Abstract
Bipolar disorder is an often-severe mental health condition characterized by alternation between extreme mood states of mania and depression. Despite strong heritability and the recent identification of 64 common variant risk loci of small effect, pathophysiological mechanisms remain unknown. Here, we analyzed genome sequences from 41 multiply-affected pedigrees and identified variants in 741 genes with nominally significant linkage or association with bipolar disorder. These 741 genes overlapped known risk genes for neurodevelopmental disorders and clustered within gene networks enriched for synaptic and nuclear functions. The top variant in this analysis – prioritized by statistical association, predicted deleteriousness, and network centrality – was a missense variant in the gene encoding D-amino acid oxidase (DAOG131V). Heterologous expression of DAOG131V in human cells resulted in decreased DAO protein abundance and enzymatic activity. In a knock-in mouse model of DAOG131, DaoG130V/+, we similarly found decreased DAO protein abundance in hindbrain regions, as well as enhanced stress susceptibility and blunted behavioral responses to pharmacological inhibition of N-methyl-D-aspartate receptors (NMDARs). RNA sequencing of cerebellar tissue revealed that DaoG130V resulted in decreased expression of two gene networks that are enriched for synaptic functions and for genes expressed, respectively, in Purkinje neurons or granule neurons. These gene networks were also down-regulated in the cerebellum of patients with bipolar disorder compared to healthy controls and were enriched for additional rare variants associated with bipolar disorder risk. These findings implicate dysregulation of NMDAR signaling and of gene expression in cerebellar neurons in bipolar disorder pathophysiology and provide insight into its genetic architecture.
Similar content being viewed by others
Introduction
Bipolar disorder is a severe mental health condition affecting 1–2% of the population and characterized by alternation between extreme mood states of mania and depression. Bipolar disorder is strongly familial, with 8 to 10-fold relative risk in the first-degree relatives of probands, ~70% concordance among monozygotic twins, and broad-sense heritability in the range of 60–80% [1]. While genome-wide association studies (GWAS) of common variants have identified 64 risk loci for bipolar disorder, causal mechanisms remain elusive due to the small effect sizes of common risk variants [2].
Identifying rare risk variants, some of which may have larger effects, would aid in the discovery of disease mechanisms, development of more precise disease models, and advancement of novel therapeutic targets. Several studies have reported rare variants in case-control cohorts and multiply-affected pedigrees with bipolar disorder [3,4,5,6,7,8,9]. These variants were enriched in a variety of functional categories related to neuronal functions and suggested overlap with risk genes for related psychiatric disorders such as schizophrenia and autism spectrum disorders. However, both family studies and case-control cohorts of bipolar disorder indicate extensive polygenicity. Also, there is evidence that many rare variants contributing to risk for bipolar disorder are missense or non-coding. This makes it challenging to identify risk genes since missense and non-coding variants can either be gain- or loss-of-function (or have no effect) whereas truncating variants are loss-of-function. Underlying mechanisms remain unclear since very few of these variants have been functionally characterized [6, 10].
Methods
Genetic association and linkage analysis
The Bipolar Genome Study [4] (BiGS) dataset consists of genome sequences of 200 individuals from 41 multiply-affected families, of whom 108 were affected with bipolar disorder or recurrent major depression. Whole-genome sequencing was performed to >40× depth by Complete Genomics (San Jose, CA). Details of this cohort and sequencing strategy have been previously described [4, 11]. Previously, we reported a candidate gene-based analysis of these data [4]. Here, we extended our analysis to all protein-coding genes. Variant annotation was performed in two phases. Initial variant annotation and statistical genetic analyses were performed in 2014 using annotations available at that time. The DAO G131V variant was initially identified and selected for experimental validation based on this first phase of analysis. As variant annotation resources have matured considerably since those initial analyses, we updated our analysis in April 2021 using current gene models, allele frequency databases, and variant deleteriousness scores, resulting in the re-annotation or elimination of a small number of variants. The network modeling and gene set enrichment analyses presented here were performed using these updated annotations.
The first phase of variant annotation was performed using QIAGEN’s Ingenuity Variant Analysis, Kaviar [12], and custom variant annotation tools in the Family Genomics Toolkit (familygenomics.systemsbiology.net/software), as previously described [4]. The updated annotation was performed using the Ensembl Variant Effect Predictor, GRCh37 release 103 [13]. Because indels from Complete Genomics genome sequences are not always reliable, our analysis focused on single-nucleotide variants. We selected variants with global allele frequencies <5% in the 1000 Genomes Phase 3 dataset (across all populations) [14]. No additional ancestry-specific threshold criteria were applied. We further selected variants with the following effects relative to both UCSC knownGene models (Phase 1) and Ensembl release 103 protein-coding transcripts (update): stop gained, stop lost, start lost, splice donor variant, splice acceptor variant, missense, 3’ untranslated region variant, 5’ untranslated region variant. These variants were further annotated with CADD to predict their deleteriousness [15].
Affection status models are described in our previous publication [4]. Briefly, we considered all individuals with a diagnosis of bipolar disorder type 1, bipolar disorder type 2, or schizoaffective disorder bipolar type to be affected. Individuals with recurrent or single episodes of major depression were also considered to be affected if (i) the best-supported pre-determined linkage peak for that pedigree [11] supported an inheritance model that included major depression, and they were not married-in; or (ii) they had offspring with bipolar disorder and were not married-in to a pedigree.
Genetic associations of variants with affection status were calculated by two approaches. For variants with allele frequencies >1% in our dataset – primarily those in very large families or present in more than one pedigree – we computed associations with linear mixed models using EMMAX [16], controlling for the polygenic background using a Browning-Nichols empirical kinship matrix as a covariate. To improve statistical power, this analysis integrated the 200 genomes from bipolar disorder pedigrees with an additional 254 Complete Genomics genomes of population controls, originally ascertained on a variety of non-neurological diseases, from an in-house collection of >1,200 genomes at the Institute for Systems Biology, as described in our previous publication [4]. This analysis extends an analysis of candidate genes described in our previous publication to all protein-coding genes, and additional details are described therein [4].
The BiGS study was designed to incorporate data from pre-determined linkage scans, and in many pedigrees, we had genome sequences from only one or a few of several affected individuals. Thus, variants that co-segregate with bipolar disorder in these pedigrees are observed just one or a few times in our WGS dataset. We inferred the segregation patterns for these variants by integrating our WGS with pre-determined linkage regions derived from pedigree-specific linkage analysis using 4,500 SNPs genotyped in a larger number of individuals from each pedigree [11]. Specifically, we identified variants within the bounds of the pre-determined linkage peaks that were shared by the affected individual(s) with WGS and not by unaffected individuals with WGS. For this analysis, we considered only coding variants, as we cannot rely as extensively on genetic association in variant selection. Fine-mapping was performed with the Family Genomics Toolkit [17, 18], combining evidence from genotype call quality score, allele frequency in the population, and predicted deleteriousness. Up to ten variants were reported for each pedigree.
Variant prioritization via network analysis
We used gene interaction networks to identify clusters of functionally related genes and prioritize specific variants. For this purpose, we started with the 741 genes derived from association testing in the BiGS cohort. In addition, for validation, we included 121 bipolar disorder-associated genes from the Bipolar Exome Study (BipEx) [9]. BipEx is an exome sequencing study for which the current freeze includes n = 14,210 cases and 14,422 controls. Results from BipEx were downloaded from bipex.broadinstitute.edu on July 9, 2021. We selected 121 genes with P < 0.05 and odds ratio > 1 from Fisher’s exact tests examining gene burden of ultra-rare protein-truncating variants. We note that the BiGS and BipEx studies differ in important ways that impact gene discovery. Since BipEx is a case-control cohort, most rare variants are observed in a single participant. Therefore, gene burden tests that combine the effects of multiple variants in a gene are utilized to test for significance. Because gene burden tests lose statistical power in the presence of allelic heterogeneity, gene-level analyses in BipEx focused on protein-truncating variants, which have more uniform loss-of-function effects than missense variants. By contrast, our analyses within the BiGS cohort identified statistical associations with disease status via familial transmission of specific variants and included additional categories of functional variants. Due to these differences in analytical strategies, BipEx yielded fewer statistically significant genes than BiGS, despite a larger sample size.
Protein-protein interaction (PPI) networks were derived from GeneMANIA [19]. We downloaded the GeneMANIA combined human PPI network, which consists of 6,979,631 PPI among human proteins from integration of a wide variety of underlying studies. Using these data, we tested whether bipolar disorder-associated genes were more likely to interact with each other in this network than would be expected if by chance. We summarized these interactions as the total number of direct interactions among the bipolar disorder-associated genes. We calculated an empirical p-value based on a background distribution derived by permuting the edges in the full GeneMANIA network 1,000 times. The empirical p-value for the observed number of interactions is the number of permutations in which these metrics are larger than the observed metrics, divided by the total number of permutations. First, this analysis was applied to the 741 genes from BiGS. Next, we used the genes from BipEx to validate that networks discovered in BiGS overlap bipolar disorder risk genes identified in an independent cohort. Finally, we combined the genes from BiGS and BipEx to produce a comprehensive network with 855 genes.
We searched for gene communities (modules) amongst our bipolar disorder-associated genes, again considering either the genes from BiGS and BipEx separately, or the 855 genes from the combination of the two cohorts. A community is defined as a group of genes with a high number of within-community PPI and a lower number of between-community PPI. The starting point for this analysis was a weighted graph of PPI from GeneMANIA, including direct interactions among the risk genes, as well as “one-hop” interactions in which two genes from our list are connected via a shared interaction with any other human gene. We detected communities in this graph using Leiden clustering, with a resolution of 0.6, implemented using the igraph and leiden R packages [20, 21]. This analysis revealed two major communities, as well as two smaller communities with fewer than 20 genes, which were dropped from downstream analyses. We annotated each of the major communities using Gene Ontology terms derived from the org.Hs.egGO2ALLEGS object in the org.Hs.eg.db R package. Gene set enrichment analysis was performed using Fisher’s exact tests. In addition, we computed the centrality of each gene within its community based on eigencentrality, computed within igraph. Community detection was highly robust to the edge weight parameter, and in our primary analysis direct interactions were assigned a weight 10x greater than one-hop interactions. As expected, smaller sub-communities could be identified at higher resolution. Similar communities were also obtained using Louvain clustering. All of the major findings hold true in all of these versions of the analysis.
We developed a combined ranking system to prioritize variants for functional validation. First, we ranked variants by the p-value from linear mixed modeling or fine-mapping of linkage peaks (for the latter analysis, we derived this p-value from the best LOD score within the linkage region). Second, we ranked variants by their predicted deleteriousness, based on CADD scores [15]. Variants derived from BipEx were assigned a “proxy” CADD score of 30, a typical score for protein-truncating variants. Third, we ranked variants by the gene’s eigencentrality in the PPI network. The final rank is the unweighted average of these three scores.
Validation of DAO G131V genotypes in human genomes
DAOG131V (rs768676371) genotypes in members of the multiply-affected pedigree, initially determined by genome sequencing and imputation from linkage scans, were validated using custom TaqMan assays run on an ABI 7900HT Fast Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA) and sequence-specific primers (Supplementary Table 1). One member of the family, a nephew of the proband with a diagnosis of bipolar disorder type, was newly ascertained as part of the current study. A blood sample was collected from this individual and used for genotyping. The remaining genotypes were determined using genomic DNA from lymphoblastoid cell lines obtained from the Coriell Institute for Medical Research, Camden, NJ.
