Abstract
Transition from fetal to postnatal life requires significant changes in cardiac, pulmonary, and hepatic blood flow. As such, there must be changes in vascular control in these vascular systems. Vascular resistance, a major contributor to blood flow, is mediated in the ductus arteriosus and pulmonary vasculature by endothelial nitric oxide synthase (eNOS). This study was conducted to determine the ontogeny of hepatic eNOS expression and activity. Additionally, the expression and activity of inducible nitric oxide synthase (iNOS) was measured to determine whether perinatal hypoxia resulted in detectable levels. NOS mRNA and proteins were determined by reverse transcription PCR assay and semiquantitative Western blot analysis, respectively. NOS activity was measured by the formation of [14C]-citrulline from [14C]-arginine. Localization of eNOS within the liver was determined by immunohistochemistry. eNOS mRNA was detectable at low levels at 18-d gestation and increased after birth, reaching a maximum level (4.5-fold increase) at 20 d of life. Parallel patterns for eNOS protein and activity were seen, with 6.9-fold and 16.1-fold increases, respectively. In the prenatal rat, eNOS was localized to areas of extramedullary hematopoiesis, with little signal in the sinusoids. Postnatally, there was a decrease in staining in the hematopoietic cells and a gradual increase in the staining of the endothelium of the sinusoids and central veins. iNOS mRNA and protein could not be detected at any age. eNOS expression and activity are developmentally regulated, increasing after birth coincident with an initial relative hypoxia and an increase in shear forces upon closure of the ductus venosus.
Similar content being viewed by others
Main
NO is a short-lived free radical that influences physiologic processes in essentially every organ and tissue. Some of the roles of NO include neuromodulator, prevention of clotting, mediation of bactericidal and tumoricidal activity of macrophages, and regulation of blood pressure. NO is enzymatically synthesized from l-arginine by three known NOS isoforms: constitutively expressed eNOS (or NOS-3), nNOS (or NOS-1), iNOS (or NOS-2) (1–3). NO production by eNOS is primarily regulated by fluctuations in intracellular calcium levels. In the liver under normal conditions, only eNOS is present, produced by the sinusoidal endothelial cells (4, 5). The resulting low level of NO production is a major contributor of the basal vascular tone in the normal liver (4, 5).
eNOS plays a role in control of vascular tone in most vascular beds (6). eNOS participates in the control of vascular tone during the transition from fetal circulation to postnatal circulation in the ductus arteriosus (7) and lung (8). The role of eNOS in control of the ductus arteriosus is age dependent. The effects of eNOS are more pronounced in determining vascular tone of the ductus arteriosus in the preterm animal than in the near-term or adult animal (7, 9). During the transition period, blood flow to the lungs is increased by a number of factors, including closure of the ductus arteriosus and dilation of the pulmonary vasculature. The latter is under the control of eNOS. Expression of eNOS in the lung is highest at the time of birth, possibly serving to optimize the capacity for pulmonary vasodilation during transition (5, 8). Hepatic blood flow also changes significantly postnatally. Fetal hepatic blood flow derives from the umbilical vein (75%), portal vein (15–20%), and hepatic artery (5–10%), with 50% of the umbilical vein blood flow bypassing the liver via the ductus venosus. After birth, the portal vein (67–75%) and the hepatic artery (25– 33%) supply hepatic blood flow. In the immediate postnatal period, the percentage oxygen saturation (%O2 sat) of the hepatic artery increases, however, hepatic artery flow does not increase. In contrast, portal vein flow increases 3-fold after birth, but up to 40% continues to be shunted through the ductus venosus (10, 11). Complete closure of the ductus venosus may not occur for several days. Thus, in the immediate postnatal period there is a decrease in oxygen delivery to the liver. As the ductus venosus closes, there is increased oxygen delivery to the liver, as well as increased shear forces in the hepatic sinusoids. Previous research has shown that expression of eNOS mRNA in the liver is up-regulated both by hypoxia (12) and increased shear forces (5).
To date, the ontogeny of hepatic eNOS has not been studied. We propose that there are age-related differences in the expression of hepatic eNOS in the developing rat, reflecting changes in oxygen delivery and shear forces as they pertain to transition from fetal to postnatal circulation. The aim of this study was to determine the pattern of the eNOS expression and activity in the liver of the developing rat.
MATERIAL AND METHODS
Chemicals.
