Abstract
The role of microorganisms in the gastrointestinal tract has undergone significant modification in the past few decades with new observations from clinical, epidemiologic, and basic science research. We now know that the perception of these gut microbes as pathogens or even as commensals is somewhat outdated. It is becoming increasingly clear that the gut microbiome plays an important role in a host of activities including digestion, protection from potentially pathogenic organisms, and the regulation and development of the host immune system. The complex interactions between microbes and host combined with recent clinical observations and epidemiologic trends may point to the convergence of two well-supported (though imperfect) hypotheses: the “hygiene hypothesis” and the “fetal programming hypothesis.” We are beginning to understand that exposure to microbes before conception, during gestation, and in the neonatal period have profound effects on the developing immune system. Recent observations from a variety of fields help support the expansion of the “fetal programming hypothesis” to a host-microbe corollary that microbe-host interactions at critical windows influence the future immune phenotype, the maintenance of immune health, and the development of immune-mediated disease.
Similar content being viewed by others
Main
The “hygiene hypothesis” was initially formed as an explanation for the observed rise in the incidence of acute appendicitis coinciding with improvements in sanitation during the industrialization of Western societies in the early 20th Century (1–3). Observations supporting the theory's relevance to atopic diseases were noted as early as the mid-1970s by Gerrard et al. (4), but the expansion of the hypothesis to its current and more popularized form is credited to Strachan (5), who noted that an increasing number of siblings in a household and specifically the number of older siblings, both markers of high microbial exposure, were protective against the development of hay fever. The hypothesis presently states that the recent increase in T-helper cell (Th) type 2-mediated diseases over the past half-century can be explained, at least in part, by decreased exposure to microbial antigens early in life through improved sanitation and the relative sterility of the modern world. Decreased antigenic exposure has adverse effects on the budding immune system and increases the likelihood of developing atopic disease (6).
The resident gut microbial flora and its constant and active communication with the gastrointestinal immune compartment seem to play an important role in immunologic programming and when abnormal or inappropriate can lead to the development of allergic diseases. It is also becoming more clear that intestinal microflora influence the development of both Th1- and Th2-mediated diseases through their effect on regulatory cells and pathways that, when intact, help prevent disease-associated aberrant immune responses (7). Gastrointestinal tract microbes also play an integral role in the development of Th1 and Th2 balance. Examples include mice that are maintained in germ-free conditions tend to have Th2 dominant immune responses. Sellon et al. (8) showed that IL-10 deficient mice raised in germ-free conditions do not develop the expected spontaneous Th1-mediated colitis while the same mice reared in specific pathogen-free bacteria do. Although the hygiene hypothesis by itself is an imperfect theory and cannot explain all of the recent increases in certain diseases, there is support for one of its primary predictions that host-microbe interactions that occur early in life have long-term effects on the development of disease across populations.
Barker et al. are credited with the “developmental origins of adult disease” or “fetal programming” hypothesis (9), which grew from epidemiologic observations linking LBW and prenatal under-nutrition to adult diseases such as coronary artery disease (10), type 2 diabetes (11), hypertension (12), and stroke. Interpreted more generally, the hypothesis suggests that events and exposures occurring in utero, at birth, and in early childhood can have profound and long-term effects on the adult phenotype. The roots of the hypothesis lie in developmental plasticity during gestation and early childhood, a process through which one genotype can lead to various phenotypes in response to environmental signals during critical periods of development (9). Although the original hypothesis specifically relates to fetal nutrition and growth, the concept of environmental conditions at critical windows programming the development of disease can and has been expanded well beyond the original observations into a more general “Barker hypothesis.” Maternal dietary factors (13–15) and maternal smoking (16) can modify neonatal immune responses and influence the development of immune-mediated disease. Figure 1 illustrates a range of maternal and environmental factors that may influence fetal immune development and are implicated in short- and long-term immune health. Considering the recent rise in certain immune-mediated disorders, of particular interest is the exposure of the fetus and neonate to microbes and the resultant effects, both deleterious and beneficial, on developmental immune programming. The mechanisms underlying the microbial influences on immune development are beginning to be elucidated with many studies focused on the intrauterine milieu and on epigenetic inheritance. Epigenetic dysregulation, in particular, has been shown to be an important factor in the development of many human diseases, and the role of gene-environment interactions in immune function and immune-mediated disease is just beginning to be established (17).
Maternal exposure to microbes, the sequential colonization of the gastrointestinal tract after birth, the influence on that colonization by birth conditions and exposures in the first few weeks of life, and the influence of prebiotics and probiotics all support the adaptation of the “fetal programming” hypothesis to a host-microbe corollary that specific (though not yet defined) interactions between the microbes and host during critical periods of immune development (including interactions between the fetus and the maternal immune system and microbiome during gestation) may have consequences well into adulthood.
Gestation
Placental mammals are faced with a difficult task during pregnancy. The maternal immune system must not only actively eliminate potentially harmful microbial stimuli but also be tolerant of the fetus and protect it from immune rejection. Maternal health during gestation has significant effects on the health of offspring and nutritional, toxic, genetic, metabolic, and infectious factors all contribute to the eventual newborn phenotype.
