Abstract
Leukemia stem cells (LSCs) are responsible for acute myeloid leukemia (AML) chemotherapy resistance and relapse. Here, we discovered that miR-34c-5p, a microRNA central to the senescence regulation network, was significantly down-regulated in AML (non-acute promyelocytic leukemia, non-APL) stem cells compared to that in normal hematopoietic stem cells (HSCs). The lower expression of miR-34c-5p in LSCs was closely correlated to the adverse prognosis and poor responses to therapy of AML patients. Increased miR-34c-5p expression induced LSCs senescence ex vivo, prevented leukemia development and promoted the eradication of LSCs in immune deficient mice. Mechanistically, forced expression of miR-34-5p induced senescence in LSCs through p53-p21Cip1-Cyclin-dependent kinase (CDK)/Cyclin or p53-independent CDK/Cyclin pathways. Exosome-mediated transfer of miR-34c-5p was one of the reasons for miR-34c-5p deficiency in LSCs. Furthermore, miR-34c-5p could increase its intracellular level by inhibiting exosome-mediated transfer via a positive feedback loop through RAB27B, a molecule that promotes exosome shedding. Overall, this study establishes a new strategy for treatment of AML patients by targeting LSCs to reinitiate senescence via increased miR-34c-5p expression. This miRNA-mediated tumor stem cell senescence could also have important therapeutic value in other malignancies.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) is one of the most common and fatal hematopoietic malignancies in adults. Despite achieving a high remission rate with current treatment regimens, most patients still relapse and die of the disease. There have been reports of a rare subset of leukemia cells, which have been termed leukemia stem cells (LSCs), that is more resistant to regular chemotherapy than bulk leukemia populations and possesses self-renewal capacity and the ability to initiate leukemia [1]. Thus, finding strategies to specifically eliminate LSCs might be necessary to cure the disease permanently.
Cellular senescence, a state of irreversible growth arrest, is an important barrier that prevents tumor cells from transformation and proliferation; senescence induction may be an effective approach for cancer treatment [2]. There are four senescence-inducing signals, specifically, DNA damage, oncogenic expression, telomere attrition, and oxidative stress [3, 4]. Our previous studies found that activation of the ROS-p38 MAPKα axis and DNA damage response (DDR) could induce hematopoietic stem cells (HSCs) senescence [5,6,7,8]. Our further research indicated that LSCs had higher levels of ROS, p38 MAPKα activity and DNA damage but lower senescence than normal HSCs [9]. Researchers have also found that leukemogenic fusion-genes induced senescence in normal hematopoietic stem and progenitor cells (HSPCs) but not in LSCs [10]. All this evidence suggests that mechanisms may exist that uncouple the connection between senescence induction and oxidative stress, DNA damage and oncogenic gene expression signaling pathways in myeloid LSCs.
MicroRNA (miRNA) is small non-coding RNA that restrains the expression of target genes by either preventing translation of the target mRNA or causing its degradation [11]. Recently, several independent studies reported that miR-34c was involved in cell cycle regulation and senescence [12,13,14,15]. Furthermore, utilizing pathway analysis of senescence-associated miRNA, it was found that miR-34c had the potential to serve as a key regulator of all four of the above senescence induction pathways [16]. However, to the best of our knowledge, it has not been determined whether miR-34c is implicated in senescence initiation of LSCs. Our preliminary experiments revealed that the expression level of miR-34c-5p was significantly lower in AML stem cells compared to that in HSCs, leading to a novel hypothesis that increasing miR-34c-5p could induce LSC senescence and eventually promote the eradication of LSCs.
Recent studies have shown that LSCs phenotypes are variable among specimens derived from different AML patients [17, 18]. However, CD34+CD38− cells are still the most commonly used LSCs population because they are the most potent leukemia-initiating population in various xenotransplantation and serial transplantation experiments [19,20,21]. The percentage of CD34+CD38− cells in the KG-1a cell line, which was established from the bone marrow (BM) of a 59-year-old man with erythroleukemia [22], can reach more than 95%. Therefore, the KG-1a cell line is regarded as a LSCs-enriched cell line [23,24,25]. Here, we report for the first time that a lower miR-34c-5p level exists in AML (non-APL) CD34+CD38− cells compared to that in normal CD34+CD38− hematopoietic cells, which is associated with an adverse prognosis and worse therapeutic outcome of AML patients, and increased miR-34c-5p might induce senescence and promote in vivo eradication of AML stem cells through p53-p21Cip1-CDK/Cyclin or p53-independent CDK/Cyclin pathways and RAB27B, a critical regulatory protein in the exosome-release process [26, 27], to inhibit exosome-mediated miR-34c-5p trafficking.
