTAT3/STAT3 expression analyzed by Western blot following the indicated treatments (analyzed by unpaired two-tailed t-tests, n=3).
PTPRT promoter methylation is associated with increased sensitivity to STAT3 inhibition in HNSCC cells
To evaluate whether PTPRT promoter methylation may serve as a predictive biomarker for STAT3 targeted therapies, we first determined EC50 values for selective STAT3 inhibitors in a panel of eight HNSCC cell lines (Figures 6a and b) that exhibit varying levels of PTPRT promoter methylation as determined by MSP (summarized in Figures 6c and d). Figures 6e and f illustrate that PTPRT promoter methylation and sensitivity to Stattic (a STAT3 SH2 domain inhibitor) or JSI-124 (a JAK/STAT3 pathway inhibitor) are significantly correlated (P<0.05), indicating that HNSCC tumors that exhibit PTPRT promoter hypermethylation may be most sensitive to treatment with STAT3 inhibitors. A similar trend was observed with the JAK2 inhibitor LY2784544, though this observation was not statistically significant (Supplementary Figure 2). Notably, we observe no direct correlation between PTPRT promoter methylation and pSTAT3 expression in this cell line panel, suggesting that high pSTAT3 expression per se may not be predictive of sensitivity to STAT3 inhibitors. Thus, inhibition of STAT3 directly rather than through upstream kinases may be more efficacious in tumors with PTPRT promoter hypermethylation.
Increased PTPRT promoter methylation is associated with increased sensitivity to STAT3 inhibition. (a, b) Dose-response curves for eight HNSCC cell lines treated with increasing concentrations of Stattic or JSI-124. MTT assays were performed after 72 h of treatment. (c, d) Tables of EC50 values and fractional methylation determined by MSP for the cell lines treated in a and b, sorted from least to most sensitive. (e, f) The PTPRT fractional methylation level of the cells treated in a and b correlates with sensitivity to Stattic (n=8, Pearson r=−0.7916, P<0.05, R2=0.5178) and JSI-124 (n=8, Pearson r=−0.8224, P<0.02, R2=0.6763).
We next sought to determine whether PTPRT methylation may serve as a predictive biomarker of sensitivity to STAT3 inhibition in a heterotopic tumorgraft model of HNSCC. We inoculated 16 nude mice with 3 × 106 BICR 18 cells (which exhibit total PTPRT unmethylation) on one flank, and 3 × 106 UMSCC 1 cells (which exhibit near total PTPRT methylation) on the other flank. After 10 days, the unmethylated cells had not formed any tumors, whereas the methylated cells had formed palpable masses in 15/16 mice (mean volume=100.3 mm3). As BICR 18 cells are the only HNSCC cells identified to date with total (or near total) PTPRT unmethylation, we are therefore unable to determine the relative sensitivities of unmethylated versus methylated HNSCC cells in vivo. Nevertheless, starting on day 10 post-inoculation, 8 mice were randomized to receive 50 mg/kg Stattic by oral gavage 5 times per week, with serial tumor measurements performed 3 times per week. After 9 days of treatment, a large and statistically significant (P<0.01) reduction in tumor volume was observed in the Stattic treatment arm relative to the no treatment group (Supplementary Figure 3), confirming that a PTPRT-methylated HNSCC heterotopic tumorgraft is indeed sensitive to STAT3 inhibition in vivo.
Discussion
The survival rate for HNSCC patients has remained stagnant in recent decades despite advances in the understanding of the biological underpinnings of this disease and improved therapeutic strategies. In 2006, the Food and Drug Administration approved cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, for the treatment of HNSCC patients.7 Unfortunately, cetuximab has produced limited success in the clinic, at least in part owing to the lack of predictive biomarkers, including epidermal growth factor receptor expression or copy number gain.8 There is therefore a need for the identification of both novel targets for pharmacologic inhibition and biomarkers for sensitivity to emerging therapeutics. STAT3 is a promising target for pharmacologic inhibition and STAT3 inhibitors are being tested in early phase clinical trials.2 We previously reported that STAT3 activation contributed to cetuximab resistance in HNSCC preclinical models and tumors from patients treated on cetuximab-containing protocols.9 It is increasingly apparent that most molecular targeted agents will be most effective in subgroups of patients identified by specific genomic/transcriptomic/proteomic characteristics. The identification of biomarkers that identify those individuals most likely to benefit from these agents would allow the design of more scientifically rational clinical trials.