There is building evidence that maternal immune status and maternal exposure to microbes during pregnancy affects the development of the fetal immune system. Blümer et al. (18) showed in a murine model that maternal exposure to lipopolysaccharide during gestation resulted in less allergic sensitization and airway inflammation in their offspring. The most convincing evidence in humans comes from studies showing that offspring born to mothers living in farming environments (a marker for high maternal microbial exposure during gestation) are protected from the development of asthma (19) and have an up-regulation of receptors of the innate immune system (20). Human offspring of mothers exposed to pets during pregnancy have lower cord blood IgE levels at birth (21), which may be protective against the development of allergic disease. The mechanisms behind such protections are being investigated, and maternal Toll-like receptor (TLR) signaling has recently been shown to be an important participant (22). Maternal infections with parasites (23), viruses (24), and exposure to viral antigens (25) during gestation have also been recently shown to modulate the immune response in the offspring in both helpful and harmful ways. These data, along with observations from probiotic intervention studies (to be discussed in detail below), show that maternal microbial exposure during gestation may lead to long-term health consequences for the offspring by influencing fetal immune development.
The Maternal/Fetal Interface and In Utero Priming
The role of maternal dietary avoidance in the prevention of atopic diseases in offspring has been well studied and hotly debated over the past few decades as is evidenced by a recent change in recommendations from the American Academy of Pediatrics against routine avoidance of specific dietary antigens (26) largely based on results from a 2006 Cochrane review (27). Some studies have even shown that maternal dietary and allergen avoidance during pregnancy results in an increased risk of allergy (28,29). At the crux of the issue of maternal allergen avoidance is the ongoing debate over whether prenatal allergen exposure leads to in utero priming of the fetal immune system or not. Although not directly related to microbes, a review of the current literature on this topic yields important clues to the potential mechanisms behind the influence of microbes, as well as other environmental exposures, on the developing immune system. To date, there is evidence supporting both sides of the discussion.
In utero priming garners backing from studies showing an association between cord blood IgE levels and the development of atopic disease (30) and the presence of allergen-specific IgE in cord blood of neonates (31). However, the clinical relevance of cord blood IgE has been called into question as it does not seem to consistently correlate with or predict the development of disease (32). Others have shown that cord blood allergen-specific IgE is not of fetal origin but rather due to maternal contamination likely at the time of delivery (33), although one recent study claims to have controlled for maternal contamination (34). More support for fetal immune priming comes from studies showing allergen induced T-cell reactivity in cord blood (35,36), whereas other studies show a lack of a quantitative relationship between maternal allergen exposure and T-cell responses in their newborn offspring (37). The potential mechanism behind in utero priming could include transport of small, yet immunogenic allergen fragments (38) or IgG/allergen complexes across the placenta to prime naïve fetal T cells. Environmental allergens have been documented both in cord blood and amniotic fluid (39). Although it is well known that maternal IgG readily crosses the placenta, IgG/allergen complexes have also been shown to cross (40) and may exert fetal immune reactivity through binding with FcRn (neonatal Fc receptor) known to be distributed on placental cells and on human fetal intestine (41).
It is also now known that the role of maternal IgG itself is no longer limited to the passive protection of the fetus against harmful environmental exposures. These antibodies also provide the developing fetus with a wealth of maternal immunologic memory and experience. Maternal antibodies influence the selection of T- and B-Cell repertoires in the offspring that are conserved well into adulthood. They also aid in the suppression of IgE antibody responsiveness, which seems to be long lasting (42). The maternal-fetal unit is infinitely complex, and more study is needed, e.g. the impact of the cytokine milieu, other soluble immune factors, and microbes at the maternal/fetal interface. However, the framework for the mechanisms underlying the influence of microbes and other stimuli on the developing immune system exists.
Gastrointestinal Immunity
The intestine is now recognized as the largest and perhaps most influential immune organ in the body. It is charged with actively responding to potentially harmful pathogens and antigens, while creating and maintaining tolerance (systemic unresponsiveness) to other antigens and to potentially beneficial commensal and symbiotic bacteria.
The gastrointestinal mucosal barrier must be selective in what molecules and signals are allowed to transfer across it. However, the role of the intestinal epithelium goes far beyond just that of a physical barrier. Mucosal barrier components such as transmembrane TLRs and cytoplasmic Nucleotide-binding oligomerization domains (NOD) receptors are members of the innate immune system that act as microbial pattern recognition receptors (43). These families of receptors bind novel ligands encountered in the gut lumen and they have an important role in the interaction of luminal microbes with host-immune defense, immune cell recruitment, and mucosal inflammation (Fig. 2). The importance of these families of receptors in inflammation and tissue homeostasis is most famously exemplified by the association of certain NOD2 gene mutations with Crohn's Disease, a chronic inflammatory disorder (44), and by the development of more severe experimental colitis in various TLR knockout mice (45). NOD1 recognition of intestinal microbiota-derived peptidoglycan has been shown to enhance systemic innate immunity (46), illustrating the influence of intestinal microflora far beyond the gut. In addition, TLR recognition of specific commensal bacteria has been shown to be required for the maintenance of intestinal homeostasis (47), lending support to the symbiotic relationship between certain microbes and the host.
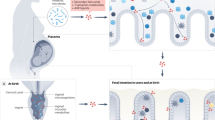
Microbe-driven T-cell differentiation: mucosal dendritic cells (DCs) sample bacterial antigens from the intestinal lumen via Toll-like receptors (TLRs) and are activated. Activated/mature DCs produce cytokines that activates naïve T cells (Th0) to mature into a balanced T-helper cell response [Th1, Th2, and regulatory T cell (Treg)]. MHC, major histocompatibility complex. Reprinted from Walker WA Functional Food Reviews 1:13–19. Copyright © 2009, BC Decker Inc, with permission.
