Abstract
Chimeric antigen receptor (CAR) T cells targeting CD22 (CD22-CAR) provide a therapeutic option for patients with CD22+ malignancies with progression after CD19-directed therapies. Using on-site, automated, closed-loop manufacturing, we conducted parallel Phase 1b clinical trials investigating a humanized CD22-CAR with 41BB costimulatory domain in children and adults with heavily treated, relapsed/refractory (r/r) B-ALL. Of 19 patients enrolled, 18 had successful CD22-CAR manufacturing, and 16 patients were infused. High grade (3–4) cytokine release syndrome (CRS) and immune effector-cell-associated neurotoxicity syndrome (ICANS) each occurred in only one patient; however, three patients experienced immune-effector-cell-associated hemophagocytic lymphohistiocytosis-like syndrome (IEC-HS). Twelve of 16 patients (75%) achieved CR with an overall 56% MRD-negative CR rate. Duration of response was overall limited (median 77 days), and CD22 expression was downregulated in 4/12 (33%) available samples at relapse. In summary, we demonstrate that closed-loop manufacturing of CD22-CAR T cells is feasible and is associated with a favorable safety profile and high CR rates in pediatric and adult r/r B-ALL, a cohort with limited CD22-CAR reporting.
Similar content being viewed by others
Introduction
CD19 surface downregulation has emerged as a key resistance mechanism following CD19-targeted therapies in pediatric and adult B-acute lymphoblastic leukemia (B-ALL) [1, 2]. CD22, a B-cell-specific surface sialoglycoprotein commonly found on B-ALL [1, 3,4,5], is a promising CAR target in lymphoid malignancies. A previous phase I study of CD22-specific CAR T cells in pediatric and young adult B-ALL conducted at the National Cancer Institute (NCI) resulted in 40 of 57 (70%) patients achieving a morphologic complete remission (CR) rate, with 35 patients (87.5%) achieving undetectable minimal residual disease (MRD) by flow cytometry [6]. The NCI study was the first CAR T-cell trial targeting an alternative antigen to CD19 that accomplished comparable outcomes in pediatric B-ALL, with lower rates of neurotoxicity and a higher rate of immune effector-cell-associated hemophagocytic lymphohistiocytosis-like syndrome (IEC-HS) relative to published CD19-CAR T-cell outcomes [7, 8]. Manufacturing modifications with CD4/CD8 positive selection resulted in increased toxicity despite conserved CAR T-cell dosing. The NCI single-center trial was limited to children and young adults aged 4–30 years.
To establish both the feasibility of manufacturing CD22-CARs using the NCI CD22-CAR vector with an alternative automated closed-loop manufacturing approach and the safety of this CAR in a variety of lymphoid malignancy populations, we conducted parallel Phase 1b clinical trials in pediatric and adult cohorts of patients with either relapsed/refractory (r/r) B-ALL or B-cell non-Hodgkin lymphoma. We now report on the outcomes of the r/r pediatric and adult B-ALL cohorts treated on these trials.
Methods
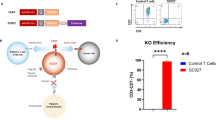
We conducted parallel Phase Ib clinical trials (NCT04088864: “CD22-CARs in Children and Young Adults with B Cell Malignancies” and NCT04088890: “Autologous CD22 CARs in Adults w/ Recurrent or Refractory B Cell Malignancies”) at Stanford University. Trials were designed to test manufacturing feasibility and safety of CD22-CARs engineered with a fully humanized CD22 single chain variable fragment, a 4-1BB costimulatory domain, and a CD3ζ activation domain, for use in the treatment of children and adults with r/r B-ALL. Eligible patients were required to have B-ALL either refractory to two lines of therapy or to have relapsed after achieving prior CR. Patients were required to have measurable disease at time of enrollment; patients with MRD-only disease were eligible if MRD testing revealed detectable disease on two samples obtained at least 14 days apart. Expression of CD22 on leukemic blasts by flow cytometry or immunohistochemistry was required without specification of minimum expression threshold. Patients who had undergone prior CD19-CAR therapy were eligible if at least 30 days had elapsed and <5% of circulating CD3+ cells expressed previous CAR.