Unless otherwise stated, all chemicals used in this study were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). l-[14C]-arginine (0.1 mCi/mL) was obtained from American Radiolabeled Chemicals (St. Louis, MO, U.S.A.). α-[32P]-dATP (10 mCi/mL, 3000 Ci/mmol) was purchased from Amersham Health (Arlington Heights, IL, U.S.A.). Oligonucleotide primers for detection of individual NOS isoform mRNA were synthesized by DNA International, Inc. (Lake Oswego, OR, U.S.A.).
Animal care and collection of tissue.
Pregnant Sprague Dawley rats were obtained at 14 d of gestation (term = 22 d) and kept in plastic cages with free access to food and water with alternating 12-h periods of light and darkness. In experiments requiring fetal tissue, the dam was killed by CO2 narcosis and cervical dislocation, followed by delivery of fetuses by cesarean section. In experiments requiring neonatal tissue, pups were kept with the mother with free access to breast-feeding before weaning, and standard rat chow and water post weaning. At selected ages, pups were killed by CO2 narcosis and cervical dislocation. The time points were gestational ages of 18 d and 20 d, and postnatal ages of day of birth, 1 d, 5 d and 20 d. Adult, male Sprague Dawley rats (200–250 g) were used as the time point for adults, in an effort to avoid any potential effect of estrous on expression of NOS. The care and sacrifice of these animals was as described for the pregnant rats. The study was approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center.
Measurement of eNOS and iNOS mRNA.
Total RNA was extracted from liver using TRIzol Reagent (Invitrogen, Carlsbad, CA, U.S.A.), and 1.5 μg of total RNA was reverse transcribed into cDNA using Reverse Transcription System (Promega, Madison, WI, U.S.A.), using random hexamer as primers. The reaction was carried out at room temperature for 10 min and at 42°C for 60 min, followed by termination at 95°C for 5 min and ice for 5 min. The resulting cDNA was stored at −70°C until the time of amplification.
Specific cDNA were amplified by PCR in the presence of Taq DNA polymerase and oligonucleotide primer pairs designed for targeting eNOS and iNOS cDNA. α-[32P]-dATP was used for labeling the amplified cDNA. The reaction was performed in a final volume of 50 μL, consisting of 50 mM KCL, 10 mM Tris-Cl, 1.5 mM MgCl2, 0.2 mM dNTP, 1 μCi α-[32P]-dATP, 1 unit Taq DNA polymerase, 0.5 μM each primer, and 2 μL cDNA. The PCR reaction condition was 3 min at 94°C, 29 cycles at 94°C for 30 s, 55°C for 40 s, 72°C for 1 min, and then 72°C for 10 min, followed by 4°C.
The sequences of the primer pairs for eNOS and iNOS are as follows (13, 14):
-
eNOS:
-
5′-TCC AGT AAC ACA GAC AGT GAC-3′ (sense)
-
5′-CAG GAA GTA AGT GAG AGC-3′ (anti-sense)
-
iNOS:
-
5′-CTC ACT GGG ACT GCA CAG AA-3′ (sense)
-
5′-TGT TGA AGG GTG TCG TGA AA-3′ (anti-sense)
The size of the amplified DNA fragments for eNOS and iNOS are 693 bp and 127 bp, respectively.
PCR products were subjected to a 5% PAGE. To insure that equal amounts of reverse-transcribed cDNA were applied to the PCR reaction, the primer pairs of β-actin (CLONTECH, Palo Alto, CA, U.S.A.) were included in the PCR reaction as a reference (764 bp). The gels were first stained with ethidium bromide, photographed, and then exposed to Kodak BIOMAX MS film. A 100-bp DNA ladder marker was used to identity the molecular weight of the targeted DNA. The film was scanned and analyzed with a densitometer and the ImageQuant software (Molecular Dynamics, Sunnyvale, CA, U.S.A.). The ratio of the intensity of the eNOS band to that of β-actin was used to semiquantitatively compare NOS expression between groups (15). To insure that the reverse transcription (RT)-PCR reaction was not run to saturation, PCR was performed over a range of cycles for both eNOS and β-actin. The RT-PCR reaction was performed at 29 cycles, which was in the linear region of the cycles.
Western blotting for eNOS and iNOS.
