Abstract
Objective
To assess the association between opioid exposure during therapeutic hypothermia (TH) for perinatal hypoxic-ischemic encephalopathy (HIE) and in-hospital outcomes.
Study design
In this retrospective cohort study, linked data were accessed on infants ≥36 weeks gestation, who underwent TH for HIE, born from 2010–2016 in 23 Neonatal Intensive Care Units participating in Children’s Hospitals Neonatal Consortium and Pediatric Health Information Systems. We excluded infants who received opioids for >5 days.
Results
The cohort (n = 1484) was categorized as No opioid [240(16.2%)], Low opioid (1–2 days) [574 (38.7%)] and High opioid group (HOG, 3–5 days) [670 (45.2%)]. After adjusting for HIE severity, opioids were not associated with abnormal MRI, but were associated with decreased likelihood of complete oral feeds at discharge. HOG had increased likelihood of prolonged hospital stay and ventilation.
Conclusion
Opioid exposure during TH was not associated with abnormal MRI; its association with adverse short-term outcomes suggests need for cautious empiric use.
Similar content being viewed by others
Introduction
Limited preclinical data suggest that the efficacy of therapeutic hypothermia (TH) for perinatal hypoxic-ischemic encephalopathy (HIE) in certain species may be improved with sedation-analgesia [1,2,3]. However, there are accompanying concerns about the impaired clearance of sedative-analgesics during TH [4, 5], and potential respiratory and neurologic adverse effects.
Among the randomized controlled trials (RCTs) of TH for HIE, only one mandated opioid treatment for all infants [6], while another trial recommended morphine infusions or chloral hydrate if infants “appeared distressed” [7]. The rate of administration of sedation-analgesia during TH increased significantly from 43 to 55% in a more recent trial, after adjustment for HIE severity and center [8,9,10,11]. In an international observational study, 83% of infants with encephalopathy received preemptive morphine during TH [12]. According to survey data, preemptive opioid infusion during TH was used in 86% of ventilated and 50% non-ventilated infants in 52 United Kingdom (UK) centers; corresponding rates from 34 North American centers were 50 and 34% respectively [13]. Among neonates ≥35 weeks gestational age (GA) who underwent TH at 125 Neonatal Intensive Care Units (NICUs) in the United States (US) between 2007 and 2015, opioid administration rate was 64%, and increased from 38 to 68% over the 8-year period [14]. Parents report conflicting expectations around the use of morphine during TH in HIE [15]. While alleviation of discomfort was an “unmet” expectation, parents experienced considerable apprehension about the “routine” use of morphine [15]. The increasing use of morphine during TH, despite limited evidence of either benefit or harm, seems to be largely empiric and provider-driven.
The Children’s Hospitals Neonatal Consortium (CHNC) is a collaborative of regional NICUs with the goal of optimizing clinical practice through recognition and critical review of areas of practice variability. In the current study, we investigated the association between opioid exposure during TH in infants with HIE and in-hospital outcomes including brain injury on magnetic resonance imaging (MRI).
Methods
In this retrospective cohort study, we used linked data from the CHNC and the Pediatric Health Information Systems (PHIS). CHNC prospectively captures detailed in-hospital data from all infants admitted to 34 participating regional NICUs [16]. The Institutional Review Board at each institution approved participation in CHNC and associated research studies; the study was performed in accordance with the Declaration of Helsinki. PHIS contains detailed administrative and billing data from 23 participating CHNC institutions [17, 18]. Methods ensuring data quality for both databases have been previously reported [17, 19,20,21]. CHNC and PHIS data were linked at the patient level using unique identifiers unavailable to investigators.
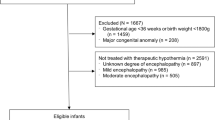
CHNC was used to identify infants born at ≥36 weeks GA, with birthweights ≥1800g, between January 2010 and December 2016, who were diagnosed with HIE by the NICHD or Vermont Oxford Network (VON) criteria and underwent TH [22]. Infants were excluded for major congenital anomalies, non-perinatal event, admission >24 h of age, readmission after a prior admission for TH, open records, or, if linkage to PHIS was not possible. PHIS data were used to quantify opioid cost and duration during TH across the various sites. To focus on opioid use primarily for sedation-analgesia during TH, we excluded infants who received opioids beyond 5 days of age. Each center had either no guideline or its own sedation and opioid use guideline.
