Abstract
Background:
The lack of sensitive and specific biomarkers for prostate cancer (PCa) has led to over-diagnosis and overtreatment with uncertain benefit. Therefore, biomarkers for early diagnosis that can distinguish aggressive from indolent tumors and that can detect metastatic or recurrent disease are needed. Long noncoding RNAs (lncRNAs) are non-protein-coding RNA species. lncRNAs are dysregulated in many diseases including PCa and are emerging as major players in cancer development. lncRNAs have several features that make then suitable as both biomarkers and therapeutics, and lncRNAs regulate critical cancer hallmarks in prostate epithelial cells including proliferation and survival.
Methods:
The PubMed database was searched using the terms 'long noncoding RNA', 'biomarker' and 'prostate cancer'. Known lncRNAs implicated as biomarkers and potential therapeutic targets in PCa are reviewed.
Results:
We comprehensively review several lncRNAs with potential as biomarkers for PCa. lncRNAs including PCA3, PCATs, SChLAP1, SPRY4-IT1 and TRPM2-AS are upregulated in PCa and are cancer specific; they are, therefore, attractive lead candidate biomarkers for clinical application. Several lncRNA therapeutics are currently being investigated by several companies for the treatment of various cancers including PCa. Small interfering RNAs, antisense oligonucleotides, ribozymes, deoxyribozymes and aptemers are few promising technologies for future lncRNA bases therapeutics.
Conclusion:
lncRNA expression is altered in cancer. Aberrant regulation promotes tumor formation, progression and metastasis. lncRNAs can use as tumor markers for PCa and may be attractive novel therapeutic targets for the diagnosis and treatment of PCa.
Similar content being viewed by others
Introduction
The United States Preventive Services Task Force did not recommend PSA as a routine screening test for prostate cancer (PCa) because of its uncertain benefit-to-risk profile.1 More sensitive and specific diagnostic PCa biomarkers are needed: this is a high-priority area for the PCa research community. Furthermore, once diagnosed, prognostic and predictive biomarkers are also required to stratify patients so that further interventions can be tailored to each individual patient to avoid overtreatment and undertreatment.
The shared clinical decision-making process is still limited by the difficulty in distinguishing indolent and aggressive cancers in a large proportion of men. The introduction of novel molecular diagnostic tools into clinical practice to more accurately detect and predict the behavior of localized PCa was embraced by the urologic community.2, 3 Here, we focus on the role and promise of long noncoding RNAs (lncRNAs) as next-generation diagnostic, prognostic and predictive biomarkers in PCa and their possible use as therapeutic targets.
lncRNAs in tumor biology
Recent innovations in high-throughput technologies have contributed to valuable progress in the molecular characterization of PCa.4, 5 Although molecular tumor biology is often still considered in 'linear' terms of transcription, translation and the action of oncoproteins, one of the major messages from the Human Genome Project was that only about 1.5–2% of our genome is protein coding.6 Genome-wide association studies have been used in PCa and other cancers as an unbiased method to identify alleles associated with PCa risk.7 The Encyclopedia of DNA Elements (ENCODE) project revealed that as much as 70% of the genome is transcribed as non-protein-coding RNA, including as lncRNAs (over 200-nucleotide long),8 and most transcribed products are functional rather than transcriptional noise. The number of recognized lncRNA genes is growing, with the most recent comprehensive transcriptome study identifying 58 648 lncRNAs genes.9 Although the biological roles of lncRNAs, sometimes referred to as the ‘dark matter of the genome’, are not nearly as well understood as protein-coding mRNAs, it rapidly became clear that they play important roles in almost every aspect of cancer biology.10, 11, 12 Our understanding of lncRNA function has increased rapidly but large knowledge gaps remain, in particular, about how lncRNAs are regulated and how they regulate other genes and proteins. From the translational perspective, lncRNAs are of particular interest because of their high tissue and tumor specificity, making them good candidates for biomarkers.11, 13, 14 Although lncRNAs are found in many non-human species they are generally expressed at negligible levels, and some human lncRNAs are thought to have solely arisen from primates.15 lncRNAs are expressed at much lower levels and with much higher cell-type specificity than mRNAs.16 Non-protein-coding DNA accounts for about 98% of the human genome, which can be transcribed in functional ncRNAs (Figure 1).15
Structure of the human genome. Adapted from Ronnau et al.15 The human genome consists of ~2% protein-coding sequences and ~98% noncoding sequences, which can be transcribed in noncoding RNAs (ncRNAs). ncRNAs can be grouped into long noncoding RNAs (lncRNAs) and small ncRNAs, depending on the size. Small ncRNAs consist of microRNAs (miRNAs), piwi-interacting RNAs (piRNAs), ribosomal RNAs (rRNAs), small Cajal body-specific RNAs (scaRNAs), small interfering RNAs (siRNAs), small nuclear RNAs (snRNAs), small nucleolar RNAs (snRNAs) and transfer RNAs (tRNAs). miRNAs can also be derived from lncRNAs and snoRNAs (highlighted in red). A full color version of this figure is available at the Prostate Cancer and Prostatic Diseases journal online.