The interaction of the microbes and immune cells of the gastrointestinal tract also effects the development and maturation of both the innate and adaptive immune system. Mazmanian et al. (48) showed that a bacterial polysaccharide (PSA) from Bacteroides fragilis, a specific colonizer of the gastrointestinal tract, has direct immunomodulatory activities including correction of systemic T-cell deficiencies and Th1/Th2 imbalance and has a role in immune maturation by directing lymphoid organogenesis. Hooper et al. (49) showed that angiogenin 4 (Ang4), a product of the commensal bacterium Bacteroides thetaiotaomicron, acts as a mediator of host defense in the intestine. Finally, it is becoming accepted that commensal microflora can induce an anti-inflammatory immune response through induction of regulatory T cells that help guide Th1 and Th2 balance (50). This is supported by the demonstration of impaired oral tolerance induction in germ-free mice which later regain their ability to be tolerized after reconstitution of their microflora with Bifidobacteria during infancy (51). Commensal gut microflora DNA (gfDNA) has also been shown to limit regulatory T-cell conversion through their interaction with TLR9 and thus play a role in intestinal homeostasis (52). These examples highlight the importance of reciprocal interactions between microbes and the host and the significance of this symbiosis with regard to human immune health.
Colonization in the Peripartum Period
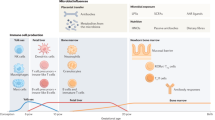
The adult human intestinal tract contains 1014 bacteria with a density of between 1011 and 1012 microbes/mL of luminal content (53). Each microbiome is unique and each is also fairly stable over time (54). By 1 year of age, the infant microbiome appears to be fairly similar to that of adults. However, during that first year, the infant intestinal microbiota is far more impressionable and far less stable. Initial intestinal colonization provides an enormous microbial stimulus to the host, which leads to profound changes in intestinal development and defense (Fig. 3). At the time of birth, the neonate passes from the relatively sterile conditions within the amnion into an environment filled with microbes. Exposure of the infant's mucosal surfaces to the mother's vaginal and fecal flora, in addition to other environmental exposures, is thought to begin the complex process of intestinal colonization. There is wide variability in colonization across individuals and over time, and initial colonization seems to be dependent on the bacterial population to which the infant is first exposed. Bacteria begin to appear in feces within a few hours of life. With vaginal delivery, facultative anaerobes such as Escherichia coli and Streptococcus dominate in the first few days of life, taking advantage of the abundance of oxygen (55). As oxygen begins to be deprived, the facultative bacteria are replaced by anaerobic bacteria such as Bacteroides, Clostridium, and Bifidobacterium, typically by 1–2 wk of age (56).
Cross section of a noncolonized human fetal small intestine (left) and the same cross section of a fully colonized infant (right). Note the contrast in active proliferation, epithelial maturity, and abundance of lymphoid elements. Reprinted from Walker WA Functional Food Reviews 1:13–19. Copyright © 2009, BC Decker Inc, with permission.
Neonatal intestinal colonization is influenced by numerous perinatal factors including mode of delivery, composition of early feeding (breast milk versus formula), and antibiotic use. More importantly, the resulting differences in early microflora composition may have lasting effects on immune function. For example, infants born via cesarean section are more frequently colonized with Klebsiella, Enterobacter, and Clostridium than those born vaginally (57) and less frequently colonized by Bifidobacterium and Bacteroides (58). These differences in colonization may help account for the increased risk of asthma (59), atopy, and allergic disease (60) in children born by cesarean section.
The next major event that impacts the pattern of microbial colonization and immune status is the initiation of feeding. Although results are somewhat less consistent, it does appear that breast-fed infants are colonized with less Escherichia coli, Clostridium difficile, and Bacteroides than formula-fed infants. Breastfeeding provides many immune-related benefits for the infant including short-term protection from gastrointestinal illness (61), respiratory illness and infections (62), and otitis media (63). These advantages have been attributed to a range of different components of human milk including immunoglobulins, antimicrobial peptides, growth factors, nutrients, lysozyme, lactoferrin, and complement (64). These effects may also be due, at least in part, to the observed differences in microbial colonization in the neonatal period.
Intestinal colonization is also influenced by the use of antibiotics early in life. In premature infants, the duration of antibiotic treatment in the first month of life correlates with decreased bacterial diversity (65), and certain antibiotic regimens have been shown to decrease anaerobic bacteria and result in an overgrowth of specific organisms such as Klebsiella (66) and Staphylococci (67). Term infants given antibiotics in the first month of life have decreased colonization with Bifidobacteria and Bacteroides fragilis (58). These differences in microbiota might contribute to the somewhat disputed clinical observation, supported by one meta-analysis (68), that antibiotic use in the newborn period may increase the risk of atopic disease later in life. Once again, although the mechanism is not clear and more study is needed, these examples show that microbe-host interactions during critical periods of development may have long-lasting effects on the future immune phenotype.
Bifidobacteria are one of the better studied intestinal colonizers. As previously mentioned, they are less abundant colonizers of neonates exposed to antibiotics and those born via cesarean section. Colonization with Bifidobacteria seems to play an important role in humoral immune development exemplified by its role in the maturation of the mucosal salivary secretory IgA system (69) and its association with more circulating IgA and IgM secreting cells (70). In addition, Bifidobacteria are generally found in lower numbers in the feces of infants who go on to develop atopy (71) and thus may have long-term effects on an immune phenotype. This effect is likely species-specific as allergic infants have been reported to be colonized with different Bifidobacterium species than their healthy counterparts (72).
Intestinal Colonization and Specific Disease States
Recent advances in culture-independent techniques to better identify gut microflora have led to a wealth of investigations into patterns of colonization in specific disease states and the potential role for gut microbiota in the etiology and course of immune-mediated diseases.
Necrotizing enterocolitis (NEC).