Protein structure-based modeling of the DAO G131V variant
The thermodynamic impact of the G131V single-nucleotide variant (SNV; chr12:109283989 G/T) in DAO was estimated using protein structure-based simulations of protein stability [22]. Modeling was performed using various DAO crystal structures obtained from the Protein Data Bank [23], including: 2DU8 [24], 2E48 [24], 2E49 [24], 2E4A [24], 2E82 [24], 3CUK [25], 3G3E [26], 3W4I [27], 3W4J [27], 3W4K [27], 3ZNN [28], 3ZNO [28], 3ZNP [28], 3ZNQ [28]. For both reference and mutated proteins, the sidechain and backbone conformations of each protein domain were sampled at least 100 times using a Monte Carlo procedure to minimize the estimated total free energy of folding for the protein [29]. The estimated change in energy upon mutation (∆∆G) was calculated as the total free energy of the mutated protein minus that of the wild-type protein.
Site-directed mutagenesis
The hDAO missense variant, G131V, was introduced into the human DAO cDNA (GenBank sequence: BC029057) by site-directed mutagenesis and cloned into a pCMV3-C-Myc vector (HG13372-CM, Sino Biological) using the Quick Change II Site-Directed Mutagenesis kit and sequence-specific primers (Supplementary Table 1), as per manufacturer’s recommendations (Agilent Technologies). Mutagenized plasmid was transformed into chemically competent E. coli strain DH5α. Four colonies were picked randomly, and extracted plasmids were sequenced to determine mutant vs. reference genotype and to confirm absence of off-target mutations. Subsequently, we performed transfection experiments using two correctly mutagenized plasmids to express DAOG131V. The parental non-mutagenized plasmid was transfected for expression of reference DAO.
Cell culture and transient transfection
HEK293T cells (HCL4517, Dharmacon) were maintained in flasks with DMEM (10569010, ThermoFisher scientific) supplemented with 10% fetal bovine serum (F4135, Sigma-Aldrich) at 37 °C, 5% CO2. For transfection, cells were plated at a seeding density of 5 × 105 cells to reach a confluency of 50-70% on the day of transfection. Xfect (631317, Takara Clontech) was used to transfect cells with 7.5 µg pCMV3-hDAO-C-Myc (reference or G131V) or with pCMV3-C-Myc negative control vector (CV014, Sino Biological). The growth medium was replaced 4-hr post-transfection.
Generation of a knock-in mouse model for the DAO G131V variant
Cyagen (Santa Clara, CA) was contracted to generate a genetically precise knock-in mouse carrying the DAOG131V variant at the endogenous mouse locus. Exon 5 of the mouse Dao gene located on mouse chromosome 5 was selected as the target site for Cas9 genome editing. The G130V (GGC to GTC) mutation sites in donor oligo was introduced into exon 5 by homology-directed repair (Supplementary Fig. 2). Cas9 mRNA, guide RNA (gRNA) generated by in vitro transcription, and donor oligo were co-injected into fertilized eggs of C57BL/6 N mice for knock-in mouse production (Supplementary Table 1). The pups were genotyped by PCR, followed by sequence analysis and TaiI restriction analysis. Three correctly edited pups were used for F1 breeding. We received four male and two female F1 heterozygous founders from Cyagen and expanded this colony for use in in vivo experiments. DaoG130V/+ and Dao+/+ mice used in behavioral experiments were derived from eight breeding pairs with F2 DaoG130V/+ sires and C57BL/6 N (Dao+/+) dams obtained from Charles River Labs (Wilmington, MA). DaoG130V/G130V, DaoG130V/+, and Dao+/+ mice used for biochemical and transcriptomic studies were derived from three breeding pairs between F2 DaoG130V/+ mice. All experimental procedures using these mice were approved by the University of Maryland Baltimore Animal Care and Use Committee and were conducted in full accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Genotyping of Dao G130V knock-in mice
All pups were weaned on postnatal day (PD) 23, and tail clips were collected. Crude lysates were prepared by overnight incubation of the tail clips in direct lysis reagent (Viagen Biotech) and proteinase K (Viagen Biotech) at 55 °C. On the following day, the reaction was deactivated by incubation at 85 °C for 45 min. PCR-based genotyping employed the primers listed in Supplementary Table 1. The amplicons were then purified by ExoSAP-IT PCR product clean up kit (Thermo Fisher) followed by Tail restriction analysis (Thermo Fisher).
qPCR
Total RNA was isolated from transfected HEK293T cells and from post-mortem tissues of 8–15-week-old mice using the RNeasy Plus Mini Kit (74134, Qiagen) and the Nucleospin RNA isolation kit (Clontech), respectively. 500 ng total RNA was reverse transcribed using Maxima H Minus cDNA Synthesis Master Mix (M1681, Thermo Scientific). Quantitative PCR (qPCR) was performed using KAPA SYBR FAST qPCR Master Mix Kit (KK4601, Kapa Biosystems) and 10 µM each of forward and reverse transcript-specific primers (Supplementary Table 1), using a CFX 384 real time system (Bio-Rad). The program consisted of one cycle of 95 °C for 3 min, 40 cycles of 95 °C for 3 s, 60 °C for 40 s (annealing and polymerization) followed by melting curve analysis. Data were analyzed using CFX Maestro software (Bio-Rad). Relative mRNA levels for Dao were normalized to the geometric mean of the endogenous controls ActB, Gapdh, and Rpl13a using the 2^-ΔΔCt method [30].
Western blot
The cerebellum, medulla, hippocampus, cortex, and kidney of 8–15-week-old mice were dissected, flash frozen in liquid nitrogen, and stored at −80 °C. Cell lysates and tissue lysates were prepared in ice cold radioimmunoprecipitation assay (RIPA) lysis buffer containing Halt Protease and phosphatase inhibitor cocktail (78440, Thermo Scientific). Protein concentration was determined using Pierce BCA Protein Assay Kit (Thermo Scientific) per manufacturer’s recommendations. The cell (16 µg) and tissue lysates (100 µg brain; 50 µg kidney) were then subjected to electrophoresis using Bolt Mini Gels (Life Technologies) and blotted on a polyvinylidene difluoride membrane using Mini Blot Module transfer system (Life technologies) as per manufacturer’s recommendations. The membranes were blocked with TBS blocking buffer (Licor) for 1 h prior to overnight incubation with anti-MYC (Abcam, ab32), anti-DAO (ab187525), anti-GAPDH (ab83956) or anti-beta tubulin (ab6046) antibodies. On the following day, the membranes were washed four times with TBS-t (1X TBS containing 0.1% Tween 20) followed by 1 h incubation at room temperature with IRDye secondary donkey anti-rabbit (Licor, 926-32213), anti-mouse (926-68072), or anti-chicken (926-68075) antibodies. The proteins were then visualized using Odyssey CLx Imaging system and quantified with Image Studio software.
DAO activity assay
DAO activity was assayed using Amplex red assays, as described [31]. Briefly, transfected HEK293T cells were harvested and homogenized in ice cold sodium phosphate buffer (50 mM Na2HPO4; pH 7.4). Ten µl of the homogenate was added to sodium phosphate buffer containing 100 µM Amplex red, 0.25 U/ml horseradish peroxidase, 50 mM D-serine, with or without 10 µM FAD, to a final volume of 20 µl. After 1 h incubation at 37 °C, the fluorescence was measured on a fluorescence plate reader (Tecan) at an excitation and emission wavelength of 544 nm and 590 nm, respectively. Controls on each plate included a control without cell homogenate, a control without D-serine, and a control with 100 µM of the DAO inhibitor, 6-methyl-benzo[d]isoxazole-3-ol (MolPort-027-640-512). The fluorescence values of cell homogenates were calculated by subtracting their fluorescence values from fluorescence values of controls without D-serine.
Immunofluorescence
Immunostaining was performed as previously described [32]. Briefly, HEK293T cells were plated on coverslips and transfected with pCMV3-hDAO-c-Myc, as above, and co-transfected with 7.5 µg of pCAG-SpCas9-GFP-U6-gRNA (Addgene plasmid #79144) to further confirm transfection efficiency. The growth medium was replaced 4 h post-transfection. The coverslips were subsequently washed with 1X PBS 48 h post-transfection and fixed with 4% paraformaldehyde for 10 min at room temperature. Fixed cells were permeabilized using 0.1% TritonX 100 (Sigma) and non-specific sites blocked with 10% donkey serum for 1 h at room temperature. The cells were then incubated overnight at 4 °C with mouse monoclonal anti-MYC (1:1000 Abcam ab32) primary antibody, followed by incubation with secondary antibody donkey anti-mouse Alexa568 (Molecular Probes). Coverslips were imaged in Keyence BZ-X700 fluorescent microscope.
Behavioral characterization of Dao G130V knock-in mice
After weaning, mice were group housed with four male or five female mice per cage and reared to 8-12 weeks of age under standard conditions (12 h light-dark cycle) with food and water available ad libitum. Mice were housed in the facility for approximately two weeks before any behavioral experiments were conducted. Testing was conducted in order of the least to most stressful procedure [33] (Supplementary Fig. 4I). All experiments were conducted, analyzed, and scored by experimenters blind to the conditions.
Sucrose preference test
The sucrose preference test (SPT) was used to assess hedonic responses to natural reward both before (baseline) and after the learned helplessness test (see below). The interval between the two sucrose preference tests before and after the learned helplessness test was approximately one week. Mice were single housed in a cage fitted with two identical drinking bottles, containing either water or a 1% sucrose solution. Mice were allowed to drink freely for two consecutive days, and the sucrose-containing bottle was counterbalanced across animals and day. Measurements were taken between 5:00 and 7:00 PM and data from the second day were analyzed. Sucrose preference was calculated as a percentage of sucrose intake relative to the total volume of sucrose and water that was consumed. A reduction in sucrose consumption relative to water indicates a reduction in sucrose preference, which is an index of anhedonia, or a lessened ability to experience pleasure [34].
Nest building test
The nest building test (NBT) was used to assess nest construction, which is sensitive to genetic mutations and environmental conditions that are thought to underlie pathological disease states [35]. Approximately 1 h before the dark phase, mice were transferred into individual testing cages that contained wood-chip bedding and 3 g of new square nestlet material. On the following day, nests were given a score from 1 to 5, ranging from an untouched nestlet (1) to a complete nest (5), and any remaining un-shredded nesting material was weighed [36].
Open field test
The open field test (OFT) was used to assess locomotor activity and anxiety-like behavior [37]. Mice were individually placed into an open field arena (49 × 49 × 49 cm; San Diego Instruments, San Diego, CA) for 60 min (∼300 lux). The test was recorded by an overhead digital camera, and distance traveled, velocity, and time spent in the center area (as defined as the 50% most center area of the chamber) of the arena was scored using TopScan (CleverSys Inc; Reston, VA). More time spent near the perimeter of the arena (i.e., thigmotaxis) as opposed to the center is considered an anxiety-like response in this task.
Elevated plus maze
The elevated plus maze (EPM) was used to assess anxiety-like behavior, and is based on the innate desire of mice to be in enclosed spaces [38]. The EPM is comprised of two open arms and two closed arms (39 × 5 cm; Stoelting Co, Wood Dale, IL) elevated 50 cm above the ground. Mice were placed in the center zone of the area facing an open arm and allowed to freely explore the apparatus for 5 min (∼15 lux). The test was recorded by an overhead digital camera, and time spent in the open and closed arms was scored using TopScan (CleverSys Inc; Reston VA). Less time spent in the open arms is considered an anxiety-like response in this task.
