Abstract
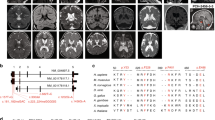
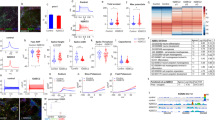
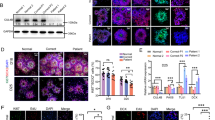
A homozygous mutation in the inositol monophosphatase 1 (IMPA1) gene was recently identified in nine individuals with severe intellectual disability (ID) and disruptive behavior. These individuals belong to the same family from Northeastern Brazil, which has 28 consanguineous marriages and 59 genotyped family members. IMPA1 is responsible for the generation of free inositol from de novo biosynthesis and recycling from inositol polyphosphates and participates in the phosphatidylinositol signaling pathway. To understand the role of IMPA1 deficiency in ID, we generated induced pluripotent stem cells (iPSCs) from patients and neurotypical controls and differentiated these into hippocampal dentate gyrus-like neurons and astrocytes. IMPA1-deficient neuronal progenitor cells (NPCs) revealed substantial deficits in proliferation and neurogenic potential. At low passage NPCs (P1 to P3), we observed cell cycle arrest, apoptosis, progressive change to a glial morphology and reduction in neuronal differentiation. These observations were validated by rescuing the phenotype with myo-inositol supplemented media during differentiation of patient-derived iPSCs into neurons and by the reduction of neurogenic potential in control NPCs-expressing shIMPA1. Transcriptome analysis showed that NPCs and neurons derived from ID patients have extensive deregulation of gene expression affecting pathways necessary for neurogenesis and upregulation of gliogenic genes. IMPA1 deficiency did not affect cell cycle progression or survival in iPSCs and glial progenitor cells or astrocyte differentiation. Therefore, this study shows that the IMPA1 mutation specifically affects NPC survival and neuronal differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association: Washington, DC, USA, 2000.
Durkin M. The epidemiology of developmental disabilities in low income countries. Ment Retard Dev Disabil Res Rev. 2002;8:206–11.
Emerson E. Poverty and people with intellectual disabilities. Ment Retard Dev Disabil Res Rev. 2007;13:107–13.
Durkin MS, Hasan ZM, Hasan KZ. Prevalence and correlates of mental retardation among children in Karachi, Pakistan. Am J Epidemiol. 1998;147:281–8.
Leonard H, Wen X. The epidemiology of mental retardation: challenges and opportunities in the new millennium. Ment Retard Dev Disabil Res Rev. 2002;8:117–34.
Vissers LE, Gilissen C, Veltman JA. Genetic studies in intellectual disability and related disorders. Nat Rev Genet. 2016;17:9–18.
Ropers HH. Genetics of intellectual disability. Curr Opin Genet Dev. 2008;18:241–50.
Musante L, Ropers HH. Genetics of recessive cognitive disorders. Trends Genet 2014;30:32–9.
Figueiredo T, Melo US, Pessoa ALS, Nobrega PR, Kitajima JP, Rusch H, et al. A homozygous loss-of-function mutation in inositol monophosphatase 1 (IMPA1) causes severe intellectual disability. Mol Psychiatry 2016;21:1125–9.
Harripaul R, Vasli N, Mikhailov A, Rafiq MA, Mittal K, Windpassinger C, et al. Mapping autosomal recessive intellectual disability: combined microarray and exome sequencing identifies 26 novel candidate genes in 192 consanguineous families. Mol Psychiatry 2017;23:973–84.
Resnick AC, Saiardi A. Inositol polyphosphate multikinase: metabolic architect of nuclear inositides. Front Biosci 2008;13:856–66.
Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29.
Hakim S, Bertucci MC, Conduit SE, Vuong DL, Mitchell CA. Inositol polyphosphate phosphatases in human disease. Curr Top Microbiol Immunol. 2012;362:247–314.
Ohnishi T, Ohba H, Seo KC, Im J, Sato Y, Iwayama Y, et al. Spatial expression patterns and biochemical properties distinguish a second myo-inositol monophosphatase IMPA2 from IMPA1. J Biol Chem. 2007;282:637–46.
Berridge MJ, Downes CP, Hanley MR. Neural and developmental actions of lithium: a unifying hypothesis. Cell 1989;59:411–9.
Atack JR, Broughton HB, Pollack SJ. Inositol monophosphatase-a putative target for Li+ in the treatment of bipolar disorder. Trends Neurosci 1995;18:343–9.
Sade Y, Toker L, Kara NZ, Einat H, Rapoport S, Moechars D, et al. IP3 accumulation and/or inositol depletion: two downstream lithium’s effects that may mediate its behavioral and cellular changes. Transl Psychiatry 2016;6:e968.
Saiardi A, Mudge AW. Lithium and fluoxetine regulate the rate of phosphoinositides synthesis in neurons: a new view of their mechanisms of action in bipolar disorder. Transl Psychriatry. 2018;8:175.
Cryns K, Shamir A, Van Acker N, Levi I, Daneels G, Goris I, et al. IMPA1 is essential for embryonic development and lithium-like pilocarpine sensitivity. Neuropsychopharmacology 2008;33:674–84.
Fisher SK, Novak JE, Agranoff BW. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem. 2012;82:736–54.
Ohnishi T, Murata T, Watanabe A, Hida A, Ohba H, Iwayama Y, et al. Defective craniofacial development and brain function in a mouse model for depletion of intracellular inositol synthesis. J Biol Chem. 2014;289:10785–96.
Ropers HH. Genetics of early onset cognitive impairment. Annu Rev Genomics Hum Genet 2010;11:161–87.
Okita K, Yamakawa T, Matsumura Y, Sato Y, Amano N, Watanabe A, et al. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells 2013;31:458–66.