Materials and methods
Patient samples, AML cell lines, and CD34+CD38− cell isolation
The primary AML patient and normal volunteer BM samples were obtained from Union Hospital (Wuhan, China). Human cord blood (hCB) samples were obtained from full-term pregnant women who underwent elective caesarean section at Tongji Hospital (Wuhan, China). All samples were collected with the consent of the patients and the approval of the local ethics committee. KG-1a, THP-1 (from a patient with AML-M4) [28], and KASUMI-1 cell lines (from a patient with AML-M2) [29] were from the American Type Culture Collection (ATCC, USA). All the cell lines were tested for mycoplasma contamination yearly using a Mycoplasma Stain Assay Kit (Beyotime, China). CD34+CD38− cells were isolated from BM, hCB samples, and AML cell lines by following the instructions of CD38 and CD34 MicroBead Kits (Miltenyi, German) as previously described [30].
Cell culture, transduction of AML stem cells with miRNA or siRNA or lentivirus
Synthetic miR-34c-5p mimic and a miRNA negative control or RAB27B siRNA and siRNA negative control were purchased from Ambion (Applied Biosystems, Carlsbad, CA, USA). Third generation lentivirus particles were generated by Genomeditech Company (Shanghai, China). Additional details are provided in Supplementary Materials and Methods.
Total RNA extraction, qPCR, Western blot analysis, transcriptome sequencing, and Luciferase assays
The detailed experimental procedures are provided in Supplementary Materials and Methods.
Analysis of senescence and apoptosis
CFSE-mediated tracking of KG-1a cells division was performed as previously described [7]. Additional details and the procedures for detections of EdU and SA-β-gal activity are provided in Supplementary Materials and Methods. Apoptosis was investigated with an Annexin V/PI staining and flow cytometry assay as previously described [30].
Isolation of apoptotic bodies (AB), microvesicles (MV), and exosomes (Exo)
AB, MV, and Exo were isolated using differential centrifugation protocols [31, 32]. Additional details are provided in Supplementary Materials and Methods.
Serial xenotransplantation of KG-1a cells in NOD/SCID or NOG mice
The detailed experimental procedure is provided in Supplementary Materials and Methods.
Statistical analysis
All data were analyzed using GraphPad Prism 6.0 from GraphPad Software (San Diego, CA, USA). Each experiment was performed at least three times, the samples of AML patients and mice for each experiment were not less than five. The samples from cell lines for each experiment were not less than three. For two sets of data, two-paired student’s t-test was used to assess the statistical significance. For more than two data sets, the variance similar between the groups was statistically compared, one-way ANOVA followed by Sidak’s multiple comparison test was used to determine the differences. To examine the influence of two different categorical independent variables on one continuous dependent variable, two-way ANOVA followed by Tukey’s multiple comparisons test was used. P-values < 0.05 were considered to be statistically significant. *P<0.05; **P<0.01; ***P<0.001. ns, not significant.
Results
miR-34c-5p is down-regulated in primary AML CD34+CD38− cells and is associated with adverse prognosis and poor therapeutic efficacy in AML patients
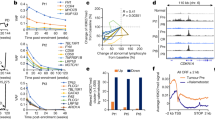
We detected miR-34c-5p expression levels in CD34+CD38− cells from primary AML patients, who were classified into better, intermediate, and poor prognosis groups (Supplementary Table S1) according to National Cancer Center Network (NCCN) guidelines [33], hCB, normal volunteers BM, and three AML cell lines (KG-1a, THP-1, and KASUMI-1) using qPCR. A significantly lower expression level of miR-34c-5p in LSCs was found in the poor and intermediate prognosis groups compared with that in the better risk group and normal control (Fig. 1a). However, miR-34c-5p expression had no significant differences between different AML FAB subtypes (Supplementary Figure S1a). To investigate whether miR-34c-5p expression was correlated with the therapeutic efficacy of the initial induction chemotherapy, patients under a unified regimen of cytarabine and daunorubicin were analyzed, and it was found that a higher miR-34c-5p expression in AML CD34+CD38− cells corresponded to a better therapeutic efficacy for AML patients (Fig. 1b, Supplementary Figure S1b). To sum up, these data suggest that LSCs exhibit low miR-34c-5p expression, and the decreased miR-34c-5p expression level is a potential adverse prognostic factor and closely related to poor therapeutic efficacy in AML patients.