Data regarding demographic, maternal, and birth characteristics, HIE severity, electroencephalographic (EEG) and amplitude-integrated EEG (aEEG) studies, and TH were abstracted according to the CHNC manual of operations [16]. Presence or absence of intracranial hemorrhage, infarct/stroke, white matter injury, deep gray matter injury (defined as injury to putamen, globus pallidus or thalamus) and cortical injury were abstracted from the site radiologist’s interpretation of the initial MRI after rewarming at the CHNC site. MRI findings were categorized as normal or abnormal. The primary outcome was abnormal MRI. Severe MRI injury, based on the presence of either cortical or deep gray matter injury (the most predictive combination of individual MRI findings), has been previously described in this data set and was found to be significantly associated with death or NDI at or beyond 12 months of age [23]. Secondary outcomes included severe MRI injury, survival with normal MRI, length of stay, duration of mechanical ventilation and extent of oral feeding at discharge, categorized as complete oral feeds, partial with tube, partial without tube, and no oral feeds, among survivors. Testing results of Bayley Scales of Infant Development, Third Edition (BSID-III), were available at ≥335 days of age in only a subset [186 (13%)] of infants. Neurodevelopmental impairment (NDI) was defined as the presence of any of the following: BSID-III composite cognitive score <85 or composite motor score <70, deafness, blindness, or gross motor delay defined as a GMFCS score ≥2; death or NDI was considered a secondary outcome [24].
The PHIS Clinical Transaction Classification (CTC) pharmacy codes corresponding to opioids (all opioids, and morphine and fentanyl individually) were used to quantify duration (number of hospital days with any opioid CTC code assigned within the initial 5 days of life) of opioid exposure per patient. Infants were categorized based on the duration of use as No opioid group (NOG), Low (1–2 days) Opioid group (LOG) and High (3–5 days) opioid groups (HOG). Additional PHIS data on duration of exposure to sedatives (benzodiazepines-lorazepam and midazolam, and dexmedetomidine), and antiepileptic drugs (AEDs) were considered as covariates in our regression analyses. Standardized opioid-related costs per patient were calculated according to a previously described cost master index and adjusted for wage and price index [16, 25]. All costs were inflated to 2012 dollars; standardized unit cost for each CTC code was defined as the median cost across all participating hospitals and calculated per patient and for each medication.
Statistical analyses included Fisher’s exact and Kruskal-Wallis tests to compare categorical and continuous variables in the three groups of opioid exposure. Jonckheere-Tepstra test was used to compare opioid use over time. Fisher’s exact and Wilcoxon Rank Sum Test were used for post-hoc testing, and results evaluated at alpha of 0.017 to protect the 3 simultaneous pairwise comparisons with an experiment-wise alpha of 0.05. Logistic regression was used for the outcome of abnormal MRI and generalized linear models for gamma distribution and log link were developed for intercenter variation in opioid exposure, lengths of stay and mechanical ventilation. Further, as a sensitivity analysis, we also assessed the association between opioid costs as a continuous variable (to capture the variability) and outcomes using regression analysis. For feeding at discharge, multinomial logistic regression was used. All analyses were adjusted for center, HIE severity, AED days and sedative days. Data were analyzed using SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC).
Results
Study cohort
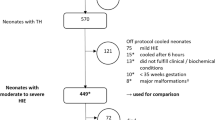
Among 129,253 infants included in the CHNC during the study period, 3391 neonates were diagnosed with HIE, and 2812 underwent TH. After applying exclusion criteria (n = 313), 2499 neonates remained eligible and 1859 (74%) were linked to their PHIS data. After excluding those who received opioids beyond 5 days of age (n = 375), 1484 neonates formed our study cohort (Supplementary Fig. 1 online). They were stratified as NOG [240 (16.2%)], LOG [574 (38.7%) and HOG [670 (45.2%)]. Duration of opioid exposure was 1 day in 286 (19.3%), 2 days in 288 (19.4%), 3 days in 254 (17.1%), 4 days in 271 (18.3%) and 5 days in 145 (9.8%) infants. In infants exposed to any opioids (n = 1244), the associated median [IQR] cost was $42.40[17.80–71.20] and duration was 3 [2,3,4] days. Of the opioid-exposed infants, 919 (61.9%) neonates received morphine and 588 (39.6%) received fentanyl with similar median costs (morphine $28.80[11.30–57.50], fentanyl $27[9.10–60.30]) and durations (morphine 2 [1,2,3] days, and fentanyl 2 [1,2,3,4] days).