NOS protein level was determined by Western blot analysis. Supernatant samples collected from the NOS activity assay were boiled with loading buffer for 5 min and separated by 7.5% SDS-PAGE, then transferred to nitrocellulose membranes using a Bio-Rad Electroblotter (Bio-Rad, Hercules, CA, U.S.A.). Monoclonal mouse anti-eNOS antibody (1:1000) and anti-iNOS (1:500) (Transduction Laboratories, Lexington, KY, U.S.A.) were used to detect NOS isoforms in the liver using the Enhanced Chemiluminescence System (Amersham Health). For semiquantitative comparison of the different age groups, samples were compared with β-tubulin. Monoclonal anti-β-tubulin antibody 3F3-G2 was a kind gift provided by Dr. J.L. Lessard, University of Cincinnati. Densitometric quantification of Western blot signal intensity of autoradiograms was performed using Molecular Analyst Software (Bio-Rad). The molecular weights for eNOS, iNOS, and β-tubulin are 140 kD, 130 kD, and 110 kD, respectively.
Measurement of eNOS and iNOS activity.
NOS activity, estimated by formation of [14C]-citrulline from [14C]-arginine, was determined according to the method of Bredt and Snyder (16), with modifications. Briefly, liver was homogenized in a 1:3 weight:volume ice-cold lysis buffer containing 50 mM HEPES (pH 7.4), 1 mM EDTA, 0.1 mg/mL aprotinin, 0.1 mg/mL leupeptin, 0.1 mM pepstatin A, 1 mM phenanthroline, and 1 mM PMSF. The homogenate was centrifuged at 20,000 ×g for 30 min at 4°C and NOS activity was measured in the supernatant within 2 h of preparation. Twenty-five microliters of supernatant, 200 μL of reaction buffer (50 mM Tris-Cl, pH 7.4, 1 mM citrulline, 50 mM valine, 10 μM FAD, 1 mM NADPH, 100 μM de-gassed BH4, 1 mM CaCl2, and 300 nM calmodulin), and 25 μL of [14C]-arginine (0.01 μCi) in 180 μM nonradiolabeled l-arginine were mixed and incubated for 30 min at 37°C. The reaction was terminated by the addition of ice-cold stop buffer containing 20 mM HEPES and 2 mM EDTA, pH 5.5. Incubation was also performed with 4 mM EGTA instead of CaCl2 and calmodulin for Ca2+-independent NOS activity. Duplicate samples were run in the presence and absence of 1 mM NG-nitro-l-arginine methyl ester (l-NAME) for all samples to assure that the measured radioactivity represented NOS activity. [14C]-citrulline in each sample was separated from the mixture by ion-exchange chromatography using Dowex 50W/50X-400 (sodium form) column. The eluent was collected and mixed with 10 mL of scintillation cocktail for radioactivity quantitation by liquid scintillation spectrometry. The results were expressed as picomoles citrulline per microgram protein per minute. The protein content of each sample was determined by the Bradford method with BSA as the standard.
Immunohistochemistry for hepatic eNOS..
Sections of liver tissue were immunostained with eNOS antibody using an ABC kit from Vector Laboratories (Burlingame, CA, U.S.A.) according to the manufacturer's recommendations. Briefly, 3-μL thick slices of formalin-fixed, paraffin-embedded liver were mounted on slides. The slides were deparaffinized, followed by suppression of endogenous peroxidase activity by immersion in methanol containing 2% H2O2 for 30 min. Antigen retrieval was achieved by heating in 0.01 M citric acid, pH 6.0, for 20 min in a Handy Steamer (Black & Decker, Model HS 800). Nonspecific binding was blocked with 10% horse serum in PBS at room temperature for 1 h. The sections were washed in PBS with 0.05% Tween-20 thrice for 2 min each time, followed by incubation overnight at 4°C with mouse anti-eNOS antibody (1:50) in PBS containing 4% horse serum. The sections were washed in PBS twice for 2 min each time, followed by incubation for 1 h at room temperature with biotinylated goat anti-mouse IgG (1:200) in PBS containing 1.5% horse serum. Next, the slides were incubated with avidin-biotin-peroxidase conjugate for 30 min at room temperature; the colored reaction product was developed by incubation for 7 min with 0.05% diaminobenzidine in 0.01% H2O2 in PBS. The slides were counterstained with hematoxylin. Negative controls were carried out under the same conditions using mouse IgG instead of eNOS antibody.
Statistical methods.
We used a repeated measures ANOVA to examine differences in the means for the seven time intervals. Because of suspected heterogeneity of the variances, we transformed the data to a natural logarithm scale to perform the ANOVA. A global test aimed at assessing significant differences in the responses over time was considered significant if p < 0.05. After a significant global test, pair-wise differences were assessed using a Bonferroni approach. In addition, a test for linear trend was reported as an indication of general increase in response over time.