Enrolled patients underwent T-cell apheresis and CD22-CAR was manufactured using an automated closed CliniMACS Prodigy lentiviral transduction system (Miltenyi Biotec, Bergisch Gladbach, Germany) [9] previously used to successfully create single and dual-targeted CARs [10, 11] at Stanford University or Miltenyi Biotec. Lymphodepletion consisted of fludarabine (30 mg/m2/day IV on Days −5, −4, −3) and cyclophosphamide (500 mg/m2/day on Days −5, −4, −3) followed by fresh or cryopreserved CD22-CAR infusion. CD22-CAR was dosed at 1 × 106 cells/kg (±20%) as this same construct with alternative manufacturing was previously tested and deemed safe at 1 × 106 cells/kg in children [12]. CAR dose de-escalation to 3 × 105 cells/kg was embedded in both trials if a DLT was observed. Peripheral blood samples were collected and processed for plasma, and peripheral blood mononuclear cells were viably cryopreserved.
Manufacturing feasibility was defined by the rate of successful manufacture of the CD22-CARs produced using the ClinicMACS Prodigy system to satisfy the targeted dose level and meet required release specifications. A manufacturing success rate of >50% was considered feasible. Expected CAR side effects were managed according to institutional standards. Safety was assessed by the incidence and severity of DLTs, defined as: grade 4 CRS of any duration, grade 3 CRS lasting >7 days, grade 4 immune effector-cell-associated neurotoxicity syndrome (ICANS) of any duration, grade 3 ICANS lasting >72 h, infusion reaction ≥ grade 2 lasting >24 h, or any other grade ≥3 non-hematologic toxicity with the exceptions of expected laboratory derangements and expected, short-lived infections. An overall DLT rate of <30% was considered acceptable. Adverse events were graded according to Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. American Society for Transplantation and Cellular Therapy (ASTCT) grading systems [13] were used to define CRS and ICANS; IEC-HS was retroactively assessed per recently established criteria [14]. A CR was defined as the absence of morphologic disease (<5% bone marrow blasts and/or absence of extramedullary leukemia) at day 28 following CAR infusion and MRD was defined as absence of detectable disease by flow cytometry (threshold of 10−4). Next-generation sequencing (NGS) of ALL samples was also performed using the Adaptive clonoSEQ® assay.
Descriptive statistics were used to summarize patient and disease characteristics. Unpaired groups were compared and two-tailed p values were computed using Mann–Whitney or Wilcoxon rank-sum tests. Progression-free (PFS) and overall survival (OS) were estimated using Kaplan–Meier methods; patients were censored at time of last follow-up or if they initiated alternative treatments before experiencing disease progression. CD22-CAR expansion and persistence were assessed by established qPCR assays [6] and flow cytometry [15]. Figures were produced using GraphPad Prism® and R statistical software.
Both clinical trials were approved by the Stanford University Institutional Review Board and patients provided written informed consent to participate in accordance with the Declaration of Helsinki. All authors had access to the primary clinical trial data and contributed to analysis.
Results
Manufacturing feasibility and patient characteristics
As shown in the CONSORT Diagram (Fig. 1), 19 patients with r/r B-ALL were enrolled between 2019–2021. Two enrolled patients were not infused with CD22-CARs due to clinical factors [graft-versus-host disease (n = 1), disease progression (n = 1)] and one heavily pretreated pediatric patient was not infused due to poor ex vivo expansion and manufacturing failure. The latter patient also had high circulating blasts at time of collection, which may have impacted ex vivo T-cell proliferation. Manufacturing was successful for 18 patients (95%), with target dose (1 × 106 cells/kg) met for all products. The median time from manufacturing initiation to product release was 8 days (range 7–13 days). Pre-infusion, the CD22-CAR product was CD4-predominant but became CD8-predominant after infusion (Supplementary Fig. 1).