Studies in many solid organ and hematological malignancies including prostate, breast, lung, liver and colon cancer and leukemia have shown that lncRNAs can have tumor-suppressor and oncogenic functions by regulating gene expression.17, 18, 19 Even though our understanding of the identity, function and dysregulation of lncRNAs in cancer is in its infancy, a rapidly growing body of literature suggests that they can act as master regulators of carcinogenesis.14, 19 Several specific lncRNAs have been shown to play critical roles in the development and progression of cancer. Therefore, in addition to their putative role as biomarkers, lncRNAs are a promising new avenue in translational oncology as therapeutic targets.20, 21, 22, 23
Characteristics and functions of lncRNAs
Understanding lncRNA function requires thorough characterization of the molecular pathways that determine their production, structure and turnover.21 General lncRNA features include being transcribed by RNA polymerase II and being associated with epigenetic signatures common to protein-coding genes such as trimethylation of histone 3 lysine 4 (H3K4me3) at the transcriptional start site and trimethylation of histone 3 lysine 36 (H3K36me3) throughout the gene.21, 24 lncRNAs then undergo co-transcriptional modifications including 5′ capping, pre-lncRNA splicing and polyadenylation.21, 24 Subsequently, they fold to form secondary and/or tertiary structures, the latter thought to be the basic components of functional lncRNA motifs (Figure 2).11 Although they vary, their half-lives are, on average, less than those of mRNA.25 Nuclear lncRNAs are less stable than cytoplasmic lncRNAs, and intergenic, cis-antisense and spliced lncRNAs are generally more stable than those derived from intronic lncRNAs and unspliced single exon lncRNAs.25 lncRNAs can act as scaffolds for complex formation, providing binding sites for various protein factors. lncRNAs may also bind small molecules via RNA pockets that facilitate the formation of, or conformational changes in, lncRNA structure.11 Unpaired lncRNA sequences, or lacunas, can also act as functional, interconnecting elements by interacting with intracellular RNA and DNA.15 Lacunas can interact with small RNAs based on sequence complementarity to regulate small RNA activity,24 and they interact with mRNA and DNA to form hybrid duplexes or triplexes that provide structural motifs for protein recognition.21 This hybridization can also mimic important elements of RNA and DNA. Ultimately, lncRNAs can be further processed to generate multiple functional fragments that are shuttled to different cellular compartments to effect a plethora of other functions.11 Finally, lncRNAs have been reported to play a role in other diverse cellular functions including imprinting, epigenetic regulation, apoptosis, cell cycle control, transcriptional and translational regulation, splicing, cell development and differentiation, and aging.15, 16, 21
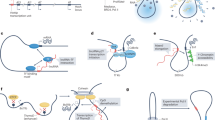
Different types and functions of long noncoding RNAs (lncRNAs). (a) HOTAIR as a scaffold for the coordination of epigenetic or histone-modifying complexes. (b) Enhancer RNA (eRNA) facilitates hormone signaling by co-operating with lineage-specific complexes such as FOXA1 and androgen receptor (AR). (c) ANRIL can directly affect transcriptional regulation of tumor-suppressor genes, although epigenetic silencing; whereas lnc-p21 can activate tumor-suppressor target genes. (d) MALAT1 contributes to post-transcriptional processing of mRNAs. (e) Direct lncRNA–mRNA interactions generate a signal for STAU1-mediated degradation of the mRNA. (f) lncRNAs serve as molecular sponges of microRNA (miRNA) to facilitate miRNA binding to each transcript (sequestration). Reprinted with permission from Prensner and Chinnaiyan.11
Clinical implementation of lncRNAs as potential next-generation biomarkers for PCa