RESULTS
Expression of eNOS mRNA.
We first performed a RT-PCR reaction for eNOS, iNOS, and β-actin at increasing numbers of cycles to insure that the reaction had not reached saturation. PCR products were seen for eNOS and β-actin, however, no PCR product was seen for iNOS up to 32 cycles. We chose 29 cycles for all future RT-PCR reactions. nNOS was not assessed in this study, as previous studies have failed to show its presence in the liver (4, 5).
Maturational change in steady-state expression of eNOS RNA as determined by semiquantitative RT-PCR is shown in Figure 1A. A representative gel (Fig. 1B) shows a single PCR product for eNOS with the predicted size of 693 bp. Similarly, a single PCR product for β-actin was seen at the predicted size of 764 bp; this band was used to determine equal amplification.
Developmental expression of eNOS RNA. (A) Semiquantitative densitometry of eNOS RNA was performed and standardized to β-actin. Values are depicted as means ± SE expressed as a percentage of eNOS at 18 d gestation; n = 5–6 for each time point; *p < 0.05 compared with G18, G20, d 0, and d 1. (B) Representative gel of RT-PCR products. β-actin was used to insure equal amplification. A single band was seen for each PCR product.
Semiquantitation using densitometry showed that eNOS mRNA was present at low levels before delivery (gestational ages 18 and 20 d) and on the day of birth. On d 1, eNOS mRNA was increased over preterm levels, however, it did not reach statistical significance. On d 5, there was a further increase in eNOS mRNA, which did reach statistical significance. Maximal expression of eNOS mRNA is seen on d 20; a 4.5-fold increase compared with 18 d gestational age. The mRNA level remained relatively constant in the adult animal. Linear trend analysis revealed a significant (p < 0.001) increase in hepatic eNOS mRNA expression from preterm to adult.
eNOS protein expression.
Maturational changes in eNOS protein expression in liver from fetal, neonatal, and adult rats are depicted in Figure 2A. The pattern of protein expression paralleled that of mRNA expression for eNOS. A representative Western blot is shown (Fig. 2B). Semiquantitative densitometry confirmed that eNOS protein is minimally expressed in the prenatal period and on the day of birth. On d 1 eNOS protein is significantly increased compared with earlier time points. There is a trend toward further increase on d 5, however it does not reach statistical significance. Maximal expression of eNOS protein is seen on d 20 (6.9-fold increase compared with 18 d gestational age); the protein level remains relatively constant in the adult animal. Linear trend analysis revealed a significant (p < 0.001) increase in hepatic eNOS protein concentration from preterm to adult.
Developmental expression of eNOS and iNOS protein. (A) Semiquantitative densitometry of eNOS protein was performed and standardized to tubulin. Values are depicted as means ± SE expressed as a percentage of eNOS at 18 d gestation; n = 5–6 for each time point; *p < 0.05 compared with G18, G20, and d 0; #p < 0.05 compared with d 1 and 5. (B) Representative Western blot. The last lane contains purified eNOS (0.9 μg) and iNOS (8 μg) as positive controls. For detection of eNOS, 150 μg of protein was loaded; for detection of iNOS, 300 μg of protein was added. Tubulin was used as a marker for equal protein loading.
eNOS activity.
The ontogeny of eNOS activity is depicted in Figure 3. The pattern of eNOS activity was similar to that of mRNA and protein expression. Activity was present in low levels before birth. On the day of birth there is a significant increase in eNOS activity. Another significant increase in activity is seen on d 5, which represents the time after closure of the ductus venosus. Maximal activity is seen on d 20 (16.1-fold increase compared with 18 d gestational age), which persisted unchanged in the adult. Linear trend analysis revealed a significant (p < 0.001) increase in hepatic eNOS activity from preterm to adult.
Expression of iNOS mRNA and protein.
There was no identifiable iNOS mRNA in the liver at any age. Similarly, there was no detectable iNOS protein at any age. A representative Western blot is shown in Figure 2B.
Localization of eNOS by immunohistochemistry.