A down-regulated miR-34c-5p in primary AML CD34+CD38− cells is associated with adverse prognosis and poor therapeutic efficacy in AML patients. miR-34c-5p expression in CD34+CD38− cells from better risk (n = 13), intermediate-risk (n = 13), and poor-risk (n = 14) AML patients and from CB (n = 7), normal volunteer BM (n = 6) samples and AML cell lines (n = 3) was analyzed using qPCR (a). miR-34c-5p expression analysis in CD34+CD38− cells from the different responses of AML patients under a unified therapeutic regimen. CR: complete remission, CR (+) (n = 11), CR (−) (n = 21) (b). The data represent the mean ± S.E.M
Increased miR-34c-5p induces senescence of AML CD34+CD38− cells ex vivo
To evaluate the senescence induction effects of enhanced miR-34c-5p expression on LSCs ex vivo, KG-1a cells were transfected via electroporation with synthetic miR-34c-5p mimic (mimic) or synthetic miRNA negative control (NC) or vehicle (Control). The transfection efficiency was determined by qPCR analysis of miR-34c-5p (Supplementary Figure S2a). Cell proliferation was assessed with cell counting, CFSE or EdU flow cytometry assays 72 h after electrotransfection. As shown in Fig. 2a, b, Supplementary Figure S2b-c, forced expression of miR-34c-5p resulted in a decreased cell number, proliferation index, and percentage of EdU+ cells. We next detected the cell cycle and cell cycle-associated proteins E2F3, Cyclin E2, c-Myc, CDK4, and c-Met [4]. Increased miR-34c-5p induced an increase in the number of G0/G1 phase cells and decreased cell cycle-associated protein levels (Fig. 2c). We further performed an Annexin V/PI flow cytometry assay and FluoReporter® lacZ Flow Cytometry for detection of apoptosis and β-galactosidase activity, respectively. As shown in Fig. 2d and Supplementary Figure S2d, up-regulation of miR-34c-5p failed to induce apoptosis but resulted in significantly increased senescence in KG-1a cells. Furthermore, two typical senescence-associated secretory factors, IL-6 and plasminogen activator inhibitor-1 (PAI-1), were elevated under treatment with miR-34c-5p mimic at 48 h post-electrotransfection (Fig. 2e). All these characteristics are identical to classical cellular senescence markers [34]. Moreover, miR-34c-5p also induced proliferation inhibition and senescence of CD34+CD38− cells from KASUMI-1 cell line and primary AML patients with different FAB subtypes (Supplementary Figure S3a-f). Taken together, these results point to senescence being selectively induced by miR-34c-5p mimic in AML stem cells.
miR-34c-5p induces senescence in KG-1a cells. KG-1a cells were transfected with miR-34c-5p mimic, negative control or vehicle via electroporation. Cells number at 1, 2, and 3 days after transfection (a), EdU flow cytometry at 72 h after transfection (b). Cell cycle distribution analyzed using PI staining followed by flow cytometry, and cell cycle-associated proteins expression analyzed via Western blotting (c), the mean fluorescence intensity (MFI) of fluorescein di-β-d-galactopyranoside (FDG) analyzed using flow cytometry (d), the senescence-associated secretory phenotype markers IL6 (left) and PAI-1 (right) detected with qPCR at 48 h after transfection (e). The data represent the mean ± S.E.M. from three replicate experiments
Increased miR-34c-5p expression prevents leukemia development and promotes eradication of AML stem cells in immune deficient mice
The in vivo anti-LSC activity induced by enhanced miR-34c-5p expression was subsequently evaluated in NOD/SCID or NOG mice. miR-34c-5p agomir treatment significantly decreased mouse weight loss and leucocyte numbers and prolonged the survival of NOD/SCID mice xenografted with KG-1a cells (Fig. 3a–c), which is supported by that the administered agomiR-34c-5p was captured by hCD34+ AML cells in NOD/SCID mice transplanted with KG-1a cells (Supplementary Figure S4a-b). To investigate the sustained effect of miR-34c-5p, KG-1a cells were transduced with lentiviruses that were empty (CTRL) or overexpressed miR-34c-5p (34cOE) and then cultured with puromycin for positive selection of lentivirus-transfected cells until the GFP+ population of the CTRL and 34cOE groups reached more than 90% (Supplementary Figure S4c) and miR-34c-5p was highly overexpressed in the 34cOE group (Supplementary Figure S4d). To investigate whether sustained expression of miR-34c-5p mimic has an influence on the self-renewal capacity of LSCs, the sublethal dose irradiated NOD/SCID mice were transplanted with 8 × 106 lentivirus-transfected KG-1a cells and euthanized at 7 weeks post-transplantation. The percentage of hCD34+CD38− or hCD34+ cells in the BM and spleen were analyzed by flow cytometry. As shown in Fig. 3d, mice injected with 34cOE-KG-1a cells presented significantly decreased hCD34+CD38− and hCD34+ cells in the BM and spleen. Then, 6.3 × 105 hCD34+ AML cells from each recipient mouse were secondarily transplanted into another sublethally irradiated NOD/SCID mouse. At 8 weeks after transplantation, BM cells were harvested from the second transplanted mice, and the percentage of hCD45+CD34+ cells was detected by flow cytometry. Our results showed that the hCD45+CD34+% in the BM was significantly attenuated in the 34cOE group compared with that in the CTRL group (Fig. 3e, Supplementary Figure S4e). The similar results were discovered in NOG mice transplanted lentivirus-transfected KG-1a cells (Supplementary Figure S4f-h). Besides, we found that miR-34c-5p also promoted ex vivo senescence induction and in vivo eradication of LSCs treated with chemotherapeutic drug (Supplementary Figure S5a-e). Cumulatively, these data indicate that increased miR-34c-5p expression effectively inhibits AML development and promotes eradication of AML stem cells in vivo.