Forced swim test
The forced swim test (FST) was used to assess behavioral despair in response to an acute inescapable swimming stress [39, 40]. Mice were placed into a cylindrical transparent plexiglass tank (30 cm × 20 cm) filled with 15 cm of water (23 ± 1 ◦C). Mice were then recorded for 6 min by a tripod video camera. Latency to immobility and total immobility were manually scored, with immobility being the minimum movement that is required for the mice to keep their head above water. Mice typically engage in escape-directed behavior when placed into water but will eventually adopt a posture of immobility. Behavioral despair is indicated by a faster latency to immobility and an increased duration of total immobility.
Learned helplessness test
The learned helplessness test (LHT) was used to assess behavioral despair in response to repeated inescapable shock stress [34]. Coulbourn shuttle boxes were used, which contained two compartments separated by a guillotine door (Coulbourn Instruments LLC, Whitehall, PA). On the first day (training), mice were placed on one side of the box and received 120 inescapable foot shocks delivered through an electric grid floor (0.3 mA, 2 s shock duration, 15 s intertrial interval). On the second day (testing), mice were placed on one side of the box and received 30 escapable shocks that coincided with the guillotine door opening (0.3 mA, 15 s shock duration, 20 s average inter-trial interval). In the first five trials, the guillotine door opened when the shock was initiated, and remained open for the duration of the shock. In the remaining trials, the guillotine door opened with a 3 s delay after initiation of the shock. In all trials, the shock was terminated as soon as the mouse escaped into the neighboring compartment. The number of escape failures were recorded and scored with Graphic State (Coulbourn Instruments, Whitehall, PA). To rule out an effect of genotype on shock sensitivity, naïve mice went through a similar procedure but were allowed to escape shock.
Rotarod test
The rotarod test (RRT) was used to assess motor learning and performance [41]. Mice were placed onto a rotarod beam (IITC Life Science Inc; Woodland Hills, CA) and were allowed to acclimatize standing on the beam for one minute. The beam then began to rotate at 4 RPM, and progressively increased to 40 RPM by the end of the trial at 5 min. Three consecutive trials occurred on each day (2 min intertrial interval) for five consecutive days. Mice were recorded by a tripod video camera, and the latency to fall from the beam was scored for each trial on each day.
MK-801-induced hyperlocomotion
MK-801 is a non-competitive N-methyl-D-aspartate receptor (NMDAR) antagonist that stimulates locomotor activity through a dopamine-independent mechanism [42]. Mice were habituated to an open field arena for 60 min (see OFT above). Mice then received a systemic injection of MK-801 (0.3 mg/kg, intraperitoneal, i.p.; Sigma-Aldrich) and were then returned to the open field for an additional 90 min. MK-801 was dissolved in pharmaceutical-grade sterile saline (0.9% NaCl) on the day of testing. The test was recorded by an overhead digital camera and distance traveled was scored using TopScan (CleverSys Inc; Reston VA). We have previously established that this dose and route of administration induces a moderate hyperlocomotor response in the absence of stereotypy [43], whereas the injection of saline alone does not influence locomotion [44].
GBR12909-Induced hyperlocomotion
GBR12909 is a dopamine transporter blocker that induces locomotor sensitization independent of NMDAR activity. Mice were habituated to an open field arena for 60 min (see OFT above). Mice then received a systemic injection of GBR12909 (16 mg/kg, i.p.; Sigma-Aldrich) and were then returned to the open field for an additional 90 min. GBR12909 was dissolved in 10% dimethylsulfoxide (DMSO; Sigma-Aldrich) on the day of testing. The test was recorded by an overhead digital camera and distance traveled was scored using TopScan (CleverSys Inc; Reston VA). This dose and route of administration is what we have previously established to induce a moderate hyperlocomotor response in the absence of stereotypy [43].
RNA sequencing
Strand-specific mRNA-seq was generated from the cerebellum of 8- to 15-week-old DaoG130V/G130V (n = 2 male and 2 female), DaoG130V/+ (n = 4 male and 4 female), and littermate C57BL/6N controls (n = 6 male and 5 female). Total RNA was extracted from flash-frozen cerebellar tissue using the RNeasy Plus Mini kit (Qiagen). Strand-specific, dual unique indexed libraries for sequencing on Illumina Novaseq were made using the NEBNext® Ultra II™ RNA Library Prep Kit for Illumina® (New England Biolabs, Ipswich, MA). Manufacturer protocol was modified by diluting adapter 1:30 and using 3 µl of diluted adapter. The size selection of the library was performed with AMPure SPRI-select beads (Beckman Coulter Genomics, Danvers, MA). Glycosylase digestion of adapter and 2nd strand was done in the same reaction as the final library amplification. Sample input for this method was PolyA enriched RNA. Enrichment was done using the NEB Poly(A) mRNA magnetic isolation module. No changes were made to the manufacturer’s protocol. Libraries were sequenced to a depth of ~50–75 million 100 bp paired-end reads on an Illumina NovaSeq6000 sequencer.
RNA-seq analysis
Following standard base-calling and adaptor trimming, we used kallisto [45] for pseudo-alignment of sequencing reads to mouse transcripts (mm10) and estimation of read counts and transcripts per million (TPM). Transcripts with a median TPM > 1 were retained for analysis. Principal component analysis and sample hierarchical clustering were used to detect outliers, and a single C57BL/6N male was removed. We used the glmFit() function in the edgeR R package to fit counts to a generalized linear model, including trended dispersion estimates [46]. We then performed likelihood ratio tests with the glmLRT() function to estimate additive effects of the DaoG130V allele (0, 1, or 2 copies), including main effects of age and sex as covariates.
We compared the RNA-seq data from mouse cerebellum to published gene expression profiles of cerebellum from bipolar disorder (n = 37), schizophrenia (n = 44), and major depression (n = 13) cases vs. non-diseased controls (n = 50) [47]. The human gene expression profiles were produced using the Affymetrix Human Gene 1.0 ST Array transcript (gene) version. We downloaded normalized data from the Gene Expression Omnibus (GSE35974) and used probe annotations from the hugene10sttranscriptcluster.db R package. We used the lmFit() and eBayes() functions from the limma R package [48] to fit linear models and estimated effects in bipolar disorder vs. non-diseased controls, as well as of any psychiatric diagnosis (combined SCZ, BD, and MDD) vs. non-diseased controls, including age, sex, post-mortem interval, and pH as covariates.
We reconstructed a consensus gene co-expression network to integrate our mRNA-seq data from DaoG130V knock-in mice. We identified one-to-one orthologs in human vs. mouse using annotations from biomaRt, and filtered both datasets to a common set of genes that were detected in both datasets. Network reconstruction was performed using the blockwiseConsensusModules() function from the WGCNA R package [49], with power = 6, corType = ‘bicor’, networkType = ‘signed’, minModuleSize = 30, mergeCutHeight = 0.3, and minKMEtoStay = 0.
Associations of modules with mouse genotype and with human psychiatric disease were computed with ROAST gene set tests [50]. For the mouse dataset, we fit a model to estimate additive effects of the DaoG130V allele (0, 1, or 2 copies), including age and sex as covariates. For the human dataset, we fit a model to estimate effects of diagnosis (any psychiatric diagnosis vs. non-diseased controls), including age, sex, post-mortem interval, and pH as covariates. We computed a combined meta-analytic p-value for each module using Fisher’s method to combine p-values, and we selected modules with a significant meta-analytic p-value at a False Discovery Rate < 0.05 and the same prevailing direction of effect (down- or up-regulated) in both datasets. For visualization purposes, we also calculated the module eigengene (first principal component) in each dataset, using the moduleEigengenes() function from WGCNA.
Gene Ontology gene set enrichment analysis of modules M5 and M10 was performed with DAVID [51]. Gene set enrichment analyses with neuropsychiatric gene lists was performed using Fisher’s exact tests. Hub genes within each module were detected based on eigencentrality, computed in the igraph R package [20].
To characterize the cell type-specific expression patterns of modules M5 and M10, we used single-nucleus RNA-seq data from mouse cerebellum [52]. Fully analyzed snRNA-seq data were downloaded from the Single Cell Portal (https://singlecell.broadinstitute.org/single_cell/study/SCP795/a-transcriptomic-atlas-of-the-mouse-cerebellum). We calculated the module eigengene expression of modules M5 and M10 within the snRNA-seq data. We then tested for enrichment of eigengene expression in each major cell type using the FindMarkers() function from the Seurat R package [53].
Statistical analysis
All experiments were performed in a randomized fashion and conducted and analyzed by experimenters who were blind to the conditions. Sample size for biochemical experiments was n = 3 (separate transfections into independently cultured cells) in human cells and n = 5 per tissue per genotype in mice. Sample size for behavioral experiments was n = 11 for male DaoG130V/+ mice and n = 12 for female DaoG130V/+ mice and for both male and female Dao+/+ mice. Sample sizes were selected to provide adequate power to detect large effects in biochemical experiments and moderate effects in behavioral experiments. No formal power analysis was conducted. Biochemical and behavioral data were analyzed with GraphPad Prism Software 9.0.1. Distributions were assessed for normality using the D’Agostino-Pearson test and for homogeneity of variance using the Brown-Forsythe Test. Data are presented as mean ± standard error of the mean (SEM) and statistical significance was defined as p < 0.05. One-way analysis of variance (ANOVA) was used when data from three or more groups were being compared. Two-way ANOVA was used when sex (male vs. female) and DaoG130V/ genotype were independent factors. Two-way repeated measures ANOVA was used when genotype and time (5-min time bins; repeated measure) were independent factors. If a significant main effect and/or interaction was detected, the Holm-Šídák post-hoc test was used to assess pairwise comparisons.
Results
Network analysis identifies a rare, nonsynonymous variant in the DAO gene associated with risk for mood disorders
We devised a network analysis and multi-parameter optimization strategy to prioritize specific bipolar disorder risk variants for functional studies (Methods). Our approach builds on the premise that risk variants for bipolar disorder are likely to cluster within networks of functionally interacting genes. Specific variants are then selected based on network centrality, predicted deleteriousness, and the strength of disease association within pedigrees.
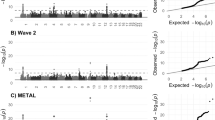
We initiated our analysis by performing linkage and association analyses to identify bipolar disorder-associated rare variants in 41 pedigrees from the Bipolar Genome Study (BiGS) [4]. We found linkage or association of bipolar disorder at a nominal level of significance (P < 0.05) to protein-truncating, missense, and non-coding variants in the 5’ and 3’ untranslated regions of 741 genes (Supplementary Table 2). We validated these 741 genes based on overlap with 23 gene sets previously implicated in risk for neuropsychiatric disorders, and we confirmed significant overlap (adjusted p-value < 0.05) with seven of these gene sets (Supplementary Table 3; Fig. 1B): loss-of-function intolerant genes [54] (gnomAD pLI > 0.9); genes associated with severe developmental disorders in the Developmental Disorder Genotype-Phenotype Database [55]; targets of the autism-associated chromatin remodeling protein CHD8 [56]; and targets of the neuronal RNA binding proteins FMRP, RBFOX1/2, RBFOX2, and CELF4 [56]. Genes identified in the BiGS cohort also include 10 of the 102 high-confidence risk genes for autism spectrum disorders from large-scale exome sequencing by the Autism Sequencing Consortium [57] – RFX3, DYRK1A, SETD5, SLC6A1, DIP2A, TCF4, GFAP, PRR12, PPP1R9B, and NSD1 (odds ratio = 2.8, P = 5.5e-3).