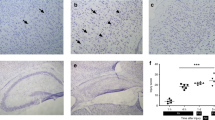
Before birth there was marked staining for eNOS in areas of extramedullary hematopoiesis (Fig. 4B). At 18 d gestation there was no staining of endothelial cells, however, at 20 d gestation there was a hint of staining of central veins. After birth there was a gradual decrease in staining of extramedullary hematopoiesis and an increase in staining of the endothelium. On the day of birth staining was obvious in the central veins, with scattered staining of the sinusoids; there was no discernable change on d 1 (Fig. 4B). On d 5, staining was more pronounced in the central veins and portal vessels, with more diffuse staining of the sinusoids (Fig. 4B). On d 20, there was an increase in staining with nearly complete staining of the sinusoids (Fig. 4B).
Immunohistochemistry of eNOS in the liver. Immunohistochemistry was performed on liver from various age rats to coincide with ages for determination of eNOS RNA, protein, and enzyme activity. For each time point one slide was stained with hemotoxylin and eosin (series A), one was stained for eNOS (series B), and one was a negative control for eNOS (series C). The number denotes the age as follows: 1 = G18, 2 = G20, 3 = d 0, 4 = d 1, 5 = d 5, 6 = d 20.
DISCUSSION
The present study determined the ontogeny of eNOS expression and activity in the rat liver. Increases in hepatic eNOS mRNA, protein level, and activity were seen from fetal to adult ages, with corresponding increase in immunostaining for eNOS in the sinusoids and central veins. eNOS was present before birth, albeit in low levels. After birth there was an increase in eNOS expression and activity, which reached a maximum at d 20 and persisted in the adult. Heretofore, developmental changes in hepatic eNOS have not been studied, whereas there have been many studies addressing the changes in hepatic blood flow during the transition from fetal to neonatal circulation. Findings of the present study can be understood in relation to known changes in blood flow and oxygen delivery during the transition period.
Developmental changes in eNOS have been reported for other organs where blood flow changes significantly during the transition from fetal to extrauterine life. In point, the ductus arteriosus is regulated in part by eNOS. In oxygen tension mimicking that of the fetus, the ductus arteriosus can respond to exogenous NO despite being unable to produce it in vitro(7). However, once exposed to the elevated neonatal oxygen tension, the ductus arteriosus can produce NO (7). The vasodilatory effect of NO on the ductus arteriosus is more prominent in preterm animals than in the near-term animals, where prostaglandin E is the major vasodilator (7, 9).
In the developing lung, there is an increase in eNOS mRNA and protein in late gestation, reaching a maximal level at 20 d gestation, followed by a decrease in expression following birth (8). It has been proposed that maximizing pulmonary eNOS expression at birth may serve to optimize the capacity for NO-mediated pulmonary vasodilation during transition (17).
Similarly, there are major changes in hepatic blood flow with birth. In utero the liver is supplied by the hepatic artery, portal vein, and umbilical vein, the latter bringing oxygenated blood to the fetus. The ductus venosus serves to shunt this oxygenated blood to the heart, brain, head, and upper body. There is preferential shunting of blood from the ductus venosus to the left side of the heart through the foramen ovale. On average, about 50% of the umbilical vein blood is shunted through the ductus venosus (18); the percentage increases with fetal hypoxia (19). Local control of blood flow to the fetal liver may not be so crucial, as the placenta plays an important role in the metabolic homeostasis of the fetus. The fetus receives a continuous infusion of nutrients via placental blood flow that is high in carbohydrates and amino acids. In fact, hepatic gluconeogenesis does not occur at significant rates in the fetus, rather fetal glucose use during intrauterine life is about equal to umbilical vein glucose uptake (20). Similarly, in utero most amino acids are transported to the fetus by the placenta. At delivery the fetus becomes biochemically self-sufficient. As such, the liver must be able to perform all aspects of protein production, metabolic homeostasis, and detoxification, making control of hepatic blood flow important after birth.
As previously mentioned, eNOS alone is expressed in the normal liver (12). Localization of eNOS is predominantly in the hepatic sinusoidal epithelium, primarily in the Golgi region of the cell (5). eNOS is important in regulating the basal vascular tone of the liver; expression of hepatic eNOS is influenced by hypoxia and shear forces (4, 5).
Our data show a 1.5-fold increase in both eNOS RNA and protein from gestational age 18 d to gestational age 20 d, without any significant change in eNOS activity. On the day of birth there was no significant increase in either eNOS RNA or protein levels compared with preterm levels, but a 6-fold increase in eNOS activity. There are several possible explanations for the discrepancy between levels of RNA and protein and activity. It may be that, although the 1.5-fold increases in RNA and protein levels were not statistically significant, they were biologically significant and accounted for the subsequent rise in eNOS activity.