NEC is an inflammatory disease of the bowel that remains a devastating cause of neonatal morbidity and mortality. Although the etiology is likely multifactorial, the end result is a marked inflammatory response that often ends in intestinal tissue necrosis. Microbial colonization seems to be required for NEC pathogenesis. Recent studies have shown differences in gut microbiota between preterm infants with and without NEC both at the time of diagnosis (73) and before disease development (74), providing some evidence for the role of microbes in disease development. Both pathogenic and commensal bacteria (75) and inflammatory stimuli (76) induce exaggerated cytokine responses from fetal intestinal epithelial cells (IECs) compared with adult IECs, which may help explain the susceptibility of premature infants to NEC. The success of some probiotics in preventing NEC in preterm infants underscores the importance of microbe-host interactions in an immune-mediated disease (77).
Atopic diseases.
Perhaps nowhere has the role of gut microbes in immune-mediated disease been more studied than in regard to atopic diseases. These studies also provide the best evidence about the critical timing of host-microbiome interactions. We know that, in mice, intestinal bacteria are required for the development of oral tolerance and that these bacteria must be introduced to the gut during the neonatal period or the effect is lost (51). This again indicates that microbe-host interactions at critical windows of time can alter the future immune phenotype. There is good evidence supporting reduced bacterial diversity (78) and differences in colonization patterns in infants with atopic diseases compared with controls (79). Overall trends point most consistently to a protective effect of Bifidobacteria and an increased risk of atopic disease in those colonized with more Enterobacteriaceae, Clostridium, Bacteroides, and Staphylococcus (79). There may be different mechanisms underlying the associations because colonization with some microbes increases the risk of atopic eczema only while others seem to increase the risk of all atopic diseases (80). With differences in microbiota fairly well established between these groups, investigators have gone on to determine whether disease outcome can be affected by manipulating the microbiota with probiotics. Although probiotics seem to have little role in the treatment of established atopic dermatitis, they may help prevent the condition. Some early studies showed an impressive reduction in the development of atopic eczema at the age of 2 y in children receiving Lactobacillus rhamnosus strain GG (LGG) during the final 4 wk of gestation (via maternal consumption) and the first 6 mo of life (81). This prevention of eczema was confirmed at ages 4 and 7 within the same cohorts (82,83). However, other studies with LGG with slightly different experimental designs have failed to show a protective effect (84). The mechanism of maternal LGG administration in reducing atopic dermatitis in offspring is not entirely clear. Probiotics alter maternal microbiota, affecting the bacteria that the newborn infant is exposed to and thus, potentially, the neonate's initial intestinal colonization patterns. There is some data to support that probiotics may affect the developing immune system in utero (85). However, other studies have shown that fetal immune responses are not affected by LGG given during pregnancy (86), suggesting the effect may be due to postnatal exposure to LGG through breast milk or via direct administration to the developing infant. Numerous other probiotics, prebiotics, and pro- and prebiotics mixtures have been evaluated as well (87). A recent meta-analysis did support a preventative effect of certain probiotics on atopic eczema (88). Probiotics have not been shown to be effective in the prevention of asthma and allergic rhinitis in humans to date. Other observations may support the importance of the colonization of the gastrointestinal tract in allergic disease including differences in the bacterial composition of intestinal flora before the onset of atopy (80), the promotion of immunologic tolerance in the neonatal period by bacterial colonization (89), and the requirement of intestinal microbial flora in the neonatal period for the induction of oral tolerance (51).
Prebiotics and Probiotics
Probiotics are live microorganisms that are beneficial to the host beyond their nutritional value. They have been shown to have favorable immunologic effects that influence both systemic and gut-associated immune responses. Many aspects of the immune system have been suggested to be effected by probiotics, including alteration of tolerance induction (90), anti-inflammatory effects via TLRs (91), Th1 skewing, augmentation of Regulatory T-cell function (92), modulation of dendritic cell function (93), and increased mucosal IgA production. Not all probiotics are alike, and it is becoming clearer that probiotic combinations are likely to be more effective than monotherapy. Some probiotics have been used successfully in the prevention and/or treatment of childhood infectious gastroenteritis (94), antibiotic associated diarrhea (95), and inflammatory bowel disease (96). Probiotics may exert their greatest effects when given during the perinatal period and during early childhood. This is supported by some of the strongest data showing prevention of immune-mediated diseases such as atopic dermatitis (97) and NEC (98) in high-risk neonates. Mechanisms of action have been proposed (99,100), but more studies are needed to determine precise pathways and safety of use. These studies, along with research into stable, probiotic-secreted products may lead to more widespread use in appropriate clinical situations in the near future as the concept of giving live bacteria to high-risk infants is still quite controversial.
Prebiotics are indigestible food ingredients, normally complex carbohydrates that benefit the host by stimulating the growth and/or activity of certain intestinal bacteria like Bifidobacterium (101). Although well-controlled trials are limited at this time, there is some evidence that prebiotics alone or in combination with probiotics may be effective in preventing atopic dermatitis in high-risk populations (102,103). More trials are clearly needed, but the promising early results support the importance of microbe-host interactions at critical periods on the development and potentially prevention of human disease.
Conclusions
Microbes, among other environmental exposures, play an important role in developmental immune programming. Although the majority of this review focused on gastrointestinal tract microbiota, postnatal immune development can also be affected by exposure to nonenteric microbes, microbial products, and nonmicrobial antigens at the interface of multiple other immune compartments (e.g. respiratory and skin epithelium), which may prove to be equally important. These different mucosal surfaces all have continuous contact with the outside world and thus all contribute to the development of an immune response and/or immune tolerance. The gastrointestinal tract seems to play a particularly significant role in the development of the immune system and the mechanisms underlying its role are being explored. For example, the development of innate immune tolerance in the neonatal intestine has recently been shown to be dependent on microRNA-146a-mediated repression and degradation of the IL-1 receptor-associated kinase (IRAK1), a TLR signaling molecule (104). Continued detailed investigation of the development of intestinal immune homeostasis and the microbiome-host relationship during the neonatal period will provide invaluable information that may better illuminate and define the windows for therapeutic intervention in the future.