Here we report that the PTPRT promoter is frequently hypermethylated in HNSCC and other cancers in association with decreased PTPRT expression. A high rate of PTPRT promoter hypermethylation has also been reported in sporadic colorectal cancer, where nearly all tumors analyzed were methylated in contrast to no observed methylation in matched normal tissues, as well as in hepatocellular carcinoma, where the PTPRT methylation levels progressively increase from adjacent tissue to tumor.10, 11 Our present findings indicate that PTPRT promoter methylation may represent a common event across many cancer types, suggesting that the development of a therapeutic strategy informed by this event may be of wide clinical benefit.
To date, the functional signaling consequences downstream of PTPRT promoter methylation have not been described, and as such, no strategies to mitigate the effects of this event have been proposed. Here we demonstrate that PTPRT promoter methylation is associated with a decrease in PTPRT mRNA expression, which in turn is associated with an increase in expression of the PTPRT substrate pSTAT3. We further evaluated PTPRT promoter methylation in an independent cohort of HNSCC and observed a similar frequency to that observed by TCGA, thus confirming the high incidence of this event. We also confirm that PTPRT methylation significantly contributes to pSTAT3 upregulation in HNSCC cells. Together, these studies suggest that HNSCC cells and tumors that exhibit high levels of PTPRT promoter methylation may be more dependent on STAT3 signaling for growth and survival, thus offering the opportunity to exploit PTPRT methylation as a predictive biomarker of sensitivity to STAT3 inhibition. Importantly, we have established an association between PTPRT promoter methylation and sensitivity to inhibitors of the STAT3 signaling pathway. These studies therefore suggest that screening for PTPRT promoter methylation may ultimately allow the identification of patients who are most likely to benefit from treatment with STAT3 targeting agents and provide a rationale for designing future clinical trials that select for patients with a high degree of PTPRT promoter methylation.
Materials and methods
Genomic and proteomic data and analysis
TCGA methylation and RNA-Seq data were obtained through R via the CGDS-R package or through the TCGA data matrix (https://tcga-data.nci.nih.gov/tcga/dataAccessMatrix.htm). Reverse-phase protein array data were obtained from The Cancer Proteome Atlas (http://app1.bioinformatics.mdanderson.org/tcpa/_design/basic/index.html). Statistical calculations were performed using GraphPad Prism 5 software (GraphPad, La Jolla, CA, USA).
MSP
Two-millimeter diameter cores were obtained from formalin-fixed paraffin-embedded HNSCC tumors under the auspices of a protocol approved by the Institutional Review Board of the University of Pittsburgh. Tumor DNA was isolated with the QIAamp DNA FFPE Tissue Kit, whereas the QIAamp DNA Mini Kit was used for isolation of DNA from cell lines, both according to the manufacturer’s instructions (Qiagen, Hilden, Germany). Bisulfite conversion of 1 μg of DNA per sample was performed using the EpiTect Bisulfite Kit according to the manufacturer’s instructions (Qiagen). MSP primers were designed using MethPrimer software (San Francisco, CA, USA).12 MSP was performed with the EpiTect MSP Kit (Qiagen). After reaction completion, products were analyzed by gel electrophoresis on a 2.5% agarose gel. Densitometry was performed using ImageJ and the fractional methylation (beta value) for a particular tumor or cell line was calculated as (methylation signal)/(methylation signal+unmethylation signal).