A Contextualization of the top 150 genes identified in bipolar disorder pedigrees and case-control cohorts in a protein interaction network. Node shape: diamond = coding; circle = non-coding. Node color: p-value for linkage or association (darkest red corresponds to P = 1.0e-5). Node size: ranking on combined evidence from statistical association, deleteriousness, and network centrality. B Enrichments of 741 genes from bipolar disorder pedigrees for neuropsychiatric risk genes and related gene sets: rare variants = risk genes from exome and genome sequencing studies; GWAS = genes at GWAS risk loci; DEGs in PFC = Differentially expressed genes in prefrontal cortex; gene networks. C DAOG131V co-segregated with bipolar disorder in a four-generation pedigree.
Rare variant associations with bipolar disorder remain poorly characterized. We compared the 741 genes identified in the BiGS cohort to 121 genes nominally associated with bipolar disorder (P < 0.05, odds ratio >1) in the largest case-control exome sequencing study to date [9] (Bipolar Exome Study, BipEx; n = 14,210 cases and 14,422 controls). We found modest overlap between genes identified in these two cohorts: PPIL2, TMEM156, SBSPON, SEMA4B, CHD1L, CHRNB2, and SCLY (odds ratio = 1.6, P = 0.17).
Next, we considered the hypothesis that genes identified by BiGS and BipEx interact within polygenic gene networks. We integrated genes from BiGS and BipEx in the context of a bipolar disorder-centered rare variant interaction network, utilizing protein-protein interactions from the GeneMania database [19]. First, we tested whether bipolar disorder-associated genes interact via PPI at a significantly higher rate than is expected by chance, based on permutations of network edges. We found that this was true for the 741 genes from BiGS (P < 0.001), for the 121 genes from BipEx (P < 0.027), and for the 855 genes when BiGS and BipEx were combined (P < 0.001). We also found an increased rate of PPI between genes identified in BipEx and BiGS (P < 0.001). Likewise, there was an increased rate of PPI between bipolar disorder risk genes and risk genes for schizophrenia identified by the SCHEMA consortium (P < 0.001). These results suggest that all these studies have identified genes within a shared polygenic network.
Leiden clustering [21] revealed that the 855 genes from BiGS and BipEx cluster into two major communities (Fig. 1A; Supplementary Table 4). Community 1 contained 428 genes and was enriched for the Gene Ontology terms “neuron projection” (54 genes, odds ratio = 2.1, P = 2.9e-6; Supplementary Table 4) and “synapse” (52 genes, odds ratio = 2.0, P = 2.3e-5). Community 2 contained 374 genes and was enriched for genes localized to the “nucleus” (216 genes, odds ratio = 2.4, P = 1.2e-16), including genes involved in “DNA repair” (27 genes, odds ratio = 2.8, P = 5.4e-6), “mitotic cell cycle” (40 genes, odds ratio = 2.3, P = 9.9e-6), and “chromatin remodeling” (13 genes, odds ratio = 3.6, P = 1.4e-4). All of these categories are also enriched among risk genes for related psychiatric disorders, such as autism spectrum disorders and schizophrenia [57,58,59].
Multi-parameter variant prioritization within this network (Supplementary Table 5) revealed the top-scoring variant overall to be rs768676371, a missense variant in DAO, encoding D-amino acid oxidase (Fig. 1C). Other top-scoring variants in Community 1 included rs3811993, a missense variant in GABRA6, encoding the GABAA receptor α-6 subunit; rs12914008, a missense variant in CHRNB4, encoding the cholinergic receptor nicotinic β-4 subunit; and rs41276505, located in the 3’ UTR of SLC6A1, encoding GABA Transporter 1. Top-scoring variants in Community 2 included rs1263034966, a missense variant in MCM7, encoding Minichromosome Maintenance Complex Component 7; chr11:125452286 C/T, a novel missense variant in EI24, encoding Etoposide Induced 2.4, an autophagy-associated transmembrane protein; and rs2455425, located in the 3’ UTR of CHD1, encoding Chromodomain-Helicase DNA-binding 1. These genes have well-established functions at synapses and in DNA repair and chromatin remodeling, consistent with the broader enrichments of each community. Importantly, both community detection and variant ranking were quite robust to parameter tuning, including analyses in which the genes from BiGS and BipEx were used separately. rs768676371 (DAO) was the top variant in most iterations of the analysis.
DAO catalyzes the degradation of D-amino acids including D-serine, a well-characterized co-agonist of the N-methyl-D-aspartate glutamate receptor (NMDAR) [60,61,62]. In mice, DAO loss-of-function caused elevated D-serine levels, increased NMDAR activity, and altered cognition and behavior [63,64,65,66]. Several previous reports described linkage of the DAO region to bipolar disorder and schizophrenia, though common variants in this region are not associated with neuropsychiatric risk [67,68,69,70,71,72]. The rs768676371 variant results in a glycine-to-valine substitution at amino acid 131 (henceforth, DAOG131V). This variant was discovered under the top linkage peak [11] in the proband of a four-generation pedigree. Using TaqMan genotyping, we confirmed the DAOG131V genotype in the proband and in three additional members of the family, all of whom had either type-1 or type-2 bipolar disorder. We also confirmed that the variant was absent in two unaffected family members. Non-parametric linkage analysis of these confirmed genotypes suggested linkage to bipolar disorder within this family (P = 7e-4). Replication analysis using exome sequences from the SCHEMA and BipEx consortia [9, 58] revealed one additional copy of DAOG131V in a schizophrenia case and none in controls. This same carrier with schizophrenia was present in the gnomAD v2.1.1 exome dataset. In addition, the gnomAD v2.1.1 dataset contains one copy of the allele in an individual from the “non-neuro” cohort (n = 104,064) whose mental health status is unknown. Thus, DAOG131V is a rare variant that we have identified exclusively or near-exclusively in individuals with bipolar disorder or schizophrenia.
Effects of DAO G131V on DAO expression and activity
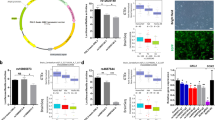
We set out to test the functional consequences of the DAOG131V missense variant. Examination of DAO crystal structures from the Protein Data Bank indicated that G131 is located in a loop domain (Fig. 2A). Protein folding simulations with Rosetta [73] substituting glycine with the bulkier valine residue resulted in a predicted increase in the free energy of the folded protein (i.e., destabilization, P = 0.004; Fig. 2B). Thus, the model predicts that DAOG131V may cause hypofunction by destabilizing the DAO protein. We tested this prediction via heterologous expression of DAOG131V or non-mutant DAO protein in the HEK293T cell line, which does not natively express DAO. In these experiments, site-directed mutagenesis was used to introduce DAOG131V into an expression plasmid for the DAO coding sequence, and all results were replicated using transient transfection with two independently mutagenized plasmids expressing DAOG131V, compared to a non-mutagenized plasmid expressing the reference sequence DAO gene. Quantification of DAO protein in transfected HEK293T cells by Western blot revealed ~30% lower levels of DAOG131V vs. non-mutant DAO protein (F = 5.7, P = 0.017, Fig. 2C; Supplementary Fig. 1B–D). By contrast, quantitative PCR (qPCR) demonstrated similar levels of DAOG131V vs. non-mutant DAO transcripts, suggesting that DAOG131V does not influence transcription or mRNA stability (Supplementary Fig. 1A). Also, DAOG131V did not alter the subcellular localization of the DAO protein in transiently transfected HEK293T cells (Supplementary Fig. 1E, F). These results suggest that DAOG131V leads to reduced abundance of the DAO protein, possibly via decreased protein stability. DAOG131V may also inhibit formation of the active DAO homodimer, as the variant is located at the dimerization interface [74].
A Structural modeling of the loop region of the DAO protein in the presence of the reference G131 allele (left) and with G131V (right). B Free energy predictions from protein folding simulations of the DAO protein with G131 vs. G131V. C Quantitation of DAO protein (Western blot) showing DAO protein abundance in HEK293T cells following transfection with DAOG131 or DAOG131V. The two bars labeled DAOG131V indicate transfections with distinct mutagenized plasmids. D Effects of DAOG131V on DAO enzymatic activity in HEK293T cells in the presence (left) or absence (right) of the co-enzyme FAD. E Abundance of DAO protein in brain regions (left) and kidney (right) from Dao+/+, DaoG130V/+, and DaoG130V/G130V mice (n = 5 / genotype). ***P < 0.001.
Next, we investigated the effect of DAOG131V on DAO enzymatic activity. The D-amino acid oxidase reaction produces hydrogen peroxide (H2O2) as a biproduct. Therefore, DAO activity is commonly quantified using Amplex red assays, which directly measure H2O2 production [75]. We detected robust H2O2 production in cell lysates from HEK293T cells expressing non-mutant DAO in the presence of D-serine substrate (Fig. 2D). H2O2 production was blocked in the presence of the DAO inhibitor 6-methyl-benzo[d]isoxazole-3-ol, confirming that this signal corresponds to DAO activity. By contrast, DAO activity was essentially undetectable in cells expressing DAOG131V. Similar results were obtained with (P < 1.0e-4) or without (P < 1.0e-4) addition of 10 μM FAD co-enzyme. Therefore, DAOG131V results in almost complete loss of DAO activity in HEK293T cells.
To investigate the effects of DAOG131V in vivo, we generated a genetically precise knock-in mouse model harboring the human mutation. The human DAO protein shares 80–85% homology across higher vertebrates and is present in most eukaryote and prokaryote genomes. Multiple sequence alignment of the DAO protein revealed that the G131 residue is conserved across all examined vertebrate and invertebrate species (Supplementary Fig. 2A). CRISPR/Cas9 genome editing was used to introduce a G-to-V point mutation into the germline of C57BL/6N mice at the homologous position in the Dao gene (Supplementary Fig. 2B–E). This position in offset by one amino acid in the human vs. mouse protein. Therefore, we refer to the resulting mouse allele as DaoG130V. Successful editing of four heterozygous DaoG130V/+ founders was confirmed by sequencing, as well as by a restriction fragment length polymorphism assay.
We assessed the effects of DaoG130V on RNA expression and protein abundance in several mouse tissues by qPCR and Western blot, respectively. Previous studies demonstrated high levels of DAO mRNA and protein primarily in astrocytes of the mouse hindbrain [76], though the activity of DAO in the human brain may be broader [77]. High expression is also observed in the kidney. As expected for Dao+/+ wildtype conditions, Dao mRNA (Supplementary Fig. 3) and DAO protein (Fig. 2E) were most abundant in kidney, followed by the two hindbrain regions examined, cerebellum and medulla. Expression of Dao mRNA and DAO protein were very low in the two forebrain regions tested, hippocampus and cortex. Dao mRNA expression in each tissue was similar in DaoG130V/+ heterozygotes and DaoG130V/G130V homozygotes vs. wildtype Dao+/+ mice (Supplementary Fig. 3), suggesting that the variant has little effect on transcription or mRNA stability. By contrast, DaoG130V caused a substantial dose-dependent reduction in the DAO protein level, which was most pronounced in the tissues of highest DAO abundance, that is, the cerebellum (F = 21.8, P = 3.0e-6), medulla (F = 22.8, P = 1.8e-6), and kidney (F = 14.6, P = 1.4e-4, Fig. 2E). Therefore, DaoG130V results in reduced levels of DAO protein in vivo, consistent with the results we observed when DAOG131V was expressed in human cells.