Intercenter variation in opioid exposure
The median [IQR] duration and costs of sedatives, AEDs and muscle relaxants are described (Supplementary Table 1 online). Varying proportions of infants (47.6–100%) in each center received opioids during TH, with median durations of 1.5−4 days (Supplementary Fig. 2 online). All centers except one used both morphine and fentanyl. The distribution of opioid-days did not differ by year (p = 0.4273). Center (p < 0.001) and death [OR 1.18; 95% C.I. 1.01–1.37, p = 0.0323] were significant predictors of overall opioid costs but HIE severity was not; 3 centers had significantly increased opioid costs, compared to the referent median center (Fig. 1). Center remained a significant predictor of morphine and fentanyl costs separately (p < 0.0001); mild HIE was associated with significantly higher fentanyl costs [OR 1.34; 95% C.I. 1.07–1.67, p = 0.0117], compared to severe HIE.
Maternal and birth characteristics
The study cohort had a median GA of 39 weeks, was more often male (57%), white (62%), outborn (97%) and born via C-section (62%). Most (73%) infants underwent intubation and ventilation in the delivery room, 93% had Apgar scores <5 at 1 min and 40% at 10 min of age. HIE severity was classified as mild in 27%, moderate in 54% and severe in 19% of cases. The three groups of opioid exposure differed in rates of maternal hypertension, fetal distress, mode of delivery and receipt of chest compressions (Table 1). Higher proportions of the LOG received epinephrine in the delivery room (29% vs. 19% in NOG and 23% in HOG), and had 10-min Apgar scores <5 (44% vs. 36% in HOG). However, the LOG also had the highest proportion of mild HIE (34% compared to 17% in NOG and 25% in HOG).
TH and outcomes data
Whole body cooling was the predominant (79%) TH modality; during transport, 15% underwent active cooling, 64% were passively cooled and 20% were not cooled. Whole body cooling was less frequent (69%) in the HOG, and transport cooling rates varied (63% in the NOG, 80% in the LOG and 84% in the HOG) (Table 2). Nearly half the infants had no comorbidities during TH; commonest comorbidities were hypothermia (>0.5 °C below target temperature) (28%), coagulopathy (21%) and hyperglycemia (16%), with the HOG having higher rates of coagulopathy and bradycardia. Muscle relaxants and naloxone were administered for a median of 1 day and, sedatives for 2 days; AED days were significantly lower in LOG, compared to HOG (5 vs. 7 days). Sedative costs were significantly higher in the NOG, compared to the groups with opioid exposure.
Clinical or EEG-confirmed seizures occurred in 43.6% of the cohort; there were no differences in seizure frequency between groups (Table 3). EEG was available at 24 h in 1154 (77.8%) infants and categorized as normal in 27%, moderately abnormal in 51% and severely abnormal in 22% cases. Severely abnormal EEG was higher in the LOG compared to HOG (21% vs. 15%). MRI was normal in 34% neonates, most often in the non-opioid exposed (unadjusted rates 41% in NOG, 31% in LOG and 34% in HOG). Deep gray matter injury was observed in 14.5% neonates overall, and was significantly higher (18% vs. 8.3%) in the HOG, compared to the NOG. Unadjusted mortality rate was 15% overall, with significant variation among groups (6% NOG, 24% LOG and 12% HOG). All infants with normal MRI survived except 3 (0.6%) in the HOG. Rates of survival with normal MRI were significantly higher (40.8%) in the NOG, compared to the LOG (30.5%) and intermediate (33.9%) in the HOG. Median durations of ventilation [5 vs. 2 days] and hospital stay [12 vs. 11 days] were both longer in the HOG, compared to LOG and NOG.
At the time of discharge, the HOG had G tubes (4.3% vs. 0.4% in NOG and 3.3% in LOG) and received supplemental oxygen (5.7% vs. 0.8% in NOG and 0.9% in LOG) more often. Also, infants in the NOG were discharged home on complete oral feeds more frequently (83% vs. 61% in LOG and 75% in HOG). Following discharge, 51.1% infants were referred to neurology, 19.1% to audiology, and 0.9% for palliative care. Speech, occupational or physical therapy referrals were documented for 14.2% infants overall, more often (18.1% vs. 10.4%) in the HOG, compared to the NOG.