Alternatively, there may be another factor that plays a role in the expression of eNOS activity at the time of delivery. A potential candidate is tetrahydrobiopterin (BH4), an essential cofactor for all three forms of NOS (21). The principal action of BH4 in intact endothelial cells is to increase the enzymatic activity of eNOS by stabilization of the enzyme, as well as to modestly increase the enzyme level (22). In the rat, the rate-limiting step in the biosynthesis of BH4 is under the control of the enzyme guanosine triphosphate (GTP) cyclohydrolase-I (23). There is variable developmental expression of both hepatic BH4 and GTP cyclohydrolase-I. In fetal mice at 20 d gestation, GTP cyclohydrolase-I is undetectable and BH4 is only 34% of adult levels, the latter presumably due to transplacental uptake from the maternal circulation (24). After birth there is seen a rapid rise in GTP cyclohydrolase-I activity and a concomitant increase in BH4 activity (24); findings that have been confirmed in other studies (25). In the context of our data, a postnatal rise in GTP cyclohydrolase-I and BH4 could stabilize the eNOS that is present and account for the significant rise in eNOS activity in the absence of a simultaneous rise in RNA and protein levels. Further rises in eNOS activity with age could be explained by increases in eNOS production, as well as further increases in BH4 activity.
Finally, the eNOS present before birth may not be biologically active. Insight into this concept can be gleaned from the localization of eNOS by immunohistochemistry. Before birth, eNOS is localized to areas of extramedullary hematopoiesis, a finding not previously reported. The potential role of eNOS in extramedullary hematopoiesis is discussed below. After delivery, eNOS is found in the endothelium of the sinusoids and central veins, where it can exert vasoactive properties.
The presence of immunoreactive eNOS in extramedullary hematopoiesis is quite interesting and raises several possible explanations. The presence of immunostaining may simply represent recognition of a protein homologous to eNOS. Alternately, it may represent eNOS that is immunoreactive but not physiologically active. An attractive hypothesis is that eNOS elaborated by extramedullary hematopoiesis serves to produce NO to support angiogenesis. Evidence supports that hematopoietic cells and endothelial cells originate from a common precursor (26). Endothelial cells, under stimulation by vascular endothelial growth factor, have an increased expression of eNOS and an increase in NO release (27). NO plays a role in angiogenesis, where it serves as an endothelial “survival factor”—inhibiting apoptosis, enhancing proliferation and migration of endothelial cells, and suppressing angiostatin (28, 29).
During transition in the immediate postgestational period there is a decrease in oxygen delivery to the liver (30). We contend that this relative hypoxia results in a further increase in eNOS expression. On d 1, eNOS mRNA, protein, and activity are increased 1.6-fold, 1.7-fold, and 1.3-fold, respectively, compared with the day of birth (d 0). Previous studies have shown that hepatic eNOS mRNA increases 2.0- and 2.6-fold in acute hypoxia induced by inhalation of 9% oxygen and 0.1% carbon monoxide, respectively (12). The response to acute hypoxia is not limited to the liver; increases in eNOS mRNA are seen in the right ventricle, lungs, and kidney, suggesting that the eNOS gene could be regulated by hypoxia (12). Similarly, eNOS mRNA and protein levels have been shown to increase in response to chronic hypoxia in the lung (31, 32).
Increases in eNOS expression occurring after d 1 cannot be explained by hypoxia. However, the increased shear forces that accompany closure of the ductus venosus may directly lead to increased expression of eNOS. In a morphologic study in neonatal rats, the ductus venosus closed completely by d 2 of life (33). With closure of the ductus venosus, the entirety of portal vein flow passes into the hepatic sinusoids. Although the liver is a low-resistance organ, there are increased shear forces associated with flow through the sinusoids (34). There is direct evidence that increased shear forces enhance eNOS activity (5, 35, 36). The resulting increase in NO production from high shear forces can lead to vasodilation and subsequent decrease in resistance. In our study, maximal eNOS expression occurs after closure of the ductus venosus. There was a slight further increase on d 20, with no further change in the adult rat.
The lack of iNOS expression is in keeping with previous studies that failed to show the presence of iNOS in normal liver (32). However, we anticipated that iNOS would be detectable immediately after birth due to the relative hepatic hypoxia seen during this time period in accordance with previous studies have shown that ischemia-reperfusion leads to an increase in expression of hepatic iNOS (37). Absence of detectable iNOS expression immediately after birth may be explained by a relatively minor hypoxia that may result in a lesser stimulus than true ischemia reperfusion. Alternatively, our measures for iNOS mRNA, protein, and activity may not have been sensitive enough to allow detection.