Cell culture
Cal27 and Detroit 562 cells were obtained from ATCC (Manassas, VA, USA). 686LN cells were obtained from Georgia Chen at MD Anderson Cancer Center (Houston, TX, USA). BICR 18 and PE/CA-PJ49 cells were obtained from Sigma-Aldrich (St. Louis, MO, USA). UMSCC cell lines were obtained from Thomas E. Cary at the University of Michigan (Ann Arbor, MI, USA). HSC-2 cells were obtained from Hideo Niwa at Nihon University (Tokyo, Japan). Cal27, Detroit 562, HSC-2, UMSCC 47 and UMSCC 22 A were maintained in DMEM (Corning, Corning, NY, USA) containing 10% fetal bovine serum (FBS; Gemini Bio-Products, West Sacramento, CA, USA). UMSCC 1 were maintained in DMEM containing 10% FBS and 0.4 μg/ml hydrocortisone (Sigma-Aldrich, St. Louis, MO, USA). BICR 18 were maintained DMEM containing 10% FBS, 0.4 μg/ml hydrocortisone and 2 mm l-glutamine (Life Technologies, Carlsbad, CA, USA). 686LN were maintained in DMEM/F12 (Life Technologies) containing 10% FBS. PE/CA-PJ49 were maintained in Iscove’s DMEM (Corning) containing 10% FBS and 2 mm l-glutamine. All cells were genotypically verified using the AmpFSTR Identifiler PCR Amplification Kit according to the manufacturer’s instructions (Life Technologies) and maintained at 37 °C and 5% CO2.
5-aza and shRNA treatment
Cal27 cells were plated at 250 000 cells per well on sixwell plates and incubated overnight. Medium was then replaced with complete medium containing 1 μM 5-aza (or 50% acetic acid in water as vehicle). Twenty-four hours later, cells were transfected with 2 μg of shRNA targeted toward PTPRT or non-targeted scrambled sequence (shScr) with 6 μl of FuGENE HD (Promega, Fitchburg, WI, USA) in 200 μl of Opti-MEM (Life Technologies) added directly to medium containing 5-aza (or vehicle). Cells were incubated for an additional 24 hours before DNA, RNA and protein were collected for analysis.
Immunoblotting
Primary antibodies for pSTAT3 and STAT3 were obtained from Cell Signaling Technology (Boston, MA, USA) and for β-tubulin from Abcam (Cambridge, MA, USA). Secondary antibodies were obtained from BioRad (Hercules, MA, USA). Densitometry was performed using ImageJ software.
Drug treatment
Cells were plated on 48-well plates at a density of 6800 cells/well and incubated overnight before treatment with increasing concentrations of Stattic (Sigma-Aldrich), JSI-124 (Calbiochem, San Diego, CA, USA), LY2784544 (Eli Lilly, Indianpolis, IN, USA), or DMSO alone as vehicle control in triplicate in the appropriate complete medium. MTT assays were performed after 72 h and data were analyzed using GraphPad Prism 5 (GraphPad).
Animals
Mice were used in accordance with a protocol approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and were housed in a facility certified by the American Association for the Accreditation of Laboratory Animal Care. Foxn1 nude mice (5–6-week old) were purchased from Harlan Laboratories (Indianapolis, IN, USA). Sixteen female mice were inoculated with 3 × 106 BICR 18 cells on the left flank, and 3 × 106 UMSCC 1 cells on the right flank. After 10 days of growth, UMSCC 1 cells had formed palpable tumors in 15/16 mice, whereas BICR 18 had formed none. At this time, eight mice were randomized to receive 50 mg/kg Stattic in 1% Tween-80 in phosphate-buffered saline by oral gavage five times per week as previously reported in an ovarian cancer model.13 Seven mice received no treatment. Tumor sizes were blindly measured by caliper in two dimensions and volumes calculated as (large measurement) × (small measurement)2. Mice were killed after measurements were taken on day 9 of treatment.