In vivo behavioral characterization of the Dao G130V variant in knock-in mice
We performed a sequence of experiments to assess the impact of the DaoG130V variant on behavioral responses in vivo. Previous studies demonstrate that mice homozygous for DAO loss-of-function mutations manifest behavioral changes relevant to mood and psychotic disorders, including increased anxiety-like behaviors [78, 79]. However, behavioral phenotypes in heterozygous mice with DAO mutations have not been reported. Given that we observed only heterozygous DaoG131V genotypes in humans, we therefore characterized the behavioral phenotypes of heterozygous DaoG130V/+ mice vs. wildtype Dao+/+ controls.
First, we assessed general locomotor activity and basal anxiety-like responses in the open field test and elevated plus maze. Both DaoG130V/+ and Dao+/+ wildtype mice displayed normal habituation to the novelty of the open field environment, with no effect of genotype on distance traveled either in males or females (males: F(1, 21) = 0.99, P > 0.1; females: F(1, 22) = 0.01, P > 0.1; Fig. 3A). DaoG130V/+ and Dao+/+ mice also responded similarly to the anxiogenic properties of the open field, as all groups spent a similar amount of time in the center zone of the arena (P > 0.1, Supplementary Fig. 4A). Similarly, neither sex (F(1, 43) = 2.5, P > 0.1) nor genotype (F(1, 43) = 0.0, P > 0.1) influenced the amount of time spent in the open arms of the elevated plus maze (Supplementary Fig. 4B). These results suggest that in heterozygous mice DaoG130V does not alter general locomotor activity and does not promote an anxiety-like phenotype in the open field test or elevated plus maze.
A, B Open field test: distance travelled per time, total distance travelled. C, D Forced swim test: latency to immobility, total immobility. E Learned helplessness: left, number of escape failures in response to escapable shock in shock-naïve mice; right, number of escape failures in response to escapable shock in trained mice who were previously exposed to repeated inescapable shock. F, G Rotarod motor learning: latency to fall per trial, mean latency to fall.
A major risk factor for the onset of psychiatric disorders, including bipolar disorder, is exposure to stress [80, 81]. To test whether the DaoG130V variant confers enhanced stress susceptibility, we assessed how well mice adapt to both forced swimming and foot shock stress. The forced swim test was used to assess escape-directed behavior in response to an acute inescapable swimming stress lasting six minutes. No differences in the latency to become immobile (Fig. 3C) were detected as a function of sex (F(1, 43) = 2.4, P > 0.1) or genotype (F(1, 43) = 0.01, P > 0.1). Similarly, no differences were detected in the total time spent immobile (Fig. 3D), as a function of either sex (F(1, 43) = 0.04, P > 0.1) or genotype (F(1, 43) = 0.18, P > 0.1). These data suggest that heterozygous DaoG130V mutations do not enhance susceptibility to acute swimming stress. However, when mice were exposed to repeated inescapable foot shock in the learned helplessness test, there was a significant main effect of genotype (F(1, 43) = 5.2, P = 2.7e-2) as well as sex (F(1, 43) = 10.3, P = 2.5e-3) on the number of times mice failed to escape shock during testing when an escape route was provided (Fig. 3E, right). Specifically, DaoG130V/+ genotype and male sex were each associated with increased escape failures, with an insignificant sex × genotype interaction (F(1, 43) = 0.98; P > 0.1). The increase in escape failures of DAOG130V mice may reflect increased stress susceptibility [82]. Although not directly assessed here, it is also possible that DAOG130V modifies conditioned fear responding (e.g., by promoting freezing over escape-directed behavior) or animals’ physiological response to repeated shock exposure (e.g., increased corticosterone or altered shock sensitivity). Given that reduced shock sensitivity could explain increased escape failures independent of changes in stress susceptibility, we conducted additional control experiments to refine our understanding of this effect. When shock-naïve mice were subjected to the same testing procedure (30 escapable shocks that coincided with the guillotine door opening), both genotypes were equally as likely to escape (Fig. 3E, left). This suggests that the effects of sex and genotype on escape failures are not driven by a reduction in shock sensitivity.
Despite differences in stress susceptibility (Fig. 3E, right), all mice displayed a comparable hedonic response to natural reward in the sucrose preference test regardless of sex (F(1, 43) = 0.35, P > 0.1) or genotype (F(1, 43) = 0.19, P > 0.1; Supplementary Fig. 4C). This suggests that the ability to experience pleasure remains intact in DaoG130V/+ mice, even after repeated inescapable shock exposure (Supplementary Fig. 4D). Similarly, no differences in nest construction in the nest building test were detected among the groups before or after the learned helplessness test (Supplementary Fig. 4E–H). Taken together, these results suggest specific effects of heterozygous DaoG130V/+ variants on susceptibility to some types of stress and not on other aspects of sensitivity to aversive or pleasurable stimuli.
Learned helplessness can be interpreted as an inability to learn the degree to which an adverse outcome is dependent on responses [83, 84]. To evaluate the ability of DaoG130V/+ mice to learn in a non-emotional context, we utilized the rotarod test, a cerebellum-dependent motor learning task. In males, there was a significant effect of time (F(14, 266) = 17.26, P < 1e-4) and a time × genotype interaction (F(14, 266) = 1.9, P = 2.5e-2) on latency to fall across the five days of the procedure (Fig. 3F, left). When assessing comparisons within a given day of training, there was a main effect of genotype on the first day of training in male mice (F(1, 19) = 6.0, P = 0.023), which suggests that the DaoG130V variant led to an initial modest reduction in motor performance. In females, there was a significant main effect of time (F(14, 308) = 22.97, P < 1e-4) on the latency to fall across the five days of the procedure (Fig. 3F, right), which suggests that both males and females improved in their motor learning over the five days of training. When comparing mean latency to fall, there was a significant main effect of sex (F(1, 41) = 11.7, P = 1.4e-3) and a trend effect of genotype (F(1, 41) = 3.0, P = 0.09; Fig. 3G). Therefore, there is little to no effect of heterozygous DaoG130V on motor learning in this task.
Since DAOG131V had decreased enzymatic activity to degrade D-serine in human cells in vitro (Fig. 2D), a co-agonist of the NMDAR, we predicted that DaoG130V may modulate NMDAR activity. To test this, we systemically administered MK-801, which is a non-competitive NMDAR antagonist that stimulates locomotor activity through a dopamine-independent mechanism [42]. Consistent with the open field results (Fig. 3A), baseline locomotor behavior (i.e., prior to MK-801 injection) did not vary as a function of genotype in male or female mice (F(1, 21) = 0.08, P > 0.1; F(1, 22) = 0.39, P > 0.1, respectively; Fig. 4A) and did vary as a function of time as mice habituated to the chamber (F(11, 231) = 13.08, P < 1e-4; F(11, 242) = 2.75, P = 2.0e-2, respectively). Male and female mice displayed significantly increased distance traveled after MK-801 injection (F(17, 357) = 21.71, P < 1e-4; F(17, 374) = 11.70, P < 1e-4, respectively; Fig. 4A). Relative to Dao+/+ wildtype mice, male DaoG130V/+ mice displayed an attenuated hyperlocomotor response to the MK-801 injection (P = 4.8e-2; Fig. 4A, left). By contrast, the hyperlocomotor response to MK-801 was not significantly different in female DaoG130V/+ vs. Dao+/+ mice (F(1, 22) = 0.08, P > 0.1; Fig. 4A, right). We conclude that heterozygous DaoG130V diminished the stimulatory actions of NMDAR antagonism in male mice.
To test the specificity of this effect, we assessed locomotor responses following administration of GBR12909, a dopamine transporter blocker that induces locomotor sensitization independent of the NMDAR. Male and female mice displayed significantly increased distance traveled after GBR12909 injection (F(17, 323) = 7.983, P < 1e-4; F(17, 374) = 13.92, P < 1e-4, respectively; Fig. 4B). We observed a non-significant trend toward enhanced hyperlocomotor responses in male DaoG130V/+ vs. Dao+/+ mice (P > 0.05, Fig. 4B). This result suggests that attenuated hyperlocomotor responses in male DaoG130V/+ mice occur specifically with MK-801 treatment and are likely related on NMDAR transmission.
Associations of Dao G131V and bipolar disorder with gene networks in the cerebellum
To gain insight into the molecular and cellular consequences of the DaoG131V variant, we performed mRNA sequencing (RNA-seq) of cerebellar tissue from 8- to 15-week-old DaoG130V/G130V, DaoG130V/+, and Dao+/+ mice. We tested for additive effects of DaoG131V genotype and detected suggestive changes in the expression of 14 genes (FDR < 20%; Supplementary Table 6), including Ncam1, encoding neural cell adhesion molecule 1, a positional candidate gene at risk loci from several recent GWAS of mood disorders [85,86,87] (log2(fold change) = 0.38; P = 9.9e-5).
We integrated our RNA-seq data from DaoG130V knock-in mice with previously published gene expression profiles of post-mortem cerebellar tissue from humans with major depressive disorder, bipolar disorder, schizophrenia, and controls with no mental illness [47] to characterize shared patterns of transcriptional dysregulation. Using Weighted Gene Co-Expression Network Analysis (WGCNA) [49], we identified 18 consensus gene co-expression modules with shared co-expression patterns in mouse and human cerebellum, involving a total of 6,107 genes (Supplementary Table 6). We tested each module for changes in expression associated with DaoG130V genotype and with psychiatric diagnosis (i.e., the three disorders combined). We then meta-analyzed the p-values to identify joint associations. Two modules were differentially expressed at an FDR < 0.05: M10 (199 genes, Pmeta = 4.3e-4; Fig. 5A) and M5 (358 genes, Pmeta = 2.6e-3, Fig. 5B). Both of these modules were down-regulated both in mice carrying the DaoG131V variant and in psychiatric cases vs. controls (Fig. 5C–F). M10 and M5 were also significantly down-regulated (FDR < 0.05) in a post-hoc contrast comparing bipolar disorder cases vs. controls (M10: P = 5e-4; M5: P = 4.0e-3).
A, B Module membership and differential expression of genes in modules M10 (A) and M5 (B). Node size corresponds to network centrality; node color corresponds to significance of differential expression (blue = down-regulated; red = up-regulated); edges correspond to Pearson correlations in mouse cerebellum. C–F Expression of M10 (C, D) and M5 (E, F) module eigengenes in cerebellar tissue from DaoG130V knock-in mice vs. wildtype controls (C, E) and in human psychiatric cases vs. unaffected controls (D, F). G, H Expression of M10 (G) and M5 (H) module eigengenes in major cerebellar cell types. WT Wildtype (Dao+/+); Het Heterozygous (DaoG130V/+), Hom Homozygous (DaoG130V/G130V), NMI No mental illness, MDD Major depressive disorder, BD Bipolar disorder, SCZ Schizophrenia, MLI Molecular layer interneuron, PLI Purkinje layer interneuron, UBC Unipolar brush cell; OPC Oligodendrocyte precursor cell.