We have shown that there is a developmental expression of eNOS in the liver, in keeping with similar findings in the vasculature of the heart and lungs. These results add to the understanding of physiologic changes in hepatic blood flow during the transition from fetal to extrauterine life. These data can serve as a baseline for further research to evaluate the effect various prenatal stressors (such as maternal hypertension, placental insufficiency, and hypoxia) on eNOS expression and hepatic blood flow in the fetal and neonatal rat.
Abbreviations
- NO:
-
nitric oxide
- NOS:
-
nitric oxide synthase
- eNOS:
-
endothelial nitric oxide synthase
- iNOS:
-
inducible nitric oxide synthase
- nNOS:
-
neuronal nitric oxide synthase
REFERENCES
Geller DA, Lowenstein CJ, Shapiro RA, Nussler AK, Di Silvio M, Wang SC, Nakayama DK, Simmons RL, Snyder SH, Billiar TR 1993 Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci U S A 90: 3491–3495
Helyar L, Bundschuh DS, Laskin JD, Laskin DL 1994 Induction of hepatic Ito cell nitric oxide production after acute endotoxemia. Hepatology 20: 1509–1515
Rockey DC, Chung JJ 1996 Regulation of inducible nitric oxide synthase in hepatic sinusoidal endothelial cells. Am J Physiol 271: G260–G267
Mittal MK, Gupta TK, Lee FY, Sieber CC, Groszmann RJ 1994 Nitric oxide modulates hepatic vascular tone in normal rat liver. Am J Physiol 267: G416–G422
Shah V, Haddad FG, Garcia-Cardena G, Frangos JA, Mennone A, Groszmann RJ, Sessa WC 1997 Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J Clin Invest 100: 2923–2930
Ignarro LJ 1989 Endothelium-derived nitric oxide: actions and properties. FASEB J 3: 31–36
Clyman RI, Waleh N, Black SM, Riemer K, Mauray F, Chen YQ 1998 Regulation of ductus arteriosus patency by nitric oxide in fetal lambs: the role of gestation, oxygen tension, and vasa vasorum. Pediatr Res 43: 633–644
North AJ, Star RA, Brannon TS, Ujiie K, Wells LB, Lowenstein CJ, Snyder SH, Shaul PW 1994 Nitric oxide synthase type I and type III gene expression are developmentally regulated in rat lung. Am J Physiol 266: L635–L641
Momma K, Toyono M 1999 The role of nitric oxide in dilating the fetal ductus arteriosus in rats. Pediatr Res 46: 311–315
Botti JJ, Edelstone DI, Caritis SN, Mueller-Heubach E 1982 Portal venous blood flow distribution to liver and ductus venosus in newborn lambs. J Obstet Gynecol 144: 303–308
Zink J, Van Petten GR 1980 Time course of closure of the ductus venosus in the newborn lamb. Pediatr Res 14: 1–3
Gess B, Schricker K, Pfeifer M, Kurtz A 1997 Acute hypoxia upregulates NOS gene expression in rats. Am J Physiol 273: R905–R910
Cai Z, Hutchins JB, Rhodes PG 1998 Intrauterine hypoxia-ischemia alters nitric oxide synthase expression and activity in fetal and neonatal rat brains. Dev Brain Res 109: 265–269
Cai Z, Xiao F, Lee B, Paul IA, Rhodes PG 1999 Prenatal hypoxia-ischemia alters expression and activity of nitric oxide synthase in the young rat brain and causes learning deficits. Brain Res Bull 49: 359–365
Miralles C, Busquets X, Santos C, Togores B, Hussain S, Rahman I, MacNee W, Agusti AG 2000 Regulation of iNOS expression and glutathione levels in rat liver by oxygen tension. FEBS Lett 476: 253–257
Bredt DS, Snyder SH 1990 Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A 7: 682–685
Shaul PW 1997 Ontogeny of nitric oxide in the pulmonary vasculature. Semin Perinatol 21: 381–392
Rudolph AM, Heymann MA 1970 Circulatory changes with growth in the fetal lamb. Circ Res 26: 289–299
Reuss ML, Rudolph AM 1980 Distribution and recirculation of umbilical and systemic venous blood flow in fetal lambs during hypoxia. J Dev Physiol 2: 71–84
Hay WW, Sparks JW, Wilkening RB, Battaglia FC, Meschia G 1984 Fetal glucose uptake and utilization as functions of maternal glucose concentration. Am J Physiol 246: E237–E242