lncRNAs appear to have fundamental biological and clinical importance in PCa: the lncRNAs implicated in PCa are presented in Table 1. PCa antigen 3 (PCA3 or DD3; marketed as the Progensa test, Gen Probe) is a highly prostate-specific gene that expresses a noncoding RNA and is overexpressed 60- to 100-fold in PCa cells.26 The PCA3 score reflects the PCA3 mRNA to PSA mRNA ratio in post-digital rectal examination urine, with a higher score associated with a high probability of a cancer-positive biopsy after a previously negative initial biopsy. This test obtained the Food and Drug Administration approval in 2012 as the first molecular test to determine the need for repeat prostate biopsies in men aged 50 years or older with a suspicion of PCa based on PSA levels and/or digital rectal examination and/or one or more previous negative biopsies.27 Vlaeminick-Guillem et al. performed a meta-analysis of 11 multicenter and single-center clinical studies (n=2737) and demonstrated area under the curve values of receiver operator characteristic curves from 0.66 to 0.77, sensitivities between 53 and 69% and specificities between 71 and 83% (Figure 3). Overall accuracy was about 66%. Wei et al. reported use of the PCA3 assay in addition to PSA-based PCa screening. In the initial biopsy setting, a PCA3 score >60 had a high positive predictive value for PCa of 0.80. In the repeat biopsy setting, a PCA3 score <20 had a high negative predictive value for PCa of 0.88 (Tables 2 and 3).28 The limitations of the test include significant intra-individual variability, only 50% cancer detection at PCA3 >100, better performance in the repeat biopsy setting, and conflicting data on the relationship between score and cancer grade using the most common threshold of 35.28, 29
The accuracy of the PCA3 score (percentage of men with positive biopsies) at different thresholds to predict prostate biopsy outcome. Adapted from Vlaeminick-Guillem et al.46
Overexpression of oncogenic lncRNAs may promote cancer hallmarks including tumor cell proliferation and metastasis, and aberrant lncRNA expression in PCa is associated with disease progression.11, 15, 16, 21 The lncRNA PCa-associated ncRNA transcript 1 (PCAT1) is highly prostate specific and was reported to be upregulated patients with high-grade localized (Gleason score ≥7) and metastatic PCa.20 Mechanistically, this association was proposed to be due to the cell proliferation-inducing effects of PCAT1 and its gene expression-suppressive effects, not least on the tumor-suppressor BRCA2. PCAT1 also promotes proliferation via cMyc by acting as a decoy for cMyc-targeting microRNAs.30 The PCAT-family members PCAT6, 7 and 18 were predictive of tumor progression via androgen receptor (AR) signaling.11, 15 PCAT6 and PCAT7 were identified in an integrated analysis of gene expression, copy number alterations and clinical data.31 Expression of both lncRNAs was greater in primary and metastatic PCa. Small interfering (siRNA)-mediated knockdown of either PCAT6 or PCAT7 reduced cell growth and soft agar colony formation in both the parental androgen-dependent LNCaP cell line and the androgen-independent LNCaP-abl sub-line.31 PCAT18 was identified in an RNA sequencing study of paired metastatic/non-metastatic PCa patient-derived xenografts.32 PCAT18 is a highly prostate-specific transcript, and its expression is induced by AR signaling. Notably, PCAT18 could be detected in plasma samples and was greater in patients with metastatic PCa. In vitro, PCAT18 knockdown by siRNA inhibited the growth of both LNCaP and C4-2 cells and had no effect on non-neoplastic BPH1 prostate cells.32
AR blockade induces tumor regression, but castration-resistant PCa frequently develops, usually representing late-stage, lethal disease. Understanding progression to castration resistance is, therefore, important. Yang et al. reported that PRNCR1 (PCa noncoding RNA1) and PCGEM1 (PCa gene expression marker 1) are involved in AR-mediated gene expression.33 PRNCR1 binds to the carboxy-terminal domain of the AR and recruits DOT1L (DOT1-like histone H3K79 methyltransferase). DOT1L activates the AR, allowing it to associate with PCGEM1, which, in turn, recruits PYGO2 (pygopus family PHD finger 2), which binds to a promoter-associated histone mark (H3K4me3) to allow the AR to activate its target genes. Notably, these lncRNAs were required for transcriptional activation of constitutively active truncated AR isoforms, suggesting a role in castration-resistant PCa. However, this in vitro study was challenged by two later studies.34, 35 Prensner et al. showed that PCGEM1 and PRNCR1 do not interact with the AR and that neither gene was prognostic in PCa.35 Using patient-derived xenografts, Parolia et al. found high PCGEM1 expression levels, but little PRNCR1.34 They further showed that PCGEM1 was uniformly distributed in PCa cell nuclei and the cytoplasm, and that localization was not altered upon AR transcriptional activation. In addition, PCGEM1 was upregulated in primary, but not metastatic, PCa.34 Therefore, the role of PCGEM1 and PRNCR1 in castration-resistant PCa remains uncertain.