Cell type specificity analysis using published scRNA-seq from mouse cerebellum [76] revealed that the M10 eigengene (its first principal component) was expressed most highly in granule neurons (Fig. 5G), and several of the most highly connected genes in the module (i.e., hub genes) were expressed almost exclusively in this cell type. Gene set enrichment analyses showed that M10 was enriched for components of membranes (97 genes, 1.5-fold enriched, uncorrected P = 5.1e-6, FDR = 0.001), especially synaptic components (17 genes, 3.5-fold enriched, uncorrected P = 2.6e-5, FDR = 0.003; Supplementary Table 7), including genes localized to both the presynaptic active zone (4 genes, 14.0-fold enriched, uncorrected P = 2.8e-3) and the postsynaptic membrane (8 genes, 3.8-fold enriched, uncorrected P = 5.3e-3). Granule neuron-specific synaptic genes in M10 with evidence of down-regulation (P < 0.05) in DaoG130V knock-in mice and/or in mood and psychotic disorders include Slc17a7, encoding the vesicular glutamate transporter VGLUT1; Grin2c, encoding the GluN2C NMDA receptor subunit; Gabrd, encoding the delta subunit of the GABAA receptor; and Sema4c, which encodes a semaphorin cell-cell signaling protein with roles in synaptic plasticity.
The M5 eigengene and many of the M5 hub genes were most highly expressed in Purkinje neurons (Fig. 5H). Like M10, M5 was enriched for synaptic genes (25 genes, 2.9-fold enriched, uncorrected P = 6.7e-6, FDR = 3.4e-4; Supplementary Table 7). The strongest enrichments were for components of the post-synaptic density (18 genes, 4.4-fold enriched, uncorrected P = 7.9e-7, FDR = 4.9e-5) and genes localized to dendrites (27 genes, 3.2-fold enriched, uncorrected P = 3.5e-7, FDR = 3.6e-5), but the module also included several genes specific to GABAergic pre-synapses. Purkinje neuron-specific synaptic genes in M5 with evidence of down-regulation in DaoG131V knock-in mice and/or in mood and psychotic disorders included Grid2, which encodes the delta-2 ionotropic glutamate receptor, Gad2, which encodes glutamate decarboxylase 2, the enzyme responsible for GABA production, and Slc32a1, a vesicular inhibitory amino acid transporter. Taken together, our RNA-seq results reveal that DaoG130V causes transcriptional down-regulation of synaptic genes expressed in granule neurons and Purkinje neurons, and these changes are recapitulated in the cerebellum of humans with mood and psychotic disorders.
To further explore the relationship of these gene networks with psychiatric disorders, we tested for overlap with known psychiatric risk genes and gene networks, as in our genomic sequence analyses, above (Supplementary Table 7). Both M10 and M5 were enriched (FDR < 0.05) for genes that are also down-regulated in the prefrontal cortex of individuals with bipolar disorder, schizophrenia, and/or autism, suggesting shared molecular pathology across these brain regions. Both M10 and M5 were also enriched for targets of the neuronal RNA binding proteins FMRP, RBFOX1/2, RBFOX3, and CELF4, which likely reflects the functions of these RNA-binding proteins in transporting nascent RNAs to synapses. Finally, we found that M5 – but notably not M10 – was enriched for neuropsychiatric risk genes identified by exome and genome sequencing studies. These included developmental disorder genes compiled by the Deciphering Developmental Disorders consortium [55] (49 genes, odds ratio = 1.5, P = 4.9e-3), as well as genes associated with schizophrenia from SCHEMA [58] (25 genes with SCHEMA P < 0.05; odds ratio = 1.6, P = 0.03). Remarkably, the most significant overlap was with “Community 1” genes from our own bipolar disorder pedigrees, described above (17 genes, odds ratio = 2.7, P = 4.7e-4). This analysis highlights several variants that could potentially influence the function of Purkinje neurons. For instance, chr3:155838590 C/T is a novel missense variant in KCNAB1 that was identified in all four sequenced bipolar disorder cases (and no unaffected individuals) from a four-generation pedigree. KCNAB1 encodes a cytoplasmic subunit that modulates the biophysical characteristics of shaker-type voltage-gated potassium channels. In the cerebellum, KCNAB1 is expressed almost exclusively in Purkinje neurons [76]. Overall, these results suggest that effects on Purkinje neuron synapses may be a recurring theme among variants contributing to risk for bipolar disorder.
Discussion
We found that hundreds of rare, potentially functional variants in genes with synaptic and nuclear functions are associated with bipolar disorder in multiply-affected pedigrees and case-control cohorts. Although we and others have described rare variants co-segregating with bipolar disorder, very few of these variants have been characterized experimentally [6, 10]. Indeed, the very large number of variants and genes detected in bipolar disorder pedigrees (we report an average of ~20 variants per pedigree) had left it unclear whether any of these variants is sufficient to cause mood phenotypes in isolation from the polygenic background. The discovery, prioritization, and characterization of a hypofunctional, bipolar disorder-associated variant, DAOG131V, provided insights into the genetics and neurobiology of bipolar disorder.
Heterozygous DaoG130V/+ knock-in mice displayed increased escape failures following inescapable foot shock. The DaoG130V-dependent reduction in escape-directed behavior was specific to a more intense stress exposure, as both male and female heterozygous mice do not manifest deficits to acute inescapable swimming stress compared to wildtype controls. These results suggest that DaoG130V may confer an enhanced susceptibility (or reduced resilience) to more severe forms of stress. Severe stress is a major risk factor for bipolar disorder [81, 88], so it is plausible that altered stress susceptibility could contribute to risk for bipolar disorder in human carriers of DaoG131V.
Not surprisingly, the behavioral changes in heterozygous DaoG130V/+ mice were more subtle and specific than published findings in mice with homozygous DAO loss-of-function variants. Unlike homozygous mice [78, 79], heterozygous DaoG130V/+ mice manifested no significant differences in innate anxiety-like responses. DaoG130V/+ mice also showed normal locomotor behavior, novelty exploration, nest building, preference for natural reward, and cerebellar-dependent motor learning. These results suggest that heterozygous DaoG130V does not influence basal behavioral responsivity to normal environmental stimuli or non-emotional motor-based learning. Since all known human carriers of DaoG131V are heterozygous, our findings may more accurately model aspects of DAO function that are relevant specifically to bipolar disorder in humans. In addition, in the context of a multiply-affected pedigree, DaoG130V occurs alongside other common and rare functional variants, and it is likely that the combined effects of these variants are required to explain the high rate of bipolar disorder and other mood disorders in this pedigree. Nonetheless, our results demonstrate that even in isolation from other genetic and environmental factors, heterozygous DaoG130V is sufficient to cause changes in behavior related to bipolar disorder endophenotypes [89, 90].
Convergent evidence suggests that DaoG131V causes dysregulation of NMDAR and related components of glutamatergic synapses in the hindbrain. At a molecular level, DaoG131V impaired degradation of D-serine in vitro. It is likely that DaoG131V also results in elevated levels of D-serine and NMDAR activity in the hindbrain, as has been observed previously in heterozygous and homozygous mice with other DAO loss-of-function mutations [64, 66]. Direct evidence for dysregulation of NMDAR signaling comes from blunted hyperlocomotor responses after pharmacological inhibition of NMDAR. This result extends previous findings in homozygous mice with Dao loss-of-function mutations [63]. At a transcriptional network level, we found down-regulation of gene networks in the cerebellum that were enriched for components of glutamatergic synapses, including the NMDAR subunit Grin2c (GluN2C). In the cerebellum, D-serine also binds and modulates a distinct ionotropic glutamate receptor, the δ2 ionotropic glutamate receptor (GluD2), to regulate synaptic plasticity [91, 92]. The gene encoding GluD2, Grid2, is a hub gene of a down-regulated gene network in DaoG130V knock-in mice. We speculate that transcriptional down-regulation of these genes is a compensatory response to sustained, elevated levels of D-serine, but additional experiments will be needed to establish the precise changes in synaptic physiology.
Our transcriptomic data suggest that dysregulated gene networks are expressed primarily in granule neurons and Purkinje neurons. Axonal projections from granule neurons, termed parallel fibers, form synapses on the dendrites of Purkinje neurons, with each fiber synapsing onto hundreds of thousands of Purkinje neurons. Plasticity of parallel fiber synapses – in part mediated by D-serine, NMDAR, and GluD2 – is critical for cerebellar learning [93]. Emotional learning in the cerebellum, as well as social behavior and cognition, is mediated by specialized circuits in the cerebellar vermis [94]. In fear conditioning paradigms, repeated foot shock resulted in long-term potentiation of parallel fiber synapses in the vermis, and this effect was attenuated in GluD2 knockout mice [95, 96]. The aversive stimulus in fear conditioning is very similar to foot shock in the learned helplessness test. Therefore, it is plausible that the increased stress susceptibility of DaoG130V/+ involves a deficit in parallel fiber synaptic plasticity.
In recent years, several studies have described rare variants that co-segregate with bipolar disorder in large, multiply-affected pedigrees, including our own previous report on these 41 pedigrees [3,4,5,6,7]. A general conclusion from these studies has been that multiply-affected pedigrees contain functional variants in many genes, enriched in broad categories relevant to neuronal functions. Similar conclusions can be drawn from exome and genome sequencing studies of bipolar disorder case-control cohorts [8, 9]. The variants that we found in synaptic genes (Community 1 in our analysis) are consistent with these previous findings. Functional enrichments for nuclear functions such as DNA damage responses and chromatin remodeling (Community 2) are also consistent with sequencing studies [57] and GWAS [59] of psychiatric disorders. Notably, protein-truncating variants in many of these same genes are associated with autism and developmental disorders, whereas the vast majority of variants that co-segregated with bipolar disorder in our dataset are missense or non-coding. Therefore, bipolar disorder may be associated with less deleterious variants in some of the same genes that cause neurodevelopmental disorders.
Evidence supporting the involvement of the cerebellum in bipolar disorder and related psychiatric disorders spans >25 years of research [97,98,99]. Human brain imaging studies indicate structural and functional changes in bipolar disorder and schizophrenia, including reduced cerebellar volume [100,101,102] and altered cortico-cerebellar functional connectivity [102,103,104]. Anatomical studies have revealed that Purkinje neurons are reduced both in number and in size in patients with psychiatric disorders [98, 105,106,107]. Our transcriptomic analyses are also consistent with previous studies demonstrating down-regulation of transcripts and proteins for synaptic genes, including GABAA receptors and NMDA receptor components [108,109,110]. A causal role is supported by numerous reports of bipolar disorder and related affective disorders in patients with cerebellar lesions [111,112,113]. Our results suggest that rare variants acting primarily through these hindbrain circuits may contribute substantially to risk for bipolar disorder.
Data availability
Genome sequences have been deposited in the BIGPOWER database and are available upon request to Dr. Jared Roach (jedroach@uw.edu). RNA-seq data have been deposited in the Neuroscience Multi-Omic Archive (http://data.nemoarchive.org/other/grant/sament/sament/dao_rnaseq/). The DaoG130V/+ mouse line has been made available through the Mutant Mouse Resource and Research Centers (RRID:MMRRC_067164-UCD). All other data are available in the main text or the supplementary materials.
References
Merikangas KR, Low NCP. The epidemiology of mood disorders. Curr Psychiatry Rep. 2004;6:411–21.
Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53:817–29.
Georgi B, Craig D, Kember RL, Liu W, Lindquist I, Nasser S, et al. Genomic view of bipolar disorder revealed by whole genome sequencing in a genetic isolate. PLoS Genet. 2014;10:e1004229.
Ament SA, Szelinger S, Glusman G, Ashworth J, Hou L, Akula N, et al. Rare variants in neuronal excitability genes influence risk for bipolar disorder. Proc Natl Acad Sci USA 2015;112:3576–81.
Goes FS, Pirooznia M, Parla JS, Kramer M, Ghiban E, Mavruk S, et al. Exome Sequencing of Familial Bipolar Disorder. JAMA Psychiatry. 2016;73:590–7.
Cruceanu C, Schmouth JF, Torres-Platas SG, Lopez JP, Ambalavanan A, Darcq E, et al. Rare susceptibility variants for bipolar disorder suggest a role for G protein-coupled receptors. Mol Psychiatry. 2018;23:2050–6.
Toma C, Shaw AD, Allcock RJN, Heath A, Pierce KD, Mitchell PB, et al. An examination of multiple classes of rare variants in extended families with bipolar disorder. Transl Psychiatry. 2018;8:65.
Jia X, Goes FS, Locke AE, Palmer D, Wang W, Cohen-Woods S, et al. Investigating rare pathogenic/likely pathogenic exonic variation in bipolar disorder. Mol Psychiatry. 2021;26:5239–50.
Palmer DS, Howrigan DP, Chapman SB, Adolfsson R, Bass N, Blackwood D, et al. Exome sequencing in bipolar disorder reveals shared risk gene AKAP11 with schizophrenia. MedRxiv. 2021:2021.03.09.21252930.
Nakajima K, Miranda A, Craig DW, Shekhtman T, Kmoch S, Bleyer A, et al. Ntrk1 mutation co-segregating with bipolar disorder and inherited kidney disease in a multiplex family causes defects in neuronal growth and depression-like behavior in mice. Transl Psychiatry. 2020;10:407.
Badner JA, Koller D, Foroud T, Edenberg H, Nurnberger JI, Zandi PP, et al. Genome-wide linkage analysis of 972 bipolar pedigrees using single-nucleotide polymorphisms. Mol Psychiatry. 2012;17:818–26.
Glusman G, Caballero J, Mauldin DE, Hood L, Roach JC. Kaviar: an accessible system for testing SNV novelty. Bioinformatics 2011;27:3216–7.
McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GRS, Thormann A, et al. The Ensembl variant effect predictor. Genome Biol. 2016;17:122.
Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, et al. An integrated map of genetic variation from 1,092 human genomes. Nature 2012;491:56–65.
Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–5.
Kang HM, Sul JH, Service SK, Zaitlen NA, Kong S-Y, Freimer NB, et al. Variance component model to account for sample structure in genome-wide association studies. Nat Genet. 2010;42:348–54.
Roach JC, Glusman G, Smit AFA, Huff CD, Hubley R, Shannon PT, et al. Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science 2010;328:636–9.
Stittrich A-B, Lehman A, Bodian DL, Ashworth J, Zong Z, Li H, et al. Mutations in NOTCH1 Cause Adams-Oliver syndrome. Am J Hum Genet. 2014;95:275–84.
Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38:W214–20.
Csardi G, Nepusz T. The igraph software package for complex network research. InterJournal, Complex Syst. 2006. 2006.
Kelly ST leiden: R implementation of the Leiden algorithm. R package version 0.3.7. 2020.
Fleishman SJ, Leaver-Fay A, Corn JE, Strauch E-M, Khare SD, Koga N, et al. RosettaScripts: A scripting language interface to the Rosetta macromolecular modeling suite. PLoS One. 2011;6:e20161.
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The protein data bank, 1999–. Int. Tables Crystallogr. Vol. F Crystallogr. Biol. Macromol., Springer; 2006.675–84.
Kawazoe T, Tsuge H, Imagawa T, Aki K, Kuramitsu S, Fukui K. Structural basis of D-DOPA oxidation by D-amino acid oxidase: alternative pathway for dopamine biosynthesis. Biochem Biophys Res Commun. 2007;355:385–91.
Sparey T, Abeywickrema P, Almond S, Brandon N, Byrne N, Campbell A, et al. The discovery of fused pyrrole carboxylic acids as novel, potent D-amino acid oxidase (DAO) inhibitors. Bioorg Med Chem Lett. 2008;18:3386–91.
Duplantier AJ, Becker SL, Bohanon MJ, Borzilleri KA, Chrunyk BA, Downs JT, et al. Discovery, SAR, and pharmacokinetics of a novel 3-hydroxyquinolin-2(1H)-one series of potent D-amino acid oxidase (DAAO) inhibitors. J Med Chem. 2009;52:3576–85.
Hondo T, Warizaya M, Niimi T, Namatame I, Yamaguchi T, Nakanishi K, et al. 4-Hydroxypyridazin-3(2H)-one derivatives as novel D-amino acid oxidase inhibitors. J Med Chem. 2013;56:3582–92.
Hopkins SC, Heffernan MLR, Saraswat LD, Bowen CA, Melnick L, Hardy LW, et al. Structural, kinetic, and pharmacodynamic mechanisms of D-amino acid oxidase inhibition by small molecules. J Med Chem. 2013;56:3710–24.
Ashworth J, Bernard B, Reynolds S, Plaisier CL, Shmulevich I, Baliga NS. Structure-based predictions broadly link transcription factor mutations to gene expression changes in cancers. Nucleic Acids Res. 2014;42:12973–83.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001;25:402–8.
Jagannath V, Brotzakis ZF, Parrinello M, Walitza S, Grünblatt E. Controversial effects of D-amino acid oxidase activator (DAOA)/G72 on D-amino acid oxidase (DAO) activity in human neuronal, astrocyte and kidney cell lines: the N-methyl D-aspartate (NMDA) receptor hypofunction point of view. Front Mol Neurosci. 2017;10:342.
Sacchi S, Bernasconi M, Martineau M, Mothet J-P, Ruzzene M, Pilone MS, et al. pLG72 modulates intracellular D-serine levels through its interaction with D-amino acid oxidase: effect on schizophrenia susceptibility. J Biol Chem. 2008;283:22244–56.
Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav. 1997;31:197–211.
Cryan JF, Mombereau C. In search of a depressed mouse: Utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–57.
Neely CLC, Pedemonte KA, Boggs KN, Flinn JM. Nest building behavior as an early indicator of behavioral deficits in mice. JoVE. 2019:e60139.
Deacon RMJ. Assessing nest building in mice. Nat Protoc. 2006;1:1117.
Seibenhener ML, Wooten MC. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. JoVE. 2015:e52434.
Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–8.
Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature 1977;266:730–2.
Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The mouse forced swim test. JoVE. 2012:e3638.
Gould TD, O’Donnell KC, Picchini AM, Dow ER, Chen G, Manji HK. Generation and behavioral characterization of β-catenin forebrain-specific conditional knock-out mice. Behav Brain Res. 2008;189:117–25.
Chartoff EH, Heusner CL, Palmiter RD. Dopamine is not required for the hyperlocomotor response to NMDA receptor antagonists. Neuropsychopharmacology 2005;30:1324–33.
Terrillion CE, Dao DT, Cachope R, Lobo MK, Puche AC, Cheer JF, et al. Reduced levels of C acna1c attenuate mesolimbic dopamine system function. Genes, Brain Behav. 2017;16:495–505.
Wu J, Zou H, Strong JA, Yu J, Zhou X, Xie Q, et al. Bimodal effects of MK-801 on locomotion and stereotypy in C57BL/6 mice. Psychopharmacol (Berl). 2005;177:256–63.
Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34:525–7.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010;26:139–40.
Chen C, Cheng L, Grennan K, Pibiri F, Zhang C, Badner JA, et al. Two gene co-expression modules differentiate psychotics and controls. Mol Psychiatry. 2013;18:1308–14.
Smyth GKG. Limma: linear models for microarray data. Bioinforma. Comput. Biol. Solut. Using R Bioconductor, New York: Springer-Verlag; 2005.397–420.
Langfelder P, Horvath S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinforma. 2008;9:559.
Wu D, Lim E, Vaillant F, Asselin-Labat ML, Visvader JE, Smyth GK. ROAST: Rotation gene set tests for complex microarray experiments. Bioinformatics 2010;26:2176–82.
Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57.
Kozareva V, Martin C, Osorno T, Rudolph S, Guo C, Vanderburg C, et al. A transcriptomic atlas of mouse cerebellar cortex comprehensively defines cell types. Nature 2021;598:214–9.
Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36:411–20.
Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020;581:434–43.
Wright CF, Fitzgerald TW, Jones WD, Clayton S, McRae JF, Van Kogelenberg M, et al. Genetic diagnosis of developmental disorders in the DDD study: A scalable analysis of genome-wide research data. Lancet 2015;385:1305–14.
Genovese G, Fromer M, Stahl EA, Ruderfer DM, Chambert K, Landén M, et al. Increased burden of ultra-rare protein-altering variants among 4,877 individuals with schizophrenia. Nat Neurosci. 2016;19:1433–41.
Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An JY, et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020;180:568–.e23.
Singh T, Poterba T, Curtis D, Akil H, Al-Eissa M, Barchas JD, et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature. https://doi.org/10.1038/s41586-022-04556-w 2022.
O’Dushlaine C, Rossin L, Lee PH, Duncan L, Parikshak NN, Newhouse S, et al. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2015;18:199–209.
Park HK, Shishido Y, Ichise-Shishido S, Kawazoe T, Ono K, Iwana S, et al. Potential role for astroglial D-amino acid oxidase in extracellular D-serine metabolism and cytotoxicity. J Biochem. 2006;139:295–304.
Oliet SHR, Mothet JP. Regulation of N-methyl-d-aspartate receptors by astrocytic d-serine. Neuroscience 2009;158:275–83.
Farrell MS, Werge T, Sklar P, Owen MJ, Ophoff RA, O’donovan MC, et al. Evaluating historical candidate genes for schizophrenia. Mol Psychiatry. 2015;20:555–62.
Hashimoto A, Yoshikawa M, Niwa A, Konno R. Mice lacking D-amino acid oxidase activity display marked attenuation of stereotypy and ataxia induced by MK-801. Brain Res. 2005;1033:210–5.
Yamanaka M, Miyoshi Y, Ohide H, Hamase K, Konno R. D-Amino acids in the brain and mutant rodents lacking D-amino-acid oxidase activity. Amino Acids. 2012;43:1811–21.
Zhang M, Ballard ME, Basso AM, Bratcher N, Browman KE, Curzon P, et al. Behavioral characterization of a mutant mouse strain lacking d-amino acid oxidase activity. Behav Brain Res. 2011;217:81–87.
Almond SL, Fradley RL, Armstrong EJ, Heavens RB, Rutter AR, Newman RJ, et al. Behavioral and biochemical characterization of a mutant mouse strain lacking d-amino acid oxidase activity and its implications for schizophrenia. Mol Cell Neurosci. 2006;32:324–34.
Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, et al. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA. 2002;99:13675–80.
Shi J, Gershon ES, Liu C. Genetic associations with schizophrenia: meta-analyses of 12 candidate genes. Schizophr Res. 2008;104:96–107.