Enhancing miR-34c-5p expression in LSCs effectively inhibits AML development and promotes LSCs eradication in vivo. After 200 cGy total-body-irradiation (TBI), NOD/SCID mice were transplanted with 8 × 106 KG-1a cells. Three weeks later, NOD/SCID mice were intravenously injected with 10 nmol miR-34c-5p agomir (34c agomir) (n = 5) or miRNA negative control (NC) (n = 5) two times a week continuously for 2 weeks. The weight (a), leukocyte number (b), and survival rate of mice (c) were recorded once a week post-transplantation. Mice only receiving TBI but no KG-1a cell transplantation (n = 5) were used as controls. The percentage of hCD34+CD38− or hCD34+cells from the BM or spleen (n = 8) at 7-week after the first transplantation (d), and the percentage of hCD45+CD34+ cells from BM cells (n = 7) at 8-week after the second transplantation (e) of CTRL and 34cOE NOD/SCID mice. The data represent the mean ± S.E.M
miR-34c-5p induces senescence in AML CD34+CD38− cells through p53-p21Cip1-CDK/Cyclin or p53-independent CDK/Cyclin pathways
To gain further insight into the mechanisms by which miR-34c-5p induces LSCs senescence, we performed transcriptome sequencing of KG-1a cells transfected with lentivirus-vectors expressing miR-34c-5p (34cOE-KG-1a) or an empty control vector (CTRL-KG-1a). We focused on the senescence-associated genes [34], including senescence regulatory genes p53, p21Cip1 (also named p21), p16, and SIRT1; the senescence-associated secretory phenotype (SASP) genes PAI-1, IL6, IL8, MMP2, and MMP9; the DNA damage response (DDR) genes 53BP1 and H2AX; and other senescence markers such as HMGA1 and DEC1. The expression of all the genes listed above was increased to some degree in 34cOE-KG-1a cells except p16 and SIRT1 (Fig. 4a), a NAD+-dependent deacetylase that represses p53 activity through deacetylation of p53 protein [4]. But, cell cycle-associated genes [4, 35], including CDK6, Cyclin D, Cyclin E2, CDK4, c-Met, and CDK1, had decreased expression in 34cOE-KG-1a cells (Fig. 4a). The selected genes from the transcriptome sequence data set were further validated by qPCR and Western blotting (Figs. 2c, e and 4b, c). Using the miRTarBase (http://www.mirbase.org), an experimentally validated miRNA-target interactions database, we found among the regulatory genes that SIRT1, CDK4, CDK6, Cyclin E2 (CCNE2), MET, E2F3, and Myc were target genes of miR-34c-5p [36,37,38,39,40,41,42,43,44,45,46]. (Supplementary Table S2).
The p53-p21Cip1-CDK/Cyclin and p53-independent CDK/Cyclin pathways are involved in miR-34c-5p-induced senescence of AML stem cells. Transcriptome sequencing (a), qPCR (b), and Western blotting (c) analysis of selected senescence-associated and cell cycle-associated genes in both 34cOE and CTRL-KG-1a cells. Explanatory diagram of signaling pathways involved in miR-34c-5p-induced LSCs senescence (d)
Studies have confirmed that p53 activation triggers the expression of pro-senescence targets such as p21, which can inhibit all CDK/Cyclin complexes and is responsible for G1 cell cycle arrest [47, 48] and cellular senescence [4]. Thus, increased miR-34c-5p could induce LSCs senescence through activation of the p53-p21-CDK/Cyclin axis by repressing its target gene SIRT1. In addition, miR-34c-5p has been demonstrated to block G0/G1 phase progression by directly repressing its targets: Cyclins D, Cyclins E2, CDK4, and CDK6. Moreover, the transcription factors c-Myc, c-Met, and E2F3, inducers of cell cycle progression and proliferation [48, 49], can also be directly repressed by miR-34c-5p. Thus, miR-34c-5p can also induce LSCs senescence in a p53-independent manner (Fig. 4d).