In addition to PCGEM1’s role in AR activation, Hung et al. recently demonstrated that it promotes cancer cell proliferation by regulating cMyc. PCGEM1 interacts directly with cMyc to regulate metabolism at the transcriptional level.36 Interestingly, PCGEM1 can be regulated by microRNAs: He et al. showed that the tumor-suppressor microRNA, miR-145, binds to PCGEM1, and miR-145 overexpression decreased PCGEM1. In addition, PCGEM1 downregulation increased miR-145 expression. This cross-talk had an effect on PCa cell proliferation and invasion.37
Second chromosome locus associated with prostate-1 (SCHLAP1) is a lncRNA that is highly expressed in 25% of PCas.38 SCHLAP1 expression was significantly associated with risk of biochemical recurrence, clinical progression and PCa-specific mortality in a study of 235 patients after radical prostatectomy (median follow-up of 8.1 years).38 In a recent study to identify metastasis-associated genes, high SCHLAP1 expression was associated with a higher risk of biochemical recurrence, metastasis and death from PCa.39 The study was robust: gene expression was measured in tissue samples in a Clinical Laboratory Improvements Amendments-certified laboratory, and the prognostic significance of SCHLAP1 was validated in three independent cohorts. In addition, SCHLAP1 expression in urine sediments was increased in intermediate- or high-risk patients compared with those at low risk of recurrence.39
The preceding discussion highlights that there are several clinically promising PCa-specific or PCa-associated lncRNAs in addition to PCA3, but none have yet been fully validated or approved for clinical practice.11, 16, 21 Recently, Lee et al. reported a set of upregulated PCa-specific lncRNAs in PC3 cell lines and patient samples compared with non-malignant prostate epithelial cells and matched normal (healthy) prostate tissues.40 Six lncRNAs (AK024556, XLOC_007697, LOC100287482, XLOC_005327, XLOC_008559 and XLOC_009911) upregulated in cell lines were also upregulated in PCa tissue samples (Gleason score >6.0) compared with matched normal tissues, and the biomarkers were also upregulated in urine samples. One of the upregulated lncRNAs, AK024556 (SPRY4-IT1), was highly upregulated in the human PCa cell line PC3 but not in LNCaP, and siRNA knockdown of SPRY4-IT1 in PC3 cells inhibited cellular proliferation and invasion and increased apoptosis.40 An RNA chromogenic in-situ hybridization assay was developed to detect long noncoding RNAs in primary PCa tissue samples, which demonstrated PCa-specific expression: SPRY4-IT1 was easily detected in all PCa samples with different Gleason scores (6–10), but there was no detectable SPRY4-IT1 in normal prostatic tissue (Figure 4). SPRY4-IT1 expression appears to be PCa specific and can be detected using standard clinical staining procedures on tissue samples.40
RNA chromogenic in-situ hybridization (CISH) analysis of SPRY4-IT1. (a) RNA-CISH staining of SPRY4-IT1 in tumor samples and matched normal tissues (formalin-fixed, paraffin embedded (FFPE) samples). Expression was visualized using alkaline phosphatase-labeled probes. (b) Quantitative PCR of SPRY-IT1 expression in tumor and matched tissue samples (FFPE samples in a). Reprinted with permission from Lee et al.40