On logistic regression analyses, HIE severity was significantly correlated with abnormal MRI as well as survival with normal MRI; opioid exposure, center, sedative and AED days did not correlate with MRI findings (Table 4). The model had an area under the curve of 0.773 with good fit (Hosmer–Lemeshow p = 0.993). There were no significant associations between opioid exposure categories and severe MRI injury. Center, high opioid exposure, HIE severity and AED days were all significantly associated with lengths of stay and ventilation in survivors and, at discharge, lesser likelihood of complete oral feeds; even low opioid exposure was associated with likelihood of receipt of tube feeds. More sedative days were also associated with prolonged ventilation. There were no significant interactions between opioid exposure and HIE severity nor opioid exposure and sedative days. The sensitivity analysis in which opioid costs were treated as a continuous variable revealed a significant association between increased opioid costs and increased duration of ventilation, a trend of an association with severe MRI injury, but not with any of the other outcomes (Table 5). These findings were consistent with the primary analysis.
A subset (n = 186) of infants had outcomes data available, including 39 (35.1%) who died and 170 (64.9%) who had neurodevelopmental follow-up with BSID-III scores documented after 11 months of age. Among them, 23 (13.5%) had NDI, of whom 19 (78.3 %) and 9 (39.1%) had BSID-III cognitive scores <85 and motor scores <70, respectively; 1 patient had abnormal hearing; and 6 (26.1%) had GMFCS scores ≥2. None of the studied variables (3-level opioid exposure, HIE severity, sedative or AED days or center) were found to be associated significantly with death or NDI (Table 5). Nor were opioid costs associated with death or NDI (Table 5).
Discussion
In the current study, we found that 86% of infants across the CHNC regional NICUs received opioids during TH for perinatal HIE, as intermittent doses or infusions, with wide intercenter variation in the proportion (48–100%) and magnitude of overall opioid exposure, as estimated by duration and costs, as well as fentanyl and morphine exposure separately. Morphine use (62%) was more prevalent than fentanyl (40%). These rates were higher than previous reports from the US [8, 10, 14], although the intercenter variation (5–100%) in exposure was similar [14]. While opioid use varied from year-to-year, there was no definite trend over the 7 year study duration.
Routine opioid administration during TH in HIE are extrapolated from recommendations in adults and older children undergoing TH following cardiac arrest that were designed to prevent shivering, maintain hypothermia, and enhance neuroprotection [13, 26, 27]. These indications may not be applicable to neonates who have non-shivering thermogenesis [28]. More recently, dexmedetomidine, an agent with potential neuroprotective effects, has been used in neonates during TH as monotherapy or an adjunct to opioids without major adverse events [29,30,31]. During the study period until 2016, dexmedetomidine was administered to less than 1% of infants, so we were unable to assess the risks or benefits of its use.
We excluded infants who received opioids beyond 5 days of age to assess opioid use which was preemptive (based on institutional protocols for care during TH) or intended to alleviate discomfort or shivering during TH, and to exclude receipt for respiratory or other indications that could persist after TH. We categorized opioid exposure as low or high based on the duration to attempt to discriminate neonates with occasional use from those, presumably, on a continuous infusion for the 3–4 duration of TH and rewarming. We found no racial differences in opioid exposure, in contrast to a previous study in which African-American neonates received opioids less frequently [14]. Infants in the three groups differed in the extent of resuscitation, HIE severity, and EEG background at 24 h, although, without any definite patterns, suggesting that the extent of opioid use was driven by practice variation, rather than clinical need. We speculate that the LOG, with low level exposure to opioids, consisted of a heterogenous population of 2 subsets of infants, those with mild HIE, and the associated irritability, and those with severe HIE, more severely abnormal EEG and MRI and high mortality, who may have received opioids around the time of death. Importantly, there was no statistical interaction between opioid costs and HIE severity.
Our main purpose was to evaluate the association between opioid exposure during TH and short-term outcomes. We found that, after adjustment for several potential confounders including HIE severity, high opioid exposure remained independently associated with prolonged NICU stay and ventilation in survivors, and tube feedings at discharge. A few previous studies have addressed this question with similar results. Neonates with moderate or severe HIE who received pre-emptive morphine infusion (n = 141) during TH had significantly longer hospital stays than those who did not (n = 28) [12]. In the NICHD RCT of TH in moderate or severe HIE, unadjusted mean lengths of stay in survivors, durations of ventilation and supplemental oxygen, and age at full oral feeds were significantly shorter in the group without sedatives or AEDs, compared to the groups that received sedatives, AEDs or both at 5 discrete time points through 72 h of age [8]. In a cohort of 282 infants with moderate or severe HIE, who were routinely intubated for the duration of TH and received pre-emptive opioids, there was no relationship between cumulative opioid dose and time at extubation [32]. In a previous database study, opioid-exposed neonates experienced greater median durations of respiratory support, both mechanical ventilation and non-invasive [14]. Our data suggest that opioids may be detrimental in a dose-dependent fashion.