miR-34c-5p directly targets RAB27B to reduce exosome secretion-mediated miR-34c-5p trafficking in AML CD34+CD38−cells
To determine if miR-34c-5p deficiency in AML stem cells is induced by vesicle-shedding, we harvested KG-1a cells and their culture supernatant at different time points and assessed miR-34c-5p expression via qPCR. Our results indicated that miR-34c-5p maintained a low expression in KG-1a cells (Fig. 5a) but an increased level in culture supernatant (Fig. 5b). Further studies showed that GW4869, an inhibitor of extracellular vesicle secretion [50], effectively increased miR-34c-5p in KG-1a cells (Supplementary Figure S6a) but decreased miR-34c-5p in AB, MV, and Exo from KG-1a culture supernatant (Supplementary Figure S6b). Together, these results confirm that extracellular vesicle-mediated miR-34c-5p secretion is an essential cause of miR-34c-5p deficiency in KG-1a cells.
miR-34c-5p directly targets RAB27B to reduce exosome secretion-mediated miR-34c-5p trafficking in AML CD34+CD38− cells. miR-34c-5p expression was analyzed via qPCR in KG-1a cells (a) and their culture supernatant (b) at 0, 12, 24, and 36 h after KG-1a cells were exchanged into fresh medium. Heatmap analysis of differential genes expression (c), qPCR and Western blotting analysis of RAB27B expression (d) between 34cOE and CTRL-KG-1a cells. Schematic of the most likely putative miR-34c-5p binding sites in the RAB27B 3′-UTR (e). A Luciferase reporter assay performed at 48 h after KG-1a cells were cotransfected with wild-type-pMIR-LUC-3’-UTR-RAB27B (WT) or mutated-pMIR-LUC-3’-UTR-RAB27B (MUT) and miR-34c-5p mimic (34c mimic) or miR-negative control (NC) (f). qPCR (g) and Western blotting (h) analysis of RAB27B expression in different treated KG-1a cells: CTRL, 34cOE, RAB-Si (CTRL-KG-1a cells transfected with RAB27B-siRNA2) and 34cOE + RAB-Si (34cOE-KG-1a cells transfected with RAB27B-siRNA2). A schematic diagram of the positive feedback between miR-34c-5p and RAB27B (i). All bars represent the mean ± S.E.M. from three replicate experiments
Another key finding of transcriptome sequencing was that among the genes differentially expressed in 34cOE-KG-1a and CTRL-KG-1a cells, RAB27B, an exosome-release regulatory gene [26], was abundantly expressed in CTRL-KG-1a cells and significantly down-regulated in 34cOE-KG-1a cells (Fig. 5c), suggesting that RAB27B is a promising target gene of miR-34c-5p. Using qPCR and Western blotting, we further confirmed that RAB27B mRNA and protein levels were decreased in 34cOE-KG-1a cells (Fig. 5d). The target-prediction program rna22 [51] revealed three putative miR-34c-5p binding sites in the 3′-UTR of RAB27B mRNA (data not shown). We selected one of the most likely binding sites according to the folding energy and P- value parameters provided by rna22 (Fig. 5e) and performed a luciferase reporter assay. We found that co-transfection of the luciferase reporter and miR-34c-5p mimic into KG-1a cells produced lower luciferase activity than cells co-transfected with miR-NC, but miR-34c-5p mimic did not reduce luciferase activity with the mutated RAB27B 3′-UTR (Fig. 5f), indicating that miR-34c-5p is a specific regulator of RAB27B. To investigate if the miR-34c-5p-RAB27B axis affects exosome secretion-mediated miR-34c-5p trafficking and miR-34c-5p synthesis, we silenced RAB27B with three different siRNAs and detected RAB27B mRNA and miR-34c-5p expression using qPCR. Interestingly, we observed a decreased RAB27B level (Supplementary Figure S6c) but an increased miR-34c-5p level (Supplementary Figure S6d) at 72 h after KG-1a cells were transfected with the different RAB27B-siRNAs. Furthermore, both single transfection of RAB27B-siRNA and co-transfection of both miR-34c and RAB27B-siRNA (34cOE + RAB-Si) significantly decreased RAB27B expression (Fig. 5g, h) but increased miR-34c-5p expression (Supplementary Figure S6e). To explore if the enhanced miR-34c-5p expression in the RAB27B-siRNA group is caused by extracellular vesicle-mediated miR-34c-5p trafficking, we isolated three different extracellular vesicles and detected their miR-34c-5p expression. It was found that 34cOE or RAB27B-siRNA significantly decreased miR-34c-5p levels in Exo but not in AB or MV (Supplementary Figure S6f). These findings reveal that RAB27B promotes Exo shedding in KG-1a cells. Unexpectedly, when we detected the level of the miR-34c-5p precursor (pre-miR-34c), which can directly reflect miR-34c-5p synthesis, no evidence indicated that pre-miR-34c expression was affected by RAB27B-siRNA (Supplementary Figure S6g). These results suggest that the up-regulated miR-34c-5p level induced by RAB27B-siRNA in KG-1a cells is due to inhibition of exosome-directed miR-34c-5p trafficking rather than to increased synthesis of miR-34c-5p. Taken together, these results suggest that positive feedback exists between miR-34c-5p and RAB27B through exosome shedding in AML stem cells (Fig. 5i).