The timing of these “critical periods” is inherently important. Animal studies showing that preconception maternal immunization protects the offspring from allergic sensitization support the importance of the prenatal period (105). Human probiotic prevention studies would point to the last month of gestation and first few months of life as the critical periods of immune development (81). Differences in microflora and the risk for atopic disease between infants born vaginally and those born by caesarean section highlight the importance of the immediate postnatal period (60) as do studies implicating breast milk components in the induction of oral tolerance and prevention of asthma (106). There may indeed be multiple windows of opportunity, and more work is clearly needed to better define them to help guide future therapeutic interventions.
More research is also needed to further elucidate how the microbiome is maintained, the associations of specific microbial species and/or components with specific clinical diseases, and the complex mechanisms of immune tolerance. New avenues of research include microbial influences on epigenetic interactions in utero and their potential effect on developmental immune programming through alterations in fetal DNA through altered histone methylation, histone acetylation, and chromatin structure (107). For example, bacterial infections have already been shown to promote DNA hypermethylation (108). It will also be important to better define communications not only between the microbe and host but also between microbes themselves. Novel research has led to new therapeutic strategies that are actively being trialed. These include polymicrobial probiotic cocktails, prebiotics, and other immunostimulatory molecules such as DNA and helminthes. New technology will allow us to address the interactions of entire microbial communities and their role in immune development and function spanning from gestation through adulthood. Ultimately, these efforts will generate new models and interventions with the goal of promoting human health and preventing human disease.
Abbreviations
- LGG:
-
Lactobacillus rhamnosus strain GG
- NEC:
-
necrotizing enterocolitis
- Th:
-
T-helper cell
- TLR:
-
Toll-like receptor
REFERENCES
Barker DJ 1985 Acute appendicitis and dietary fibre: an alternative hypothesis. BMJ 290: 1125–1127
Barker DJ, Osmond C, Golding J, Wadsworth ME 1988 Acute appendicitis and bathrooms in three samples of British children. BMJ 296: 956–958
Barker DJ 1989 The rise and fall of Western diseases. Nature 338: 371–372
Gerrard JW, Geddes CA, Reggin PL, Gerrard CD, Horne S 1976 Serum IgE levels in white and metis communities in Saskatchewan. Ann Allergy 37: 91–100
Strachan DP 1989 Hay fever, hygiene, and household size. BMJ 299: 1259–1260
Bach JF 2002 The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347: 911–920
Guarner F, Bourdet-Sicard R, Brandtzaeg P, Gill HS, McGuirk P, van Eden W, Versalovic J, Weinstock JV, Rook GA 2006 Mechanisms of disease: the hygiene hypothesis revisited. Nat Clin Pract Gastroenterol Hepatol 3: 275–284
Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB 1998 Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10 deficient mice. Infect Immun 66: 5224–5231
Barker DJ 2004 The developmental origins of chronic adult disease. Acta Paediatr Suppl 93: 26–33
Frankel S, Elwood P, Sweetnam P, Yarnell J, Smith GD 1996 Birthweight, body mass index in middle age, and incident coronary heart disease. Lancet 348: 1478–1480
Forsén T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C, Barker D 2000 The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med 133: 176–182
Curhan GC, Chertow GM, Willett WC, Spiegelman D, Colditz GA, Manson JE, Speizer FE, Stampfer MJ 1996 Birth weight and adult hypertension and obesity in women. Circulation 94: 1310–1315
Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG, Prescott SL 2003 Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol 112: 1178–1184
Olsen SF, Østerdal ML, Salvig JD, Mortensen LM, Rytter D, Secher NJ, Henriksen TB 2008 Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr 88: 167–175
Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, Bailey N, Potts EN, Whitehead G, Brass DM, Schwartz DA 2008 In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest 118: 3462–3469
Noakes PS, Hale J, Thomas R, Lane C, Devadason SG, Prescott SL 2006 Maternal smoking is associated with impaired neonatal toll-like-receptor-mediated immune responses. Eur Respir J 28: 721–729
Waterland RA, Michels KB 2007 Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr 27: 363–388
Blümer N, Herz U, Wegmann M, Renz H 2005 Prenatal lipopolysaccharide exposure prevents allergic sensitization and airway inflammation, but not airway responsiveness in a murine model of experimental asthma. Clin Exp Allergy 35: 397–402
Douwes J, Cheng S, Travier N, Cohet C, Niesink A, McKenzie J, Cunningham C, Le Gros G, von Mutius E, Pearce N 2008 Farm exposure in utero may protect against asthma. Eur Respir J 32: 603–611
Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Ublagger E, Schram-Bijkerk D, Brunekreef B, van Hage M, Scheynius A, Pershagen G, Benz MR, Lauener R, von Mutius E, Braun-Fahrländer C, Parsifal Study team 2006 Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J Allergy Clin Immunol 117: 817–823
Aichbhaumik N, Zoratti EM, Strickler R, Wegienka G, Ownby DR, Havstad S, Johnson CC 2008 Prenatal exposure to household pets influences fetal immunoglobulin E production. Clin Exp Allergy 38: 1787–1794