Eight adult and 8 pediatric patients received CD22-CARs. Among infused patients (Table 1), median age was 23 years (range, 2–62) and median number of prior therapies was 6 (range, 3–8). Five patients (31%) had extramedullary disease (EMD), of which three had central nervous system (CNS) involvement at time of CD22-CAR treatment (Supplementary Table 1A, B). Eight patients (50%) had previously documented EMD, of which five (31%) had previously documented CNS involvement during their disease course.
Thirteen patients (72%) had prior CD19-targeting agents (11 blinatumomab; 9 CD19-CAR T cells). Four of six pediatric patients responded to pre-22CAR blinatumomab, whereas 4/6 adult patients were refractory to pre-22CAR blinatumomab. Twelve patients (75%) had undergone prior allogeneic hematopoietic cell transplantation (alloHCT), and four (25%) had previously received inotuzumab ozogamicin. The median time from prior alloHCT to CD22-CAR infusion was 21 months (range 7–37), with no differences between pediatric and adult cohorts. All patients expressed CD22 by flow cytometry (median, >90%; range, 44–100%), while 6 (38%) were CD19-negative/low.
Safety
Thirteen patients (81%) experienced CRS (grade 1–2, n = 12; grade 3, n = 1) occurring a median of 8 days (range, 1–11) following CD22-CAR T-cell infusion (Table 2). ICANS occurred in two patients (13%; grade 1, n = 1; grade 4, n = 1). The one case of Grade 4 ICANS occurred within the context of VRE bacteremia and presumed aspergillosis. Three patients (19%) developed IEC-HS (Days +14, +17, and +25), with two experiencing grade 5 infections concomitantly with IEC-HS, accounting for two total DLTs. Corticosteroids, tocilizumab, and anakinra (Supplementary Table 1A, B) were used for toxicity management according to institutional guidelines with physician discretion. No anti-inflammatory agents were given preemptively for CRS. Due to evolving management practices for CRS, ICANS, and IEC-HS, formal toxicity algorithms are not included in this manuscript.
As the feasibility and safety endpoints were both met, 1 × 106 CD22-CAR T cells/kg was deemed a safe dose for future study in pediatric and adult B-ALL.
Efficacy
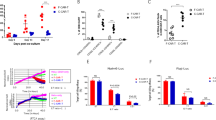
Twelve of 16 patients (75%) achieved CR, of whom nine were MRD-negative by flow cytometry (Table 2, Fig. 2A). All eight adult patients (100%) achieved CR [5 MRD-negative by flow cytometry (62.5%)], while 4/8 pediatric patients (50%) achieved CR, all of whom were MRD-negative by flow cytometry (Supplementary Table 1A, B). Five of the nine flow-MRD-negative patients were also MRD-negative by NGS (two pediatric, three adult). The median follow-up for all treated patients from time of infusion was 523 days. Nine of the 12 patients (75%) achieving CR ultimately relapsed, of whom seven were adults. Achieving MRD-negativity by NGS did not protect against relapse, as four of five patients who achieved NGS-negative CR ultimately relapsed and the one patient who did not relapse underwent alloHCT in CR. Among those who relapsed, median duration of response was 77 days (range, 27–523), without significant differences between adults and children (median 105 vs. 35 days, p = 0.11). Ten (63%) patients died (six from relapse after CR, or nonresponse to CD22-CAR; two from infection alone; two from treatment-related toxicity in the context of infection). There were no substantial differences in toxicities or in toxicity management between pediatric and adult cohorts (Supplementary Table 1A, B). The median PFS and OS were 77 days (95% CI, 31–119) and 523 days (95% CI, 108-not reached), respectively (Fig. 2B, C). The sole patient who underwent alloHCT while in post-CAR CR remains alive and disease-free.
A Swimmer plot showing the duration of response and survival post-infusion for all treated patients (N = 16). CR complete response; PR partial response; SD stable disease; PD progressive disease; MRD minimal residual disease Kaplan–Meier estimate of overall survival in all patients. B Kaplan–Meier estimate of progression-free survival in all patients. C Kaplan–Meier estimate of overall survival in all patients.