Cosentino F, Luscher TF 1999 Tetrahydrobiopterin and endothelial nitric oxide synthase activity. Cardiovasc Res 43: 274–278
Cai S, Alp NJ, McDonald D, Smith I, Kay J, Canevari L, Heales S, Channon KM 2002 GTP cyclohydrolase I gene transfer augments intracellular tetrahydrobiopterin in human endothelial cells: effects on nitric oxide synthase activity, protein levels and dimerisation. Cardiovasc Res 55: 838–849
Blau N, Niederwieser A 1985 GTP-cyclohydrolases: a review. J Clin Chem Clin Biochem 23: 169–176
Hoshiga M, Hatakeyama K, Watanabe M, Shimada M, Kagamiyama H 1993 Autoradiographic distribution of [14C] tetrahydrobiopterin and its developmental change in mice. J Pharmacol Exp Ther 267: 971–978
Yoshida YI, Eda S, Masada M 2000 Alterations of tetrahydrobiopterin biosynthesis and pteridine levels in mouse tissues during growth and aging. Brain Dev 22: S45–S49
Wagner RC 1980 Endothelial cell embryology and growth. Adv Microcirc 9: 45–75
Hood JD, Meininger CJ, Ziche M, Granger HJ 1998 VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol 274( 3 Pt 2): H1054–H1058
Murohara T, Witzenbichler B, Spyridopoulos I, Asahara T, Ding B, Sullivan A, Losordo DW, Isner JM 1999 Role of endothelial nitric oxide synthase in endothelial cell migration. Arterioscler Thromb Vasc Biol 19: 1156–1161
Dimmeler S, Hermann C, Galle J, Zeiher AM 1999 Upregulation of superoxide dismutase and nitric oxide synthase mediates the apoptosis-suppressive effects of shear stress on endothelial cells. Arterioscler Thromb Vasc Biol 19: 656–664
Townsend SF, Rudolph CD, Rudolph AM 1989 Changes in ovine hepatic circulation and oxygen consumption at birth. Pediatr Res 25: 300–304
Le Cras TD, Xue C, Rengasamy A, Johns RA 1996 Chronic hypoxia upregulates endothelial and inducible NO synthase gene and protein expression in rat lung. Am J Physiol 270: L164–L170
Li D, Laubach VE, Johns RA 2001 Up-regulation of lung soluble guanylate cyclase during chronic hypoxia is prevented by deletion of eNOS. Am J Physiol 281: L369–L376
Momma K, Ito T, Ando M 1992 In situ morphology of the ductus venosus and related vessels in the fetal and neonatal rat. Pediatr Res 32: 386–389
Lipowsky HH, Kovalcheck S, Zweifach BW 1978 The distribution of blood rheological parameters in the microvasculature of cat mesentery. Circ Res 43: 738–749
Kuchan M, Jo H, Frangos J 1994 Role of G proteins in shear stress-mediated nitric oxide production by endothelial cells. Am J Physiol 267: C753–C758
Uematsu M, Ohara Y, Navas J, Nishida K, Murphy TJ, Alexander RW, Nerem RM, Harrison DG 1995 Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol 269: C1371–C1378
Hur GM, Ryu YS, Yun HY, Jeon BH, Kim YM, Seok JH, Lee JH 1999 Hepatic ischemia/reperfusion in rats induces iNOS gene transcription by activation of NF-κB. Biochem Biophys Res Commun 261: 917–922
Acknowledgements
The authors thank Michael LeBlanc, M.D., for his critical reading of the manuscript and his many helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nowicki, M., Shi, D., Cai, Z. et al. Developmental Expression of Endothelial Nitric Oxide Synthase (eNOS) in the Rat Liver. Pediatr Res 54, 732–738 (2003). https://doi.org/10.1203/01.PDR.0000086904.52847.2A
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000086904.52847.2A
This article is cited by
-
Changes in plasma and urinary nitrite after birth in premature infants at risk for necrotizing enterocolitis
Pediatric Research (2016)
-
Arginase I expression is upregulated by dietary restriction in the liver of mice as a function of age
Molecular and Cellular Biochemistry (2015)
-
Immunohistochemical approach reveals involvement of inducible nitric oxide synthase in rat late development
Journal of Molecular Histology (2009)