Other studies have evaluated the presence of lncRNA fragments in blood specimens. In primary PCa, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) overexpression was associated with indicators of poor prognosis (high Gleason score, higher tumor-node-metastasis stage and serum PSA >20 ng ml−1), and its expression was significantly higher in castration-resistant PCa than hormone-sensitive PCa.41 Furthermore, MALAT1 expression levels could be used to distinguish biopsy-positive from biopsy-negative PCa patients (area under the curve=0.767; P<0.001). However, the sensitivity of a plasma-based MALAT1 test was very low (58.6%), even lower than that of serum PSA.41 Wang et al. investigated the potential diagnostic efficacy of urinary MALAT1 transcript detection. MALAT1 score was assessed in a retrospective discovery cohort and a prospective multicenter cohort. Compared with patients with negative biopsies, patients with positive biopsies had significantly higher MALAT1 scores, suggesting that urinary MALAT1 may be a promising diagnostic biomarker.42
Orfanelli et al. investigated TRPM2-AS, an antisense lncRNA transcript of TRPM2 that encodes an oxidative stress-activated ion channel and that is overexpressed in PCa.22 High TRPM2-AS expression and its related gene signature were associated with poor clinical outcome, the related gene signature being independent of the Gleason score. In vitro, TRPM2-AS knockdown led to PCa cell apoptosis and a transcriptional profile that indicated profound cellular stress in the dying cells, along with cell cycle arrest, an increase in intracellular hydrogen peroxide, and activation of the sense TRPM2 gene. Moreover, the action of existing therapies was associated with high TRPM2-AS levels in drug-targeted cell lines and patients. This suggests that a TRPM2-AS assay may not only be useful for the early identification of aggressive PCa tumors, but also for identifying subsets of intermediate- and high-risk patients who would benefit from targeted therapy. In parallel with PCA3, minimally invasive quantification of TRPM2-AS and/or the ind_TRPM2_AS gene signature in post-digital rectal examination urine might allow even earlier detection of high-risk tumors,22 and TRPM2-AS itself might be a 'druggable' therapeutic target.
lncRNAs as therapeutic targets
lncRNAs are, therefore, an attractive therapeutic target amenable to different technical approaches (Figure 5).16 Currently, molecular therapy tends to focus on targeting RNA, either by regulating lncRNA levels in tumor cells or by modifying their structure or mature sequence.16 RNA interference-based techniques for inhibiting lncRNAs in cancer cells are most advanced due to efficient and highly specific RNA selectivity and knockdown efficiency. The simple siRNA structure and short hairpin RNAs have made them versatile tools for RNA-specific targeting, expediting their clinical application.16 Furthermore, combining RNA interference-based techniques with gene therapy technology can, at least in theory, provide stable and persistent lncRNA inhibition. Inhibition of critical cancer-associated genes with therapeutic siRNAs has already been evaluated in different phase clinical trials. A few siRNA signatures are currently undergoing phase I evaluation including siRNA-EphA2-DOPC (targeting EphA2) in ovarian tumors (NCT01591356; www.clinicaltrials.gov), TKM-080301 (targeting PLK1) in neuroendocrine tumors and adrenocortical carcinomas (NCT01262235), and CALAA-01 (targeting RRM2) in liver cancer (NCT01437007 and NCT02191878). Other formulations using a combination of siG12D LODER and Atu027 siRNA drug with conventional chemotherapy are currently in phase II trial for its therapeutic effect in advanced pancreatic cancer (NCT01676259).