Discussion
Accumulating evidence suggests that miR-34c is aberrantly expressed in a number of solid tumors and functions as a tumor suppressor [38,39,40, 45, 52]. Our study for the first time finds that miR-34c-5p is decreased in AML CD34+CD38− cells compared to normal CD34+CD38− cells and that the lower miR-34c-5p expression level is associated with adverse prognosis and poor therapeutic efficacy in AML patients, suggesting that the miR-34c-5p level in LSCs could be a new and meaningful molecular marker to predict AML prognosis. This is supported by YANG’s report in which miR-34c was also down-regulated in AML cells [53].
We assume that senescence induction programs might provide a therapeutic strategy to eradicate LSCs. Ablain et al. first supported our assumption by finding that retinoic acid and arsenic trioxide cure individuals with acute promyelocytic leukemia (APL) through eradicating tumor cells by activating the p53 and promyelocytic leukemia proteins to induce senescence of cancer cells [54]. However, the critical molecular mechanisms involved in regulating AML, especially non-APL, stem cell senescence are still unknown. Here, we found that increased miR-34c-5p expression is an effective way to induce AML stem cell senescence.
The test of leukemia-initiating activity in vivo via xenotransplantation, especially serial transplantation experiment, is an important way to evaluate LSC function [21]. In this study, we found that increased miR-34c-5p prevented leukemia development and promoted eradication of LSCs in serial transplantation recipient mice. Thus, we demonstrated that increased miR-34c-5p could promote in vivo eradication of AML stem cells.
Our study also reports a new mechanistic link between extracellular vesicle secretion and miR-34c-5p deficiency in AML stem cells. Researchers have found that Exo released from AML cells can suppress residual HSPCs function [55]. Thus, we assume that the Exo of AML stem cells may be implicated in senescence induction of HSPCs in the leukemic hematopoietic microenvironment through exosome-mediated miR-34c-5p trafficking. Our data also demonstrated that miR-34c-5p could further increase its level through positive feedback between increasing miR-34c-5p and decreasing RAB27B levels via RAB27B-induced exosome shedding in LSCs. Altogether, our results show that RAB27B-exosome-directed miR-34c-5p trafficking could serve as a regulatory mechanism of miR-34c-5p deficiency in AML stem cells.
In conclusion, our study demonstrates that increased miR-34c-5p expression promotes eradication of AML stem cells by inducing the senescence through selective RAB27B targeting to inhibit exosome shedding. Thus, this study establishes a new strategy of treating AML patients by targeting LSCs via miRNA-mediated senescence initiation.
References
Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7.
Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–33.
Campisi J, d’Adda, di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–40.
Rufini A, Tucci P, Celardo I, Melino G. Senescence and aging: the critical roles of p53. Oncogene. 2013;32:5129–43.
Wang Y, Liu L, Zhou D. Inhibition of p38 MAPK attenuates ionizing radiation-induced hematopoietic cell senescence and residual bone marrow injury. Radiat Res. 2011;176:743–52.
Wang Y, Kellner J, Liu L, Zhou D. Inhibition of p38 mitogen-activated protein kinase promotes ex vivo hematopoietic stem cell expansion. Stem Cells Dev. 2011;20:1143–52.
Zou J, Zou P, Wang J, Li L, Wang Y. Inhibition of p38 MAPK activity promotes ex vivo expansion of human cord blood hematopoietic stem cells. Ann Hemato. 2012;l91:813–23.
Wang Y, Liu LL, Pazhanisamy SK, Li H, Meng A, Zhou DH. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med. 2010;48:348–56.
Xiao Y, Zou P, Wang J, Song H, Zou J, Liu LL. Lower phosphorylation of p38 MAPK blocks the oxidative stress-induced senescence in myeloid leukemic CD34 (+) CD38 (−) cells. J Huazhong Univ Sci Technol Med Sci. 2012;32:328–33.
Wajapeyee N, Wang SZ, Serra RW, Solomon PD, Nagarajan A, Zhu XC, et al. Senescence induction in human fibroblasts and hematopoietic progenitors by leukemogenic fusion-proteins. Blood. 2010;115:5057–60.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
Schraml E, Grillari J. From cellular senescence to age-associated diseases: the miRNA connection. Longev Health. 2012;1:10.