About 86% of deaths in our cohort were preceded by withdrawal of life-support and the primary cause of death was most often (73%) related to brain injury. The median age at death was 3.5 days, consistent with our previous report of median age of 4 days at withdrawal of life support in HIE, and likely reflecting the time for parents and clinicians to reach a decision to redirect care [33]. The higher mortality rate in the opioid exposed groups, especially the LOG (23.7% vs. 11.5% in the HOG and 6.3% in the NOG), may be confounded by opioid use for comfort during redirection of care. It is plausible that severity of neurologic injury and/or multi-organ failure may have preceded opioid exposure, withdrawal of life-support and subsequent mortality. The rates of in-hospital death in the NICHD trial were statistically not different in the groups with and without exposure to sedative-analgesics and AEDs (16–26%) [8]. Berube et al. observed lower mortality in the first 3 days in opioid-exposed infants (5%), compared to the non-exposed group (9%), and speculated that infants with severe HIE may not have had autonomic/behavioral manifestations of pain and therefore, may not have received opioids [14]. In the current study, only 10% of neonates with mild HIE and 18–19% of those with moderate to severe HIE were unexposed to opioids. While morphine administration has been associated with microglial and neuronal cell apoptosis and lower survival in small studies of rat models of hypoxia-ischemia, their association, if any, with mortality in human neonates with HIE requires further investigation [34, 35].
A third of our cohort had normal MRIs, 15% had deep gray matter injury and 10% had cortical injury. On adjusted analyses, abnormal MRI was not associated with opioid exposure. In the Magnetic Resonance Biomarkers in Neonatal Encephalopathy study, basal ganglia/thalamic injury and cortical injury were noted in 24 and 26%, respectively, of the morphine group and 14 and 11% of the non-morphine group, which were not statistically significant differences [12]. Preemptive morphine was not associated with thalamic N-acetylaspartate (NAA) concentration, and lactate/NAA peak area ratios at 1 week [12]. In a rat model of perinatal opioid exposure, a distinct signature of neuroinflammation, immune dysfunction and microstructural brain injury on MRI has been reported [36]. However, in previous neonatal HIE studies, neither preemptive morphine during TH [12], nor sedation-analgesia exposure or cumulative dose [8, 32], were associated with neurodevelopmental outcomes in early childhood. We did not find any association between death or NDI and level of opioid exposure in a subset of our cohort.
Our study has some limitations, including the retrospective design, referral bias inherent in the CHNC and relatively small subset of patients who did not receive opioids. About 13% of infants were excluded due to prolonged opioid use, possibly related to ventilation or surgery. While we did not have long-term follow-up data except in a small subset of our cohort, MRI is a reasonable surrogate of outcome in this population, and normal MRI is associated with favorable developmental trajectories. However, subtle adverse neurodevelopmental effects related to neuronal apoptosis may not be evident on early MRI. MRI findings were abstracted from clinical reports and lacked the granular detail for scoring systems; normal MRI was based on absence of abnormalities, some relatively minor. We included neonates coded as “mild HIE” undergoing TH, because this is an increasing real-world practice. There may have been coding differences in opioid use between the centers. Although we could not ascertain the precise opioid dose, timing or levels, nor whether it was a continuous infusion or a bolus dose, we had quantitative per-patient estimates of cost derived from pharmacy charges based on dose, route and scheduling of opioid administration. We recognize that these may not reflect the cumulative dose, especially in infants with impaired renal clearance. The indication for initiation and preceding pain scores were not known. We did not adjust for severity of illness at the time of opioid initiation. While we adjusted for AED and sedative durations as potential confounders, the additive effects of multiple classes of drugs could not be studied. Our categorization of opioid exposure as low and high based on the duration, although a reasonable quantitative surrogate, was not by exact cumulative dose. The association between opioid use and mortality could not be ascertained due to confounding with use during end-of-life.