Mechanisms of long noncoding RNA (lncRNA)-targeting agents. (a) Small interfering RNAs (siRNAs) target RNA molecules via complementarity to the unpaired lncRNA sequences. (b) Antisense oligonucleotides are single-stranded DNAs or RNAs designed with sequence specificity to target lncRNAs. (c) HamRz is a single-stranded RNA in neutral conditions that undergoes folding in cells to expose the binding arms. (d) Aptamers are short DNA or RNA oligonucleotides or peptides that specifically bind to their target lncRNAs. (e) Small molecules are synthesized to specifically bind to the RNA-binding pockets of lncRNAs. Reprinted with permission from Li and Chen.16
Meanwhile, other established methods for inhibiting cancer-associated RNA, including antisense oligonucleotides, ribozymes and aptamers, can also be used to modulate lncRNAs, and these techniques have unique advantages over siRNAs.16, 22 Small molecules, post-transcriptional processing pathways and microRNAs targeting lncRNAs may also be useful tools in the treatment armamentarium.11
Targeting lncRNAs by Crisper/Cas system
Recently, clustered regularly interspaced short palindromic repeats (CRISPER)/CRISPER-associated (Cas) system namely Crisper/Cas has been shown to be an efficient gene editing tool. However, this system has been used predominantly for gene modification in coding genes. Nevertheless, this system has a great potential to target noncoding RNAs in gene knockout, gene silencing (Crisperi) and gene activation (Crispera). In a recent study, Ho et al.43 show that the use of Crisper/Cas9 system by successful targeting of noncoding RNAs (microRNAs and lncRNAs). In this study, they knockout miR-21, miR-29a, lncRNAs 21 A, UCA1 and AK023948. Interestingly, PCa cell line LNCaP was one of the cell lines they used to test the Crisper/Cas9 system successfully. Shechner et al.44 reported of using a multiplexable, locus-specific targeting of long RNAs with CRISPR-Display method. In essence, they demonstrated that large functional RNA domains can be inserted in CRISPR guide RNA at multiple points. Together, these results demonstrate the possibility of knockout for noncoding genes by the Crisper/Cas system.
Conclusions
The use of lncRNAs as PCa biomarkers remains in its infancy, and further investigations and large validation studies are necessary before successful translation into the clinical setting. New lncRNAs are present in the 'gene desert' region of 8q24 (the germline susceptibility locus most frequently identified in genome-wide association study), and single-nucleotide polymorphisms found in these areas are likely to affect as yet unknown aspects of tumor biology. There is a growing body of literature on the biological functions of lncRNAs and their roles in tumorigenesis. Genome-wide association studies may provide information on the germline polymorphisms that predict an individual patient's clinical risk of developing PCa, responding to therapy, or the clinical course of their disease, as well as providing molecular information on the regulation of gene expression of key genes through lncRNAs. Several lncRNAs associated with PCa have already been identified, and these early studies demonstrate how lncRNA targeting can be used to inhibit cancer cells. Therefore, these lncRNAs are potential novel therapeutic targets. Drugs that target RNA provide the basis for precision lncRNA-based cancer therapies, but more work is required in this area for clinical translation. Given that only 5% of the human genome is 'druggable',45 the structure, folding, interactions and function of lncRNAs needs further elucidation so that the clinical benefit can be realized.
References
Klotz L . Prostate cancer overdiagnosis and overtreatment. Curr Opin Endocrinol Diabetes Obes 2013; 20: 204–209.
Sartori DA, Chan DW . Biomarkers in prostate cancer: what's new? Curr Opin Oncol 2014; 26: 259–264.
Crawford ED, Ventii K, Shore ND . New biomarkers in prostate cancer. Oncology (Williston Park) 2014; 28: 135–142.
Beltran H, Rubin MA . New strategies in prostate cancer: translating genomics into the clinic. Clin Cancer Res 2013; 19: 517–523.
Beltran H, Yelensky R, Frampton GM, Park K, Downing SR, MacDonald TY et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur Urol 2013; 63: 920–926.
Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J et al. Initial sequencing and analysis of the human genome. Nature 2001; 409: 860–921.
Chung CC, Hsing AW, Edward Y, Biritwum R, Tettey Y, Adjei A et al. A comprehensive resequence-analysis of 250kb region of 8q24.21 in men of African ancestry. Prostate 2014; 74: 579–589.
Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A et al. Landscape of transcription in human cells. Nature 2012; 489: 101–108.
Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet 2015; 47: 199–208.
Qi P, Du X . The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol 2013; 26: 155–165.
Prensner JR, Chinnaiyan AM . The emergence of lncRNAs in cancer biology. Cancer Discov 2011; 1: 391–407.
Yang L, Froberg JE, Lee JT . Long noncoding RNAs: fresh perspectives into the RNA world. Trends Biochem Sci 2014; 39: 35–43.
Bolton EM, Tuzova AV, Walsh AL, Lynch T, Perry AS . Noncoding RNAs in prostate cancer: the long and the short of it. Clin Cancer Res 2014; 20: 35–43.
Cheetham SW, Gruhl F, Mattick JS, Dinger ME . Long noncoding RNAs and the genetics of cancer. Br J Cancer 2013; 108: 2419–2425.