Liu Y-L, Wang S-C, Hwu H-G, Fann CS-J, Yang U-C, Yang W-C, et al. Haplotypes of the D-Amino Acid oxidase gene are significantly associated with schizophrenia and its neurocognitive deficits. PLoS One. 2016;11:e0150435.
Prata D, Breen G, Osborne S, Munro J, St Clair D, Collier D. Association of DAO and G72(DAOA)/G30 genes with bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:914–7.
Bass NJ, Datta SR, McQuillin A, Puri V, Choudhury K, Thirumalai S, et, 74 al. Evidence for the association of the DAOA (G72) gene with schizophrenia and bipolar disorder but not for the association of the DAO gene with schizophrenia. Behav Brain Funct. 2009;5:28.
Jönsson EG, Saetre P, Vares M, Andreou D, Larsson K, Timm S, et al. DTNBP1, NRG1, DAOA, DAO and GRM3 polymorphisms and schizophrenia: An association study. Neuropsychobiology 2009;59:142–50.
Leaver-Fay A, Tyka M, Lewis SM, Lange OF, Thompson J, Jacak R, et al. ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Methods Enzymol. 2011;487:545–74.
Molla G Competitive inhibitors unveil structure/function relationships in human D-amino acid oxidase. Front Mol Biosci. 2017;4:80.
Brandish PE, Chiu CS, Schneeweis J, Brandon NJ, Leech CL, Kornienko O, et al. A cell-based ultra-high-throughput screening assay for identifying inhibitors of D-amino acid oxidase. J Biomol Screen. 2006;11:481–7.
Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell 2018;174:1015–.e16.
Sasabe J, Suzuki M, Imanishi N, Aiso S. Activity of D-amino acid oxidase is widespread in the human central nervous system. Front Synaptic Neurosci. 2014;6:14.
Pritchett D, Hasan S, Tam SKE, Engle SJ, Brandon NJ, Sharp T, et al. d-amino acid oxidase knockout (Dao(-/-)) mice show enhanced short-term memory performance and heightened anxiety, but no sleep or circadian rhythm disruption. Eur J Neurosci. 2015;41:1167–79.
Morikawa A, Hamase K, Inoue T, Konno R, Niwa A, Zaitsu K. Determination of free D-aspartic acid, D-serine and D-alanine in the brain of mutant mice lacking D-amino acid oxidase activity. J Chromatogr B Biomed Sci Appl. 2001;757:119–25.
Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16.
Post RM, Leverich GS. The role of psychosocial stress in the onset and progression of bipolar disorder and its comorbidities: The need for earlier and alternative modes of therapeutic intervention. Dev Psychopathol. 2006;18:1181–211.
Ebner K, Singewald N. Individual differences in stress susceptibility and stress inhibitory mechanisms. Curr Opin Behav Sci. 2017;14:54–64.
Alloy L, Seligman M. On the cognitive component of learned helplessness and depression. Psychol Learn Motiv. 1979;13:219–76.
Maier SF, Seligman MEP. Learned helplessness at fifty: Insights from neuroscience. 2016;123:349–67.
Baselmans BML, Jansen R, Ip HF, van Dongen J, Abdellaoui A, van de Weijer MP, et al. Multivariate genome-wide analyses of the well-being spectrum. Nat Genet. 2019;51:445–51.
Luciano M, Hagenaars SP, Davies G, Hill WD, Clarke TK, Shirali M, et al. Association analysis in over 329,000 individuals identifies 116 independent variants influencing neuroticism. Nat Genet. 2018;50:6–11.
Atz ME, Rollins B, Vawter MP. NCAM1 association study of bipolar disorder and schizophrenia: Polymorphisms and alternatively spliced isoforms lead to similarities and differences. Psychiatr Genet. 2007;17:55–67.
Lippard ETC, Nemeroff CB. The devastating clinical consequences of child abuse and neglect: Increased disease vulnerability and poor treatment response in mood disorders. Am J Psychiatry. 2020;177:20–36.
Gould TD, Gottesman II. Psychiatric endophenotypes and the development of valid animal models. Genes, Brain Behav. 2006;5:113–9.
Lenox RH, Gould TD, Manji HK. Endophenotypes in bipolar disorder. Am J Med Genet - Neuropsychiatr Genet. 2002;114:391–406.
Kakegawa W, Miyoshi Y, Hamase K, Matsuda S, Matsuda K, Kohda K, et al. D-Serine regulates cerebellar LTD and motor coordination through the δ glutamate receptor. Nat Neurosci. 2011;14:603–13.
Naur P, Hansen KB, Kristensen AS, Dravid SM, Pickering DS, Olsen L, et al. Ionotropic glutamate-like receptor δ2 binds D-serine and glycine. Proc Natl Acad Sci USA 2007;104:14116–21.
Gao Z, Van Beugen BJ, De Zeeuw CI. Distributed synergistic Plasticity and cerebellar learning. Nat Rev Neurosci. 2012;13:619–35.
Strata P. The emotional cerebellum. Cerebellum 2015;14:570–7.
Sacchetti B, Scelfo B, Tempia F, Strata P. Long-term synaptic changes induced in the cerebellar cortex by fear conditioning. Neuron 2004;42:973–82.
Han JK, Kwon SH, Kim YG, Choi J, Kim JI, Lee YS, et al. Ablation of STAT3 in purkinje cells reorganizes cerebellar synaptic plasticity in long-term fear memory network. Elife 2021;10:1–23.
Phillips JR, Hewedi DH, Eissa AM, Moustafa AA. The cerebellum and psychiatric disorders. Front Public Heal. 2015;3:66.
Wang SSH, Kloth AD, Badura A. The cerebellum, sensitive periods, and autism. Neuron 2014;83:518–32.
Ding S-L, Royall JJ, Sunkin SM, Ng L, Facer BAC, Lesnar P, et al. Comprehensive cellular-resolution atlas of the adult human brain. J Comp Neurol. 2016;524:3127–481.
Delbello MP, Strakowski SM, Zimmerman ME, Hawkins JM, Sax KW. MRI analysis of the cerebellum in bipolar disorder: A pilot study. Neuropsychopharmacology 1999;21:63–8.
Baldaçara L, Nery-Fernandes F, Rocha M, Quarantini LC, Rocha GGL, Guimarães JL, et al. Is cerebellar volume related to bipolar disorder? J Affect Disord. 2011;135:305–9.
Lupo M, Siciliano L, Leggio M. From cerebellar alterations to mood disorders: A systematic review. Neurosci Biobehav Rev. 2019;103:21–28.
He Y, Wang Y, Chang TT, Jia Y, Wang J, Zhong S, et al. Abnormal intrinsic cerebro-cerebellar functional connectivity in un-medicated patients with bipolar disorder and major depressive disorder. Psychopharmacol (Berl). 2018;235:3187–3200.
Wang Y, Zhong S, Chen G, Liu T, Zhao L, Sun Y, et al. Altered cerebellar functional connectivity in remitted bipolar disorder: A resting-state functional magnetic resonance imaging study. Aust N. Zeal J Psychiatry. 2018;52:52.
Maloku E, Covelo IR, Hanbauer I, Guidotti A, Kadriu B, Hu Q, et al. Lower number of cerebellar Purkinje neurons in psychosis is associated with reduced reelin expression. Proc Natl Acad Sci USA 2010;107:4407–11.
Tran KD, Smutzer GS, Doty RL, Arnold SE. Reduced Purkinje cell size in the cerebellar vermis of elderly patients with schizophrenia. Am J Psychiatry. 1998;155:1288–90.
Skefos J, Cummings C, Enzer K, Holiday J, Weed K, Levy E, et al. Regional alterations in Purkinje cell density in patients with autism. PLoS One. 2014;9:e81255.
Benes FM, Berretta S. GABAergic interneurons: Implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 2001;25:1–27.
Fatemi SH, Folsom TD, Rooney RJ, Thuras PD. Expression of GABAA α2-, β1- and ɛ-receptors are altered significantly in the lateral cerebellum of subjects with schizophrenia, major depression and bipolar disorder. Transl Psychiatry. 2013;3:e303–e303.
Bullock WM, Cardon K, Bustillo J, Roberts RC, Perrone-Bizzozero NI. Altered expression of genes involved in GABAergic transmission and neuromodulation of granule cell activity in the cerebellum of schizophrenia patients. Am J Psychiatry. 2008;165:1594–603.
Lupo M, Olivito G, Siciliano L, Masciullo M, Molinari M, Cercignani M, et al. Evidence of cerebellar involvement in the onset of a manic state. Front Neurol. 2018;9:774.
Wolf U, Rapoport MJ, Schweizer TA. Evaluating the affective component of the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2009;21:245–53.
Lauterbach EC. Bipolar disorders, dystonia, and compulsion after dysfunction of the cerebellum, dentatorubrothalamic tract, and substantia nigra. Biol Psychiatry. 1996;40:726–30.
Acknowledgements
This work was supported by grants from the National Institute of Mental Health (R01 MH094483 to J.R.K.; R01 MH110437 to P.P.Z.; F31 MH123066 to L.M.R.), the National Institute of General Medical Sciences (T32 GM008181 to L.M.R.), the National Institute of General Medical Sciences Center for Systems Biology (P50 GM076547, Leroy Hood, principal investigator), the Intramural Research Program of the National Institute of Mental Health (F.J.M.), U.S. Department of Veterans Affairs Merit Awards I01BX004062 and 101BX003631 (T.D.G.), the University of Luxembourg–Institute for Systems Biology Strategic Partnership (Leroy Hood, principal investigator), a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation (S.A.A.), and seed funding from the University of Maryland School of Medicine (S.A.A.). Data and biomaterials were collected as part of 11 projects (Study 40) that participated in the NIMH Bipolar Disorder Genetics Initiative (MH59545, MH059534, MH59533, MH59553, MH60068, MH059548, MH59535, MH59567, MH059556, and 1Z01MH002810-01), which was also supported by NIH Grants P50CA89392, from the National Cancer Institute, and 5K02DA021237, from the National Institute of Drug Abuse. We thank the Coriell Institute for providing DNA samples. We thank the staff of the University of Maryland Veterinary Resources and at the Mutant Mouse Resource and Research Center at UC Davis for assistance in maintaining and archiving the DaoG130V/+ knock-in mouse line. We thank the Genomics Resource Center at the University of Maryland School of Medicine for RNA sequencing. Most importantly, we thank the families who have participated in and contributed to these studies.
Author information
Authors and Affiliations
Contributions
Conceptualization: NH, SAA. Investigation: NH, LMR, TS, JA, RL, RTO, EMH, JB, PT, JCR, and SAA. Funding acquisition: SAA, JRK, and PPZ. Supervision: DCG, DWC, HWE, ESG, FJM, JIN, PPZ, JRK, JCR, TDG, and SAA. Writing – original draft: NH, LMR, SAA. Writing – review & editing: all authors.
Corresponding author
Ethics declarations
Competing interests
Dr. Nurnberger is an investigator for Janssen. All other authors declare that they have no competing interests. The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hasin, N., Riggs, L.M., Shekhtman, T. et al. Rare variants implicate NMDA receptor signaling and cerebellar gene networks in risk for bipolar disorder. Mol Psychiatry 27, 3842–3856 (2022). https://doi.org/10.1038/s41380-022-01609-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01609-4
This article is cited by
-
Genome-wide significant risk loci for mood disorders in the Old Order Amish founder population
Molecular Psychiatry (2023)