Hannon GJ, He XY, He L. The guardian’s little helper: microRNAs in the p53 tumor suppressor network. Cancer Res. 2007;67:11099–101.
Kumamoto K, Spillare EA, Fujita K, Horikawa I, Yamashita T, Appella E, et al. Nutlin-3a activates p53 to both down-regulate inhibitor of growth 2 and up-regulate mir-34a, mir-34b, and mir-34c expression, and induce senescence. Cancer Res. 2008;68:3193–203.
Nikitin AY, Corney DC, Flesken-Nikitin A, Godwin AK, Wang W. MicroRNA-34b and microRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 2007;67:8433–8.
Lafferty-Whyte K, Cairney CJ, Jamieson NB, Oien KA, Keith WN. Pathway analysis of senescence-associated miRNA targets reveals common processes to different senescence induction mechanisms. Biochim Biophys Acta. 2009;1792:341–52.
Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17:1086–93.
Sarry JE, Murphy K, Perry R, Sanchez PV, Secreto A, Keefer C, et al. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rgc-deficient mice. J Clin Invest. 2011;121:384–95.
de Leeuw DC, Denkers F, Olthof MC, Rutten AP, Pouwels W, Schuurhuis GJ, et al. Attenuation of microRNA-126 expression that drives CD34+38− stem/progenitor cells in acute myeloid leukemia leads to tumor eradication. Cancer Res. 2014;74:2094–105.
Taussig DC, Miraki-Moud F, Anjos-Afonso F, Pearce DJ, Allen K, Ridler C, et al. Anti-CD38 antibody mediated clearance of human repopulating cells masks the heterogeneity of leukemia-initiating cells. Blood. 2008;112:568–75.
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukemia after transplantation into SCID mice. Nature. 1994;367:645–8.
Koeffler HP, Billing R, Lusis AJ, Sparkes R, Golde DW. An undifferentiated variant derived from the human acute myelogenous leukemia cell line (KG-1). Blood. 1980;56:265–73.
Fuchs D, Daniel V, Sadeghi M, Opelz G, Naujokat C. Salinomycin overcomes ABC transporter-mediated multidrug and apoptosis resistance in human leukemia stem cell-like KG-1a cells. Biochem Biophys Res Commun. 2010;394:1098–104.
Zhang Y, Chen HX, Zhou SY, Wang SX, Zheng K, Xu DD, et al. Sp1 and c-Myc modulate drug resistance of leukemia stem cells by regulating survivin expression through the ERK-MSK MAPK signaling pathway. Mol Cancer. 2015;14:56.
She M, Niu X, Chen X, Li J, Zhou M, He Y, et al. Resistance of leukemic stem-like cells in AML cell line KG1a to natural killer cell-mediated cytotoxicity. Cancer Lett. 2012;318:173–9.
Pfeffer SR. Two Rabs for exosome release. Nat Cell Biol. 2010;12:3–4.
Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–25.
Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T, et al. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982;42:1530–6.
Tashiro S, Asou H, Hamamoto K, Otsuji A, Kita K, Kamada N. Establishment of a human acute myeloid leukemia cell line (Kasumi-1) with 8;21 chromosome translocation. Blood. 1991;77:2031–6.
Peng DY, Song H, Liu LB. Resveratrol-downregulated phosphorylated liver kinase B1 is involved in senescence of acute myeloid leukemia stem cells. J Huazhong Univ Sci Technol Med Sci. 2015;35:485–6.
Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2:20677.
Bautista D, Rodriguez LS, Franco MA, Angel J, Barreto A, et al. Caco-2 cells infected with rotavirus release extracellular vesicles that express markers of apoptotic bodies and exosomes. Cell Stress Chaperon. 2015;20:697–708.
O’Donnell MR, Abboud CN, Altman J, Appelbaum FR, Arber DA, Attar E, et al. Acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:984–1021.
Salama R, Sadaie M, Hoare M, Narita M. Cellular senescence and its effector programs. Genes Dev. 2014;28:99–114.
Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25:5220–7.
Bhatnagar S, Chertkow H, Schipper HM, Yuan Z, Shetty V, Jenkins S, et al. Increased microRNA-34c abundance in Alzheimer’s disease circulating blood plasma. Front Mol Neurosci. 2014;7:2.
Whisnant AW, Bogerd HP, Flores O, Ho P, Powers JG, Sharova N, et al. In-depth analysis of the interaction of HIV-1 with cellular microRNA biogenesis and effector mechanisms. MBio. 2013;4:e000193.
Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;7:193–9.
Suzuki H, Yamamoto E, Nojima M, Kai M, Yamano HO, Yoshikawa K, et al. Methylation-associated silencing of microRNA-34b/c in gastric cancer and its involvement in an epigenetic field defect. Carcinogenesis. 2010;31:2066–73.