CD22-CAR T-cell kinetics
Median peak CAR T-cell expansion occurred on Day +14 (Fig. 3A). Higher peak CAR T-cell levels associated with a longer time to progression (Supplementary Fig. 2A), presence of IEC-HS (Supplementary Fig. 2B), a trend towards longer response duration, and a trend towards higher grade CRS (Supplementary Fig. 2C). Greater cumulative levels of CAR T cells (area under the curve) in the first month following infusion also showed a trend towards longer response duration (Supplementary Fig. 3A), but not with CRS grade or presence/absence of IEC-HS (Supplementary Fig. 3B, C). At relapse, flow cytometry revealed downregulated CD22 expression in 4 of 12 (33%) available samples (Supplementary Table 2), and two patients (17%) lost all CD22 expression. Five patients had CAR T-cell persistence beyond Day +28; of these, 2 had CAR T cells detectable at 6 months post-infusion (Supplementary Fig. 4). Six patients had detectable CD22 CAR T cells at time of relapse. Of these patients with circulating CAR T cells, two patients relapsed with CD22-downregulated disease, three patients relapsed with CD22-positive disease, and one patient did not have disease CD22 expression available. Baseline features of T-cell exhaustion (Supplementary Fig. 5A, B), phenotype (Supplementary Fig. 6) and activation marker expression (Supplementary Fig. 7) were characterized and stratified by clinical response. Higher levels of IL-2 production by CD22 CAR T cells exposed to CD22-expressing ALL cell lines in vitro was significantly associated with clinical response to therapy (Supplementary Fig. 8). These analyses were limited by small sample size and warrant further study.
A CD22-CAR cellular kinetic profile. Median values and interquartile ranges (Q1 and Q3 by the shaded regions) are shown. B (G) In vivo CAR expansion at peak is CD8+ CAR+ predominant (median CD8+ 98 vs CD4+ 9). C Association between peak CD22-CAR expansion and time until progression (≤3 months vs >3 months).
Discussion
Commercial CAR T-cell products for B-ALL currently target CD19 [16, 17]. CAR T cells targeting alternative antigens are urgently needed for patients with CD19 downregulation and those lacking adequate response to CD19-specific therapies. Single-institutional data (N = 58) limited to children and young adults ≤ age 30 was paradigmatic in establishing high efficacy (CR 70%) and tolerability of CD22-specific CAR T cells [6]. Our Phase Ib parallel clinical trials conducted in both children and adults adds to this literature by (1) demonstrating that this same CD22-CAR vector can be effectively used in a closed system platform to manufacture CD22-CAR T cells with a consistent 7–10 day manufacturing turnaround time; (2) further extending pediatric CD22-CAR T-cell reporting beyond a single institution; (3) expanding this experience to adults with r/r B-ALL; and (4) establishing a safe Phase 2 dose of this therapeutic when produced using the ClinicMACS Prodigy system in children and adults with r/r B-ALL.
Among the 16 B-ALL patients treated on our trials, we observed very low rates of high-grade CRS/ICANS (ASTCT criteria) with only one patient (6%) experiencing ≥ grade 3 CRS and one patient experiencing grade ≥ 3 ICANS (6%). Relative to tisagenlecleucel and specifically brexucabtagene autoleucel, the respective commercial CD19-targeting CAR products for pediatric and adult r/r B-ALL, these rates of severe toxicity appear particularly low [8, 18]. Interestingly, three patients (19%) developed IEC-HS, which is lower than the rates of hemophagocytic lymphohistiocytosis (HLH) seen in the NCI study [6], but is significant and further suggests that regardless of manufacturing platform, either the CD22 target or this CAR construct may be associated with greater IEC-HS than CD19-targeting CARs. This is particularly relevant in the context of overall low rates of severe CRS seen on our trials. Concomitant infection may impact CAR-related inflammation, as the two patients who died from treatment-related toxicity had simultaneous bacteremia (with coagulase-negative staphylococcus and vancomycin-resistant enterococcus, respectively).