References
Siegel R, Ma J, Zou Z, Jemal A . Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29.
Peyser ND, Grandis JR . Critical analysis of the potential for targeting STAT3 in human malignancy. OncoTargets Ther 2013; 6: 999.
Julien S, Dubé N, Hardy S, Tremblay M . Inside the human cancer tyrosine phosphatome. Nat Rev Cancer 2011; 11: 35–49.
Lui VWY, Peyser ND, Ng PK-S, Hritz J, Zeng Y, Lu Y et al. Frequent mutation of receptor protein tyrosine phosphatases provides a mechanism for STAT3 hyperactivation in head and neck cancer. Proc Natl Acad Sci USA 2014; 111: 1114–1119.
Zhang X, Guo A, Yu J, Possemato A, Chen Y, Zheng W et al. Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T. Proc Natl Acad Sci USA 2007; 104: 4060–4064.
Benson E, Li R, Eisele D, Fakhry C . The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol 2014; 50: 565–574.
Leemans CR, Braakhuis BJ, Brakenhoff RH . The molecular biology of head and neck cancer. Nat Rev Cancer 2011; 11: 9–22.
Licitra L, Mesia R, Rivera F, Remenár E, Hitt R, Erfán J et al. Evaluation of EGFR gene copy number as a predictive biomarker for the efficacy of cetuximab in combination with chemotherapy in the first-line treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck: EXTREME study. Ann Oncol 2011; 22: 1078–1087.
Sen M, Joyce S, Panahandeh M, Li C, Thomas SM, Maxwell J et al. Targeting Stat3 abrogates EGFR inhibitor resistance in cancer. Clin Cancer Res 2012; 18: 4986–4996.
Laczmanska I, Karpinski P, Bebenek M, Sedziak T, Ramsey D, Szmida E et al. Protein tyrosine phosphatase receptor-like genes are frequently hypermethylated in sporadic colorectal cancer. J Hum Genet 2013; 58: 11–15.
Gao W, Kondo Y, Shen L, Shimizu Y, Sano T, Yamao K et al. Variable DNA methylation patterns associated with progression of disease in hepatocellular carcinomas. Carcinogenesis 2008; 29: 1901–1910.
Li L-C, Dahiya R . MethPrimer: designing primers for methylation PCRs. Bioinformatics 2002; 18: 1427–1431.
Ji T, Gong D, Han Z, Wei X, Yan Y, Ye F et al. Abrogation of constitutive Stat3 activity circumvents cisplatin resistant ovarian cancer. Cancer Lett 2013; 341: 231–239.
Acknowledgements
This work was supported by the National Cancer Institute at the National Institutes of Health (grant numbers P50 CA097190 and R01 CA077308 to JRG); and by the National Institute for Dental and Craniofacial Research at the National Institutes of Health (grant number F31 DE024007 to NDP). The authors wish to thank Mark H Gage for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Peyser, N., Freilino, M., Wang, L. et al. Frequent promoter hypermethylation of PTPRT increases STAT3 activation and sensitivity to STAT3 inhibition in head and neck cancer. Oncogene 35, 1163–1169 (2016). https://doi.org/10.1038/onc.2015.171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.171
This article is cited by
-
Downregulation of PTPRT elevates the expression of survivin and promotes the proliferation, migration, and invasion of lung adenocarcinoma
BMC Cancer (2024)
-
First genome-wide association study of non-severe malaria in two birth cohorts in Benin
Human Genetics (2019)
-
Targeting the IL-6/JAK/STAT3 signalling axis in cancer
Nature Reviews Clinical Oncology (2018)
-
Identification of subsets of actionable genetic alterations in KRAS-mutant lung cancers using association rule mining
Cellular Oncology (2018)
-
Mechanism of action of selective inhibitors of IL-6 induced STAT3 pathway in head and neck cancer cell lines
Journal of Chemical Biology (2017)