Ronnau CG, Verhaegh GW, Luna-Velez MV, Schalken JA . Noncoding RNAs as novel biomarkers in prostate cancer. BioMed Res Int 2014; 2014: 591703.
Li CH, Chen Y . Targeting long non-coding RNAs in cancers: progress and prospects. Int J Biochem Cell Biol 2013; 45: 1895–1910.
Qiu MT, Hu JW, Yin R, Xu L . Long noncoding RNA: an emerging paradigm of cancer research. Tumour Biol 2013; 34: 613–620.
Pennisi E . Cell biology. Lengthy RNAs earn respect as cellular players. Science 2014; 344: 1072.
Deng G, Sui G, Noncoding RNA . in oncogenesis: a new era of identifying key players. Int J Mol Sci 2013; 14: 18319–18349.
Prensner JR, Chen W, Iyer MK, Cao Q, Ma T, Han S et al. PCAT-1, a long noncoding RNA, regulates BRCA2 and controls homologous recombination in cancer. Cancer Res 2014; 74: 1651–1660.
Pickl JM, Heckmann D, Ratz L, Klauck SM, Sultmann H . Novel RNA markers in prostate cancer: functional considerations and clinical translation. BioMed Res Int 2014; 2014: 765207.
Orfanelli U, Jachetti E, Chiacchiera F, Grioni M, Brambilla P, Briganti A et al. Antisense transcription at the TRPM2 locus as a novel prognostic marker and therapeutic target in prostate cancer. Oncogene 2014; 34: 2094–2102.
Petrovics G, Zhang W, Makarem M, Street JP, Connelly R, Sun L et al. Elevated expression of PCGEM1, a prostate-specific gene with cell growth-promoting function, is associated with high-risk prostate cancer patients. Oncogene 2004; 23: 605–611.
Liu D, Xu B, Chen S, Yang Y, Zhang X, Liu J et al. Long non-coding RNAs and prostate cancer. J Nanosci Nanotechnol 2013; 13: 3186–3194.
Clark MB, Johnston RL, Inostroza-Ponta M, Fox AH, Fortini E, Moscato P et al. Genome-wide analysis of long noncoding RNA stability. Genome Res 2012; 22: 885–898.
Tomlins SA . Urine PCA3 and TMPRSS2:ERG using cancer-specific markers to detect cancer. Eur Urol 2014; 65: 543–545.
Hessels D, Schalken JA . The use of PCA3 in the diagnosis of prostate cancer. Nat Rev Urol 2009; 6: 255–261.
Wei JT, Feng Z, Partin AW, Brown E, Thompson I, Sokoll L et al. Can urinary PCA3 supplement PSA in the early detection of prostate cancer? J Clin Oncol 2014; 32: 4066–4072.
Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: does PCA3 testing for the diagnosis and management of prostate cancer improve patient health outcomes? Genet Med 2014; 16: 338–346.
Prensner JR, Chen W, Han S, Iyer MK, Cao Q, Kothari V et al. The long non-coding RNA PCAT-1 promotes prostate cancer cell proliferation through cMyc. Neoplasia 2014; 16: 900–908.
Du Z, Fei T, Verhaak RG, Su Z, Zhang Y, Brown M et al. Integrative genomic analyses reveal clinically relevant long noncoding RNAs in human cancer. Nat Struct Mol Biol 2013; 20: 908–913.
Crea F, Watahiki A, Quagliata L, Xue H, Pikor L, Parolia A et al. Identification of a long non-coding RNA as a novel biomarker and potential therapeutic target for metastatic prostate cancer. Oncotarget 2014; 5: 764–774.
Yang L, Lin C, Jin C, Yang JC, Tanasa B, Li W et al. lncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature 2013; 500: 598–602.
Parolia A, Crea F, Xue H, Wang Y, Mo F, Ramnarine VR et al. The long non-coding RNA PCGEM1 is regulated by androgen receptor activity in vivo. Mol Cancer 2015; 14: 46.
Prensner JR, Sahu A, Iyer MK, Malik R, Chandler B, Asangani IA et al. The IncRNAs PCGEM1 and PRNCR1 are not implicated in castration resistant prostate cancer. Oncotarget 2014; 5: 1434–1438.