Jehee FS, Takamori JT, Medeiros PF, Pordeus AC, Latini FR, Bertola DR, et al. Using a combination of MLPA kits to detect chromosomal imbalance in patients with multiple congenital anomalies and mental retardation is a valuable choice for developing countries. Eur J Med Genet 2001;54:425–32.
Yu DX, Di Giorgio FP, Yao J, Marchetto MC, Brennand K, Wright R, et al. Modeling hippocampal neurogenesis using human pluripotent stem cells. Stem Cell Rep. 2014;2:295–310.
Marchetto MC, Belinson H, Tian Y, Freitas BC, Fu C, Vadodaria K, et al. Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol Psychiatry 2017;22:820–35.
Santos R, Vadodaria KC, Jaeger BN, Mei A, Lefcochilos-Fogelquist S, Mendes APD, et al. Differentiation of inflammation-responsive astrocytes from glial progenitors generated from human induced pluripotent stem cells. Stem Cell Rep. 2017;8:1757–69.
Stern S, Santos R, Marchetto MC, Mendes APD, Rouleau GA, Biesmans S, et al. Neurons derived from patients with bipolar disorder divide into intrinsically different sub-populations of neurons, predicting the patients’ responsiveness to lithium. Mol Psychiatry 2018;23:1453–65.
Freire-Maia N. Inbreeding in Brazil. Am J Hum Genet. 1957;9:284–98.
Freire-Maia N. Genetic effects in Brazilian populations due to consanguineous marriages. Am J Med Genet. 1989;35:115–7.
Fonseca LG, Freire-Maia N. Further data on inbreeding levels in Brazilian populations. Soc Biol 1970;17:324–8.
Weller M, Tanieri M, Pereira JC, Almeida ES, Kok F, Santos S. Consanguineous unions and the burden of disability: a population-based study in communities of Northeastern Brazil. Am J Hum Biol. 2012;24:835–40.
Meikrantz W, Schlegel R. Apoptosis and the cell cycle. J Cell Biochem. 1995;58:160–74.
Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004;165:347–56.
Sakamaki K, Satou Y. Caspases: evolutionary aspects of their functions in vertebrates. J Fish Biol. 2009;74:727–53.
Engeland K. Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM. Cell Death Differ. 2018;25:114–32.
Doze VA, Perez DM. G-protein-coupled receptors in adult neurogenesis. Pharm Rev 2012;64:645–75.
Varella-Nallar L, Inestrosa NC. Wnt signaling in the regulation of adult hippocampal neurogenesis. Front Cell Neurosci. 2013;7:100.
Ishibashi M, Moriyoshi K, Sasai Y, Shiota K, Nakanishi S, Kageyama R. Persistent expression of helix-loop-helix factor HES-1 prevents mammalian neural differentiation in the central nervous system. EMBO J 1994;13:1799–805.
Hirata H, Ohtsuka T, Bessho Y, Kageyama R. Generation of structurally and functionally distinct factors from the basic helix-loop-helix gene Hes3 by alternative first exons. J Biol Chem 2000;275:19083–9.
Ohtsuka T, Sakamoto M, Guillemot F, Kageyama R. Roles of the basic helix-loop-helix genes Hes1 and Hes5 in expansion of neural stem cells of the developing brain. J Biol Chem 2001;276:30467–74.
Toker L, Bersudsky Y, Plaschkes I, Chalifa-Caspi V, Berry GT, Buccafusca R, et al. Inositol related gene knockouts mimic lithium’s effect on mitochondrial function. Neuropsychopharmacology 2014;39:319–28.
Wiel C, Lallet-Daher H, Gitenay D, Gras B, Le Calvé B, Augert A, et al. Endoplasmic reticulum calcium release through ITPR2 channels leads to mitochondrial calcium accumulation and senescence. Nat Commun 2014;5:3792.
Llorente-Folch I, Rueda CB, Pardo B, Szabadkai G, Duchen MR, Satrustegui J. The regulation of neuronal mitochondrial metabolism by calcium. J Physiol 2015;593:3447–62.
Marchi S, Bittremieux M, Missiroli S, Morganti C, Patergnani S, Sbano L, et al. Endoplasmic reticulum-mitochondria communication through Ca2+ signaling: the importance of mitochondria-associated membranes (MAMs). Adv Exp Med Biol 2017;997:49–67.
Acknowledgements
This manuscript is dedicated to the family involved in this study. This study was financially supported by the CEPID-FAPESP (2013/08028-1), INCT/FAPESP (2014/50931-3) and FAPESP (process number: 2019/18469-1, 2017/19877-0, and 2016/09618-5). This work was supported in part by the National Cancer Institute (grant no. P30 CA014195) and the National Institutes of Health (grant no. R01AG05651) and by the National Cooperative Reprogrammed Cell Research Groups (NCRCRG) (grant no. U19 MH106434). The Gage laboratory is supported in part by the Leona M. and Harry B. Helmsley Charitable Trust grant no. 2017-PG-MED001, the JPB Foundation, Annette C. Merle-Smith, and the Robert and Mary Jane Engman Foundation. We also thank the Salk core facilities. This work was supported in part by the Next Generation Sequencing Core (NGS) Facility of the Salk Institute with funding from NIH-NCI CCSG: P30 014195, the Chapman Foundation and the Helmsley Charitable Trust.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Figueiredo, T., Mendes, A.P.D., Moreira, D.P. et al. Inositol monophosphatase 1 (IMPA1) mutation in intellectual disability patients impairs neurogenesis but not gliogenesis. Mol Psychiatry 26, 3558–3571 (2021). https://doi.org/10.1038/s41380-020-00862-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-00862-9