Cai KM, Bao XL, Kong XH, Jinag W, Mao MR, Chu JS, et al. Hsa-miR-34c suppresses growth and invasion of human laryngeal carcinoma cells via targeting c-Met. Int J Mol Med. 2010;25:565–71.
Hagman Z, Haflidadottir BS, Ansari M, Persson M, Bjartell A, Edsjö A, et al. The tumour suppressor miR-34c targets MET in prostate cancer cells. Br J Cancer. 2013;109:1271–8.
Lujambio A, Calin GA, Villanueva A, Ropero S, Sánchez-Céspedes M, Blanco D, et al. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Sci USA. 2008;105:13556–61.
Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–41.
Zhou BR, Guo XF, Zhang JA, Xu Y, Li W, Wu D, et al. Elevated miR-34c-5p mediates dermal fibroblast senescence by ultraviolet irradiation. Int J Biol Sci. 2013;9:743–52.
Braun J, Misiak D, Busch B, Krohn K, Hu S, Huttelmaier S. Rapid identification of regulatory microRNAs by miTRAP (miRNA trapping by RNA in vitro affinity purification). Nucleic Acids Res. 2014;42:e66.
Benassi B, Flavin R, Marchionni L, Zanata S, Pan Y, Chowdhury D, et al. MYC is activated by USP2a-mediated modulation of microRNAs in prostate cancer. Cancer Discov. 2012;2:236–47.
Zheng Y, Zhang H, Wang Y, Li X, Lu P, Dong F, et al. Loss of Dnmt3b accelerates MLL-AF9 leukemia progression. Leukemia. 2016;30:2373–84.
Cheng T. Cell cycle inhibitors in normal and tumor stem cells. Oncogene. 2004;23:7256–66.
Rokavec M, Li H, Jiang L, Hermeking H. The p53/miR-34 axis in development and disease. J Mol Cell Biol. 2014;6:214–30.
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–7.
Miranda KC, Huynh T, Tay T, Ang YS, Tam WL, Thomson AM, et al. A pattern-based method for the identification of microRNA-target sites and their corresponding RNA/RNA complexes. Cell. 2006;126:1203–17.
Hagman Z, Larne O, Edsjo A, Bjartell A, Ehrnstrom RA, Ulmert D, et al. miR-34c is downregulated in prostate cancer and exerts tumor suppressive functions. Int J Cancer. 2010;127:2768–76.
Yang DQ, Zhou JD, Wang YX, Deng ZQ, Yang J, Yao DM, et al. Low miR-34c expression is associated with poor outcome in de novo acute myeloid leukemia. Int J Lab Hematol. 2017;39:42–50.
Ablain J, Rice K, Soilihi H, de Reynies A, Minucci S, deThe H. Activation of a promyelocytic leukemia-tumor protein 53 axis underlies acute promyelocytic leukemia cure. Nat Med. 2014;20:167–74.
Hornick NI, Doron B, Abdelhamed S, Huan J, Harrington CA, Shen R, et al. AML suppresses hematopoiesis by releasing exosomes that contain microRNAs targeting c-MYB. Sci Signal. 2016;9:ra88.
Acknowledgements
This project was supported by grants from the National Natural Science Foundation of China (Nos. 81370660, 81770192, 81300412).
Author contributions
DP performed experiments, analyzed the data, and wrote the manuscript; HW, XM, and YC gathered biological samples and provided great help with mouse experiments; YL, YX, and LL conceived the study, analyzed the data and wrote the manuscript; LL and ZH provided critical evaluation of experimental data and the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Peng, D., Wang, H., Li, L. et al. miR-34c-5p promotes eradication of acute myeloid leukemia stem cells by inducing senescence through selective RAB27B targeting to inhibit exosome shedding. Leukemia 32, 1180–1188 (2018). https://doi.org/10.1038/s41375-018-0015-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-018-0015-2
This article is cited by
-
RAB27B-regulated exosomes mediate LSC maintenance via resistance to senescence and crosstalk with the microenvironment
Leukemia (2024)
-
A novel therapeutic strategy: the significance of exosomal miRNAs in acute myeloid leukemia
Medical Oncology (2024)
-
Extracellular vesicle-mediated remodeling of the bone marrow microenvironment in myeloid malignancies
International Journal of Hematology (2023)
-
ACSL1 promotes imatinib-induced chronic myeloid leukemia cell senescence by regulating SIRT1/p53/p21 pathway
Scientific Reports (2022)
-
HCP5, as the sponge of miR-1291, facilitates AML cell proliferation and restrains apoptosis via increasing PIK3R5 expression
Human Genomics (2021)