Hung CL, Wang LY, Yu YL, Chen HW, Srivastava S, Petrovics G et al. A long noncoding RNA connects c-Myc to tumor metabolism. Proc Natl Acad Sci USA 2014; 111: 18697–18702.
He JH, Zhang JZ, Han ZP, Wang L, Lv YB, Li YG . Reciprocal regulation of PCGEM1 and miR-145 promote proliferation of LNCaP prostate cancer cells. J Exp Clin Cancer Res 2014; 33: 72.
Prensner JR, Iyer MK, Sahu A, Asangani IA, Cao Q, Patel L et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat Genet 2013; 45: 1392–1398.
Prensner JR, Zhao S, Erho N, Schipper M, Iyer MK, Dhanasekaran SM et al. RNA biomarkers associated with metastatic progression in prostate cancer: a multi-institutional high-throughput analysis of SChLAP1. Lancet Oncol 2014; 15: 1469–1480.
Lee B, Mazar J, Aftab MN, Qi F, Shelley J, Li JL et al. Long noncoding RNAs as putative biomarkers for prostate cancer detection. J Mol Diagn 2014; 16: 615–626.
Ren S, Liu Y, Xu W, Sun Y, Lu J, Wang F et al. Long noncoding RNA MALAT-1 is a new potential therapeutic target for castration resistant prostate cancer. J Urol 2013; 190: 2278–2287.
Wang F, Ren S, Chen R, Lu J, Shi X, Zhu Y et al. Development and prospective multicenter evaluation of the long noncoding RNA MALAT-1 as a diagnostic urinary biomarker for prostate cancer. Oncotarget 2014; 5: 11091–11102.
Ho TT, Zhou N, Huang J, Koirala P, Xu M, Fung R et al. Targeting non-coding RNAs with the CRISPR/Cas9 system in human cell lines. Nucleic Acids Res 2015; 43: e17.
Shechner DM, Hacisuleyman E, Younger ST, Rinn JL . Multiplexable, locus-specific targeting of long RNAs with CRISPR-Display. Nat Methods 2015; 12: 664–670.
Workman P, Al-Lazikani B . Drugging cancer genomes. Nat Rev Drug Discov 2013; 12: 889–890.
Vlaeminck-Guillem V, Ruffion A, Andre J, Devonec M, Paparel P . Urinary prostate cancer 3 test: toward the age of reason? Urology 2010; 75: 447–453.
Lee GL, Dobi A, Srivastava S . Prostate cancer: diagnostic performance of the PCA3 urine test. Nature Rev Urol 2011; 8: 123–124.
Kotake Y, Nakagawa T, Kitagawa K, Suzuki S, Liu N, Kitagawa M et al. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene 2011; 30: 1956–1962.
Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell 2010; 39: 925–938.
Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol 2011; 29: 742–749.
Chung S, Nakagawa H, Uemura M, Piao L, Ashikawa K, Hosono N et al. Association of a novel long non-coding RNA in 8q24 with prostate cancer susceptibility. Cancer Sci 2011; 102: 245–252.
Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP . A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 2010; 465: 1033–1038.
Mazar J, Zhao W, Khalil AM, Lee B, Shelley J, Govindarajan SS et al. The functional characterization of long noncoding RNA SPRY4-IT1 in human melanoma cells. Oncotarget 2014; 5: 8959–8969.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mouraviev, V., Lee, B., Patel, V. et al. Clinical prospects of long noncoding RNAs as novel biomarkers and therapeutic targets in prostate cancer. Prostate Cancer Prostatic Dis 19, 14–20 (2016). https://doi.org/10.1038/pcan.2015.48
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2015.48
This article is cited by
-
LncRNA weighted gene co-expression network analysis reveals novel biomarkers related to prostate cancer metastasis
BMC Medical Genomics (2022)
-
LncRNA EBLN3P promotes the progression of osteosarcoma through modifying the miR-224-5p/Rab10 signaling axis
Scientific Reports (2021)
-
Survival analysis of immune-related lncRNA in low-grade glioma
BMC Cancer (2019)
-
Long Non-coding RNA Expression in Anaplastic Thyroid Carcinomas
Endocrine Pathology (2019)
-
Nc886 is epigenetically repressed in prostate cancer and acts as a tumor suppressor through the inhibition of cell growth
BMC Cancer (2018)