Conrad ML, Ferstl R, Teich R, Brand S, Blümer N, Yildirim AO, Patrascan CC, Hanuszkiewicz A, Akira S, Wagner H, Holst O, von Mutius E, Pfefferle PI, Kirschning CJ, Garn H, Renz H 2009 Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J Exp Med 206: 2869–2877
Santos PA, Sales IR, Schirato GV, Costa VM, Albuquerque MC, Souza VM, Malagueño E 2010 Influence of maternal schistosomiasis on the immunity of adult offspring mice. Parasitol Res 107: 95–102
Hughes CH, Jones RC, Wright DE, Dobbs FF 1999 A retrospective study of the relationship between childhood asthma and respiratory infection during gestation. Clin Exp Allergy 29: 1378–1381
Rastogi D, Wang C, Mao X, Lendor C, Rothman PB, Miller RL 2007 Antigen-specific immune responses to influenza vaccine in utero. J Clin Invest 117: 1637–1646
Greer FR, Sicherer SH, Burks AW, American Academy of Pediatrics Committee on Nutrition, American Academy of Pediatrics Section on Allergy and Immunology 2008 Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics 121: 183–191
Kramer MS, Kakuma R 2006 Maternal dietary antigen avoidance during pregnancy and/or lactation for preventing or treating atopic disease in the child. Cochrane Database Syst Rev 3: CD000133
Fälth-Magnusson K, Kjellman NI 1992 Allergy prevention by maternal elimination diet during late pregnancy—a 5 year follow-up of a randomized study. J Allergy Clin Immunol 89: 709–713
Woodcock A, Lowe LA, Murray CS, Simpson BM, Pipis SD, Kissen P, Simpson A, Custovic A, NAC Manchester Asthma and Allergy Study Group 2004 Early life environmental control: effect on symptoms, sensitization, and lung function at age 3 years. Am J Respir Crit Care Med 170: 433–439
Edenharter G, Bergmann RL, Bergmann KE, Wahn V, Forster J, Zepp F, Wahn U 1998 Cord blood-IgE as risk factor and predictor for atopic diseases. Clin Exp Allergy 28: 671–678
Nambu M, Shintaku N, Ohta S 2003 Relationship between cord blood level of IgE specific for Dermatophagoides pteronyssinus and allergic manifestations in infancy. Biol Neonate 83: 102–106
Rowe J, Kusel M, Holt BJ, Suriyaarachchi D, Serralha M, Hollams E, Yerkovich ST, Subrata LS, Ladyman C, Sadowska A, Gillett J, Fisher E, Loh R, Soderstrom L, Ahlstedt S, Sly PD, Holt PG 2007 Prenatal versus postnatal sensitization to environmental allergens in a high-risk birth cohort. J Allergy Clin Immunol 119: 1164–1173
Bønnelykke K, Pipper CB, Bisgaard H 2008 Sensitization does not develop in utero. J Allergy Clin Immunol 121: 646–651
Pfefferle PI, Sel S, Ege MJ, Büchele G, Blümer N, Krauss-Etschmann S, Herzum I, Albers CE, Lauener RP, Roponen M, Hirvonen MR, Vuitton DA, Riedler J, Brunekreef B, Dalphin JC, Braun-Fahrländer C, Pekkanen J, von Mutius E, Renz H, PASTURE Study Group 2008 Cord blood allergen-specific IgE is associated with reduced IFN-gamma production by cord blood cells: the Protection against Allergy-Study in Rural Environments (PASTURE) Study. J Allergy Clin Immunol 122: 711–716
Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG 1999 Development of allergen-specific T-cell memory in atopic and normal children. Lancet 353: 196–200
Hagendorens MM, Ebo DG, Bridts CH, Van de Water L, De Clerck LS, Stevens WJ 2004 Prenatal exposure to house dust mite allergen (Der p 1), cord blood T cell phenotype and cytokine production and atopic dermatitis during the first year of life. Pediatr Allergy Immunol 15: 308–315
Platts-Mills TA, Blumenthal K, Perzanowski MS, Woodfolk JA 2000 Determinants of clinical allergic disease. The relevance of indoor allergens to the increase in asthma. Am J Respir Crit Care Med 162: S128–S133
Szépfalusi Z, Loibichler C, Pichler J, Reisenberger K, Ebner C, Urbanek R 2000 Direct evidence for transplacental allergen transfer. Pediatr Res 48: 404–407
Holloway JA, Warner JO, Vance GH, Diaper ND, Warner JA, Jones CA 2000 Detection of house-dust-mite allergen in amniotic fluid and umbilical-cord blood. Lancet 356: 1900–1902
Casas R, Jenmalm MC, Bjorksten B 2004 Cat allergen-induced cytokine secretion and Fel d 1-immunoglobulin G immune complexes in cord blood. Clin Exp Allergy 34: 591–596
Shah U, Dickinson BL, Blumberg RS, Simister NE, Lencer WI, Walker WA 2003 Distribution of the IgG Fc receptor, FcRn, in the human fetal intestine. Pediatr Res 53: 295–301
Lemke H, Tanasa RI, Trad A, Lange H 2009 Benefits and burden of the maternally-mediated immunological imprinting. Autoimmun Rev 8: 394–399
Sanderson IR, Walker WA 2007 TLRs in the gut I. The role of TLRs/Nods in intestinal development and homeostasis. Am J Physiol Gastrointest Liver Physiol 292: G6–G10
Podolsky DK 2002 Inflammatory bowel disease. N Engl J Med 347: 417–429
Cario E, Gerken G, Podolsky DK 2007 Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132: 1359–1374
Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN 2010 Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 16: 228–231
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R 2004 Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241
Mazmanian SK, Liu CH, Tzianobos AO, Kasper DL 2005 An immunomodulatory molecule of symbiotics bacteria directs maturation of the host immune system. Cell 122: 107–118
Hooper LV, Stappenbeck TS, Hong CV, Gordon JI 2003 Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol 4: 269–273
Vael C, Desager K 2009 The importance of the development of the intestinal microbiota in infancy. Curr Opin Pediatr 21: 794–800
Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y 1997 The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol 159: 1739–1745
Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y 2008 Commensal DNA limits regulatory T-cell conversion and is a natural adjuvant of intestinal immune responses. Immunity 29: 637–649
Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO 2007 Development of the human infant intestinal microbiota. PLoS Biol 5: e177
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA 2005 Diversity of the human intestinal microbial flora. Science 308: 1635–1638
Caicedo RA, Schanler RJ, Li N, Neu J 2005 The developing intestinal ecosystem: implications for the neonate. Pediatr Res 58: 625–628
Stark PL, Lee A 1982 The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J Med Microbiol 15: 189–203
Adlerberth I, Lindberg E, Aberg N, Hesselmar B, Saalman R, Strannegård IL, Wold AE 2006 Reduced enterobacterial and increased staphylococcal colonization of the infantile bowel: an effect of hygienic lifestyle. Pediatr Res 59: 96–101
Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE 2006 Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118: 511–521
Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR 2008 A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy 38: 629–633
Bager P, Wohlfahrt J, Westergaard T 2008 Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin Exp Allergy 38: 634–642
Howie PW, Forsyth JS, Ogston SA, Clark A, Florey CD 1990 Protective effect of breast feeding against infection. BMJ 300: 11–16
Oddy WH, Sly PD, de Klerk NH, Landau LI, Kendall GE, Holt PG, Stanley FJ 2003 Breast feeding and respiratory morbidity in infancy: a birth cohort study. Arch Dis Child 88: 224–228
Dewey KG, Heinig MJ, Nommsen-Rivers LA 1995 Differences in morbidity between breast-fed and formula-fed infants. J Pediatr 126: 696–702
Labbok MH, Clark DC, Goldman AS 2004 Breastfeeding: maintaining an irreplaceable immunological resource. Nat Rev Immunol 4: 565–572
Magne F, Suau A, Pochart P, Desjeux JF 2005 Fecal microbial community in preterm infants. J Pediatr Gastroenterol Nutr 41: 386–392
Bennet R, Eriksson M, Nord CE, Zetterström R 1986 Fecal bacterial microflora of newborn infants during intensive care management and treatment with five antibiotics regimens. Pediatr Infect Dis 5: 533–539
Bonnemaison E, Lanotte P, Cantagrel S, Thionois S, Quentin R, Chamboux C, Laugier J 2003 Comparison of fecal flora following administration of two antibiotic protocols for suspected maternofetal infection. Biol Neonate 84: 304–310
Marra F, Lynd L, Coombes M, Richardson K, Legal M, Fitzgerald JM, Marra CA 2006 Does antibiotic exposure during infancy lead to the development of asthma?. Chest 129: 610–618
Sjögren YM, Tomicic S, Lundberg A, Böttcher MF, Björkstén B, Sverremark-Ekström E, Jenmalm MC 2009 Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin Exp Allergy 39: 1842–1851
Grönlund MM, Arvilommi H, Kero P, Lehtonen OP, Isolauri E 2000 Importance of intestinal colonization in the maturation of humoral immunity in early infancy: a prospective follow up study of healthy infants aged 0–6 months. Arch Dis Child Fetal Neonatal Ed 83: F186–F192
Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E 2001 Distinct patterns of neonatal gut microflora in infants developing or not developing atopy. J Allergy Clin Immunol 107: 129–134
Ouwehand AC, Isolauri E, He F, Hashimoto H, Benno Y, Salminen S 2001 Differences in Bifidobacterium flora composition in allergic and healthy infants. J Allergy Clin Immunol 108: 144–145
Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, Antonopoulos DA, Chang EB, Claud EC 2009 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J 3: 944–954
Mshvildadze M, Neu J, Shuster J, Theriaque D, Li N, Mai V 2010 Intestinal microbial ecology in premature infants assessed with non-culture based techniques. J Pediatr 156: 20–25
Claud EC, Walker WA 2008 Bacterial colonization, probiotics and necrotizing enterocolitis. J Clin Gastroenterol 42: S46–S52
Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA 2000 Inflammation in the developing human intestine: a possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci USA 97: 6043–6048
Deshpande G, Rao S, Patole S, Bulsara M 2010 Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 125: 921–930
Wang M, Karlsson C, Olsson C, Adlerberth I, Wold AE, Strachan DP, Martricardi PM, Aberg N, Perkin MR, Tripodi S, Coates AR, Hesselmar B, Saalman R, Molin G, Ahrné S 2008 Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol 121: 129–134
Penders J, Stobberingh EE, van den Brandt PA, Thijs C 2007 The role of the intestinal microbiota in the development of atopic disorders. Allergy 62: 1223–1236
Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, van Ree R, Stobberingh EE 2007 Gut microbiota composition and the development of atopic manifestations in infancy: the KOALA birth cohort study. Gut 56: 661–667
Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E 2001 Probiotics in primary prevention of atopic disease: a randomized placebo-controlled trial. Lancet 357: 1076–1079
Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E 2003 Probiotics and prevention of atopic disease; 4 year follow-up of a randomized placebo-controlled trial. Lancet 361: 1869–1871
Kalliomäki M, Salminen S, Poussa T, Isolauri E 2007 Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol 119: 1019–1021
Kopp MV, Hennemuth I, Heinzmann A, Urbanek R 2008 A randomized, double-blind, placebo-controlled trial of probiotics for primary prevention; no clinical or immunological effects of Lactobacillus GG supplementation. Pediatrics 121: e850–e856