Patients treated on these CD22-CAR trials were very heavily pretreated, with a median of 6 prior lines of therapy. Despite this, 75% of patients achieved CR with an overall MRD-negative CR rate of 56%. However, the durability of response was short, suggesting that when administered to highly pretreated patients, CD22-CARs may be best used as a bridge to hematopoietic cell transplantation (HCT). Though only one patient underwent consolidative HCT (and remains in ongoing remission), the low rate of post-CAR consolidative HCT is likely explained by prior HCT in most patients (75%). Four patients had prior exposure to inotuzumab ozogamicin. Unlike the NCI report, we did not find an association between prior inotuzumab and response rate or response duration. We did, however, find that one-third of the patients had ALL relapse with CD22 down-regulation. In addition to testing bivalent [19] or bicistronic [20] CARs, one method to avoid relapse due to antigen escape may be co-infusion of CD19 and CD22-targeting CAR products, as has been recently shown to be quite successful in both pediatric [21, 22] and adult ALL cohorts.
We were unable to identify clear predictors of toxicity or response among our patient cohort, although patients with marked expansion appeared to have improved efficacy at the cost of greater toxicity. Single-center trials of CAR therapies have been limited by smaller patient cohorts yet performing multicenter investigator-initiated CAR trials (IITs) has been hampered by numerous barriers to adopting a decentralized CAR T manufacturing model. In our trials, we were able to demonstrate feasibility of a closed manufacturing platform, which achieved 7-day manufacturing timelines in most patients without safety or toxicity concerns. This platform may facilitate enhanced CAR T-cell access [23], point-of-care manufacturing [24, 25], and scalability [26], and may facilitate future multicenter CAR T-cell IITs. Rather than conducting one clinical trial across the age spectrum, due to institutional logistics and constraints, we conducted these nearly identical clinical trials in parallel with shared regulatory and clinical trials resources. Given the unique epidemiology of ALL, we believe there is benefit in conducting CAR studies and commercialization across the age continuum, rather than distinctly in children or adults.
In conclusion, we highlight tolerability and efficacy in establishing CR following CD22-CAR T cells using closed manufacturing across children and adults with B-ALL. Heavily pretreated r/r B-ALL (and specifically CD19-negative/low disease) has poor salvageability and development of alternative agents remains a high priority.
Data availability
For original deidentified patient data or the clinical trial protocol please contact LM (lmuffly@stanford.edu).
References
Boyd SD, Natkunam Y, Allen JR, Warnke RA. Selective immunophenotyping for diagnosis of B-cell neoplasms: immunohistochemistry and flow cytometry strategies and results. Appl Immunohistochem Mol Morphol. 2013;21:116–31.
Asnani M, Hayer KE, Naqvi AS, Zheng S, Yang SY, Oldridge D, et al. Retention of CD19 intron 2 contributes to CART-19 resistance in leukemias with subclonal frameshift mutations in CD19. Leukemia. 2020;34:1202–7.
Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–74.
Shah NN, Stevenson MS, Yuan CM, Richards K, Delbrook C, Kreitman RJ, et al. Characterization of CD22 expression in acute lymphoblastic leukemia. Pediatr Blood Cancer. 2015;62:964–9.
Majzner RG, Rietberg SP, Sotillo E, Dong R, Vachharajani VT, Labanieh L, et al. Tuning the antigen density requirement for CAR T-cell activity. Cancer Discov. 2020;10:702–23.
Shah NN, Highfill SL, Shalabi H, Yates B, Jin J, Wolters PL, et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a Phase I Anti-CD22 CAR T-cell trial. J Clin Oncol. 2020;38:1938–50.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T-cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48.
Mock U, Nickolay L, Philip B, Cheung GW, Zhan H, Johnston ICD, et al. Automated manufacturing of chimeric antigen receptor T cells for adoptive immunotherapy using CliniMACS Prodigy. Cytotherapy. 2016;18:1002–11.