Nonetheless, our study has several strengths. We included a large cohort of neonates who underwent TH in geographically diverse tertiary level NICUs in North America, with capabilities of neuromonitoring with EEG, MRI, and where tracheostomy and G-tube placement were available. We had detailed clinical data through the CHNC and the PHIS linkage provided us with cost and duration data of the different drug classes during the NICU stay. Our large numbers allowed us to categorize opioid exposure by duration, which has not been done previously, and to adjust for confounders and multiple comparisons.
Conclusion
Our data in more than 1400 infants with HIE undergoing TH suggest that opioids may not enhance neuroprotection and may be associated with detrimental short-term outcomes, including prolonged ventilation, delayed achievement of oral feeds and, not unexpectedly, prolonged NICU stay. Given the dearth of evidence of their benefit and a possibility of contributory harm, widespread empiric use of opioids during TH may need to be reconsidered.
Data availability
The data that support the findings of this study are available from Childrens’ Hospitals Neonatal Database, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
References
Thoresen M, Satas S, Loberg EM, Whitelaw A, Acolet D, Lindgren C, et al. Twenty-four hours of mild hypothermia in unsedated newborn pigs starting after a severe global hypoxic-ischemic insult is not neuroprotective. Pediatr Res. 2001;50:405e11.
Haaland K, Loberg EM, Steen PA, Thoresen M. Posthypoxic hypothermia in newborn piglets. Pediatr Res. 1997;41:505e12.
Chakkarapani E, Dingley J, Liu X, Hoque N, Aquilina K, Porter H, et al. Xenon enhances hypothermic neuroprotection in asphyxiated newborn pigs. Ann Neurol. 2010;68:330e41.
Róka A, Melinda KT, Vásárhelyi B, Machay T, Azzopardi D, Szabó M. Elevated morphine concentrations in neonates treated with morphine and prolonged hypothermia for hypoxic ischemic encephalopathy. Pediatrics. 2008;121:e844–9.
Frymoyer A, Bonifacio SL, Drover DR, Su F, Wustoff CJ, Van Meurs KP. Decreased morphine clearance in neonates with hypoxic ischemic encephalopathy receiving hypothermia. J Clin Pharmacol. 2017;57:64–76.
Simbruner G, Mittal RA, Rohlmann F, Muche R. neo.nEURO.network Trial Participants. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126:e771–8.
Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. TOBY Study Group. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58.
Natarajan G, Shankaran S, Laptook AR, McDonald SA, Pappas A, Hintz SR, et al. NICHD Neonatal Research Network (NRN) Whole Body Hypothermia Subcommittee. Association between sedation-analgesia and neurodevelopment outcomes in neonatal hypoxic-ischemic encephalopathy. J Perinatol. 2018;38:1060–67.
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84.
Bonifacio SL, McDonald SA, Chock VY, Wusthoff CJ, Hintz SR, Laptook AR, et al. Differences in patient characteristics and care practices between two trials of therapeutic hypothermia. Pediatr Res. 2019;85:1008–15.
Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA. 2014;312:2629–39.
Liow N, Montaldo P, Lally PJ, Teiserskas J, Bassett P, Oliveira V, et al. Preemptive morphine during therapeutic hypothermia after neonatal encephalopathy: a secondary analysis. Ther Hypothermia Temp Manag. 2020;10:45–52.
Montaldo P, Vakharia A, Ivain P, Mendoza J, Oliveira V, Markati T, et al. Pre-emptive opioid sedation during therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2020;105:108–09.
Berube MW, Lemmon ME, Pizoli CE, Bidegain M, Tolia VN, Cotten CM, et al. Opioid and benzodiazepine use during therapeutic hypothermia in encephalopathic neonates. J Perinatol. 2020;40:79–88.
Craig AK, Gerwin R, Bainter J, Evans S, James C. Exploring parent expectations of neonatal therapeutic hypothermia. J Perinatol. 2018;38:857–64.
Murthy K, Dykes FD, Padula MA, Pallotto EK, Reber KM, Durand DJ, et al. The Children’s hospitals neonatal database: an overview of patient complexity, outcomes and variation in care. J Perinatol. 2014;34:582–6.
Massaro AN, Murthy K, Zaniletti I, Cook N, DiGeronimo R, Dizon ML, et al. Intercenter cost variation for perinatal hypoxic-ischemic encephalopathy in the era of therapeutic hypothermia. J Pediatr. 2016;173:76–83 e1.