Perez PF, Doré J, Leclerc M, Levenez F, Benyacoub J, Serrant P, Segura-Roggero I, Schiffrin EJ, Donnet-Hughes A 2007 Bacterial imprinting of the neonatal immune system: lessons from maternal cells?. Pediatrics 119: e724–e732
Boyle RJ, Mah LJ, Chen A, Kivivuori S, Robins-Browne RM, Tang ML 2008 Effects of Lactobacillus GG treatment during pregnancy on the development of fetal antigen-specific immune responses. Clin Exp Allergy 38: 1882–1890
Kopp MV, Salfeld P 2009 Probiotics and prevention of allergic disease. Curr Opin Clin Nutr Metab Care 12: 298–303
Lee J, Seto D, Bielory L 2008 Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol 121: 116–121.e11
Gaboriau-Routhiau V, Raibaud P, Dubuquoy C, Moreau MC 2003 Colonizaton of gnotobiotic mice with human gut microflora at birth protects against Escherichia coli heat-labile enterotoxin-mediated abrogation of oral tolerance. Pediatr Res 54: 739–746
van Baarlen P, Troost FJ, van Hemert S, van der Meer C, de Vos WM, de Groot PJ, Hooiveld GJ, Brummer RJ, Kleerebezem M 2009 Differential NF-kB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc Natl Acad Sci USA 106: 2371–2376
Vanderpool C, Yan F, Polk DB 2008 Mechanisms of probiotic action: implications for therapeutic applications in inflammatory bowel diseases. Inflamm Bowel Dis 14: 1585–1596
Roselli M, Finamore A, Nuccitelli S, Carnevali P, Brigidi P, Vitali B, Nobili F, Rami R, Garaguso I, Mengheri E 2009 Prevention of TNBS-induced colitis by different Lactobacillus and Bifidobacterium strains is associated with an expansion of gammadeltaT and regulatory T cells of intestinal intraepithelial lymphocytes. Inflamm Bowel Dis 15: 1526–1536
Braat H, van den Brande J, van Tol E, Hommes D, Peppelenbosch M, van Deventer S 2004 Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4 T cells via modulation of dendritic cell function. Am J Clin Nutr 80: 1618–1625
Szajewska H, Mrukowicz JZ 2001 Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr 33: S17–S25
Sazawal S, Hiremath G, Dhingra U, Malik P, Deb S, Black RE 2006 Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis 6: 374–382
Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, Kamm MA, Weismueller J, Beglinger C, Stolte M, Wolff C, Schulze J 2004 Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 53: 1617–1623
Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Kuitunen M 2007 Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: A randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 119: 192–198
Alfaleh K, Bassler D 2008 Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 1: CD005496
Claud EC, Lu L, Anton PM, Savidge T, Walker WA, Cherayil BJ 2004 Devlopmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc Natl Acad Sci USA 101: 7404–7408
Lin PW, Nasr TR, Berardinelli AJ, Kumar A, Neish AS 2008 The probiotic Lactobacillus GG may augment intestinal host defense by regulating apoptosis and promoting cytoprotective responses in the developing murine gut. Pediatr Res 64: 511–516
Boehm G, Moro G 2008 Structural and functional aspects of prebiotics used in infant nutrition. J Nutr 138: 1818S–1828S
Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G 2006 A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child 91: 814–819
Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G 2008 Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first tow years of life. J Nutr 138: 1091–1095
Chassin C, Kocur M, Pott J, Duerr CU, Gutle D, Lotz M, Hornef MW 2010 miR-146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe 8: 358–368
Fusaro AE, Brito CA, Victor JR, Rigato PO, Goldoni AL, Duarte AJ, Sato MN 2007 Maternal-fetal interaction: preconception immunization in mice prevents neonatal sensitization induced by allergen exposure during pregnancy and breastfeeding. Immunology 122: 107–115
Mosconi E, Rekima A, Seitz-Polski B, Kanda A, Fleury S, Tissandie E, Monteiro R, Dombrowicz DD, Julia V, Glaichenhaus N, Verhasselt V 2010 Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol 3: 461–474
Prescott SL, Clifton V 2009 Asthma and pregnancy: emerging evidence of epigenetic interactions in utero. Curr Opin Allergy Clin Immunol 9: 417–426
Bobetsis YA, Barros SP, Lin DM, Weldman JR, Dolinoy DC, Jirtle RL, Boggess KA, Beck JD, Offenbacher S 2007 Bacterial infection promotes DNA hypermethylation. J Dent Res 86: 169–174
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaplan, J., Shi, H. & Walker, W. The Role of Microbes in Developmental Immunologic Programming. Pediatr Res 69, 465–472 (2011). https://doi.org/10.1203/PDR.0b013e318217638a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e318217638a
This article is cited by
-
Prevalence of bronchial asthma and correlation between the chemokine receptor 3 gene polymorphism and clinical asthma phenotypes among Egyptian asthmatic children
Egyptian Pediatric Association Gazette (2023)
-
Early-life interactions between the microbiota and immune system: impact on immune system development and atopic disease
Nature Reviews Immunology (2023)
-
In silico molecular docking analysis and ADME prediction of cow urine derived 1-heneicosanol against some bacterial proteins
Vegetos (2023)
-
Developments in pediatrics in 2020: choices in allergy, autoinflammatory disorders, critical care, endocrinology, genetics, infectious diseases, microbiota, neonatology, neurology, nutrition, ortopedics, respiratory tract illnesses and rheumatology
Italian Journal of Pediatrics (2021)
-
Gestational hypertension and childhood atopy: a Millennium Cohort Study analysis
European Journal of Pediatrics (2021)