Zhu F, Shah N, Xu H, Schneider D, Orentas R, Dropulic B, et al. Closed-system manufacturing of CD19 and dual-targeted CD20/19 chimeric antigen receptor T cells using the CliniMACS Prodigy device at an academic medical center. Cytotherapy. 2018;20:394–406.
Shah NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a Phase 1 dose escalation and expansion trial. Nat Med. 2020;26:1569–75.
Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–28.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–38.
Hines MR, Knight TE, McNerney KO, Leick MB, Jain T, Ahmed S, et al. Immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome. Transpl Cell Ther. 2023;29:438.e1–438.e16.
Baird JH, Frank MJ, Craig J, Patel S, Spiegel JY, Sahaf B, et al. CD22-directed CAR T-cell therapy induces complete remissions in CD19-directed CAR-refractory large B-cell lymphoma. Blood. 2021;137:2321–5.
O’Leary MC, Lu X, Huang Y, Lin X, Mahmood I, Przepiorka D, et al. FDA Approval Summary: tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin Cancer Res. 2019;25:1142–6.
Bouchkouj N, Lin X, Wang X, Przepiorka D, Xu Z, Purohit-Sheth T, et al. FDA Approval Summary: brexucabtagene autoleucel for treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Oncologist. 2022;27:892–9.
Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398:491–502.
Spiegel JY, Patel S, Muffly L, Hossain NM, Oak J, Baird JH, et al. CAR s with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27:1419–31.
Shalabi H, Qin H, Su A, Yates B, Wolters PL, Steinberg SM, et al. CD19/22 CAR T cells in children and young adults with B-ALL: phase 1 results and development of a novel bicistronic CAR. Blood. 2022;140:451–63.
Wang T, Tang Y, Cai J, Wan X, Hu S, Lu X, et al. Coadministration of CD19- and CD22-directed chimeric antigen receptor T-cell therapy in childhood B-cell acute lymphoblastic leukemia: a single-arm, multicenter, phase II trial. J Clin Oncol. 2023;41:1670–83.
Pan J, Tang K, Luo Y, Seery S, Tan Y, Deng B, et al. Sequential CD19 and CD22 chimeric antigen receptor T-cell therapy for childhood refractory or relapsed B-cell acute lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2023;24:1229–41.
Arnaudo L. On CAR-Ts, decentralized in-house models, and the hospital exception. Routes for sustainable access to innovative therapies. J Law Biosci. 2022;9:lsac027.
Fried S, Shouval R, Varda-Bloom N, Besser MJ, Yerushalmi R, Shem-Tov N, et al. Point-of-care CAR T-cell therapy as salvage strategy for out-of-specification tisagenlecleucel. Leuk Lymphoma. 2022;63:3385–93.
de Macedo Abdo L, Barros LRC, Saldanha Viegas M, Vieira Codeço Marques L, de Sousa Ferreira P, Chicaybam L, et al. Development of CAR- therapy for B-ALL using a point-of-care approach. Oncoimmunology. 2020;9:1752592.
Abou-El-Enein M, Elsallab M, Feldman SA, Fesnak AD, Heslop HE, Marks P, et al. Scalable manufacturing of CAR T cells for cancer immunotherapy. Blood Cancer Discov. 2021;2:408–22.
Acknowledgements
The authors would like to acknowledge Ted Gooley, Ph.D. of the Fred Hutchinson Cancer Research Center for his assistance with statistical analysis as well as Nirali Shah M.D., M.H.Sc. and the National Cancer Institute for providing additional viral vector to allow more patients to enroll on the CD22-CAR studies at Stanford.
Author information
Authors and Affiliations
Contributions
All authors were responsible for data collection and analysis, revision of the manuscript, and final approval. NJ, LS, LM, and SR were primarily responsible for manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schultz, L.M., Jeyakumar, N., Kramer, A.M. et al. CD22 CAR T cells demonstrate high response rates and safety in pediatric and adult B-ALL: Phase 1b results. Leukemia 38, 963–968 (2024). https://doi.org/10.1038/s41375-024-02220-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-024-02220-y