Dizon MLV, Rao R, Hamrick SE, Zaniletti I, DiGeronimo R, Natarajan G, et al. Practice variation in anti-epileptic drug use for neonatal hypoxic-ischemic encephalopathy among regional NICUs. BMC Pediatr. 2019;19:67.
Murthy K, Yanowitz TD, DiGeronimo R, Dykes FD, Zaniletti I, Sharma J, et al. Short-term outcomes for preterm infants with surgical necrotizing enterocolitis. J Perinatol. 2014;34:736–40.
Pasquali SK, Jacobs JP, Shook GJ, O’Brien SM, Hall M, Jacobs ML, et al. Linking clinical registry data with administrative data using indirect identifiers: implementation and validation in the congenital heart surgery population. Am Heart J. 2010;160:1099–104.
Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299:2048–55.
Massaro AN, Murthy K, Zaniletti I, Cook N, DiGeronimo R, Dizon M, et al. Short-term outcomes after perinatal hypoxic ischemic encephalopathy: a report from the Children’s hospitals neonatal consortium HIE focus group. J Perinatol. 2015;35:290–6.
Peeples ES, Rao R, Dizon MLV, Johnson YR, Joe P, Flibotte J, et al. Predictive models of neurodevelopmental outcomes after neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2021;147:e2020022962.
Adams-Chapman I, Heyne RJ, DeMauro SB, Duncan AF, Hintz SR, Pappas A, et al. Follow-Up Study of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental impairment among extremely preterm infants in the neonatal research network. Pediatrics. 2018;141:e20173091.
Keren R, Luan X, Localio R, Hall M, McLeod L, Dai D, et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166:1155–64.
Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, et al. THAPCA trial investigators. therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015;372:1898–1908.
Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, et al. American Heart Association. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S768–86.
Wassink G, Lear CA, Gunn KC, Dean JM, Bennet L, Gunn AJ. Analgesics, sedatives, anticonvulsant drugs, and the cooled brain. Semin Fetal Neonatal Med. 2015;20:109–14.
O’Mara K, Weiss MD. Dexmedetomidine for sedation of neonates with HIE undergoing therapeutic hypothermia: a single-center experience. AJP Rep. 2018;8:e168–e173.
Ma D, Hossain M, Rajakumaraswamy N, Arshad M, Sanders RD, Franks NP, et al. Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharm. 2004;502:87–97.
Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med. 2004;32:1322–26.
Gundersen JK, Chakkarapani E, Jary S, Menassa DA, Scull-Brown E, Frymoyer A, et al. Morphine and fentanyl exposure during therapeutic hypothermia does not impair neurodevelopment. EClinicalMedicine. 2021;36:100892.
Natarajan G, Mathur A, Zaniletti I, DiGeronimo R, Lee KS, Rao R, et al. Children’s Hospitals Neonatal Consortium (CHNC). Withdrawal of life-support in neonatal hypoxic-ischemic encephalopathy. Pediatr Neurol. 2019;91:20–26.
Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–36.
Festekjian A, Ashwal S, Obenaus A, Angeles DM, Denmark TK. The role of morphine in a rat model of hypoxic-ischemic injury. Pediatr Neurol. 2011;45:77e82.
Jantzie LL, Maxwell JR, Newville JC, Yellowhair TR, Kitase Y, Madurai N, et al. Prenatal opioid exposure: The next neonatal neuroinflammatory disease. Brain Behav Immun. 2020;84:45–58.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed sufficiently to the intellectual content of the submission. GN had the final responsibility for the decision to submit for publication. SHE, KSL, UM, RDG, MLVD, ESP, TDY, TWW, JF, PJ, ANM, RR were all involved in reviewing study design, all acquired data from their sites, and played an important role in interpreting the results, revising the manuscript, approving the final version and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. GN, ANM, RR conceived and designed the work and drafted the initial manuscript. IZ was responsible for data review and validation, linking of databases and all data analysis.
Corresponding author
Ethics declarations
Competing interests
Author IZ is an employee of Children’s Hospitals Association, Kansas City, MO. Other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of members and their affiliations appears in the Supplementary Information.
Supplementary information
Rights and permissions
About this article
Cite this article
Natarajan, G., Hamrick, S.E., Zaniletti, I. et al. Opioid exposure during therapeutic hypothermia and short-term outcomes in neonatal encephalopathy. J Perinatol 42, 1017–1025 (2022). https://doi.org/10.1038/s41372-022-01400-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01400-x