Abstract
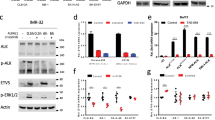
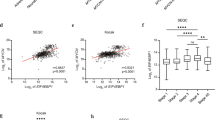
ALK mutations occur in 10% of primary neuroblastomas and represent a major target for precision treatment. In combination with MYCN amplification, ALK mutations infer an ultra-high-risk phenotype resulting in very poor patient prognosis. To open up opportunities for future precision drugging, a deeper understanding of the molecular consequences of constitutive ALK signaling and its relationship to MYCN activity in this aggressive pediatric tumor entity will be essential. We show that mutant ALK downregulates the ‘HMG-box transcription factor 1’ (HBP1) through the PI3K-AKT–FOXO3a signaling axis. HBP1 inhibits both the transcriptional activating and repressing activity of MYCN, the latter being mediated through PRC2 activity. HBP1 itself is under negative control of MYCN through miR-17~92. Combined targeting of HBP1 by PI3K antagonists and MYCN signaling by BET- or HDAC-inhibitors blocks MYCN activity and significantly reduces tumor growth, suggesting a novel targeted therapy option for high-risk neuroblastoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Louis CU, Shohet JM. Neuroblastoma: molecular pathogenesis and therapy. Annu Rev Med. 2015;66:49–63.
Cheung N-K, Dyer M. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer Nat. 2013;13:397–411.
Molenaar JJ, Koster J, Zwijnenburg DA, van Sluis P, Valentijn LJ, van der Ploeg I, et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature. 2012;483:589–93.
Mossé Y, Laudenslager M, Longo L, Cole K, Wood A, Attiyeh E, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–5.
George R, Sanda T, Hanna M, Fröhling SW II, Zhang J, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Cah Rev The. 2008;455:975–8.
Chen Y, Takita J, Choi Y, Kato M, Ohira M, Sanada M, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Cah Rev The. 2008;455:971–4.
Janoueix-Lerosey I, Lequin D, Brugières L, Ribeiro A, Pontual L, Combaret V, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Cah Rev The. 2008;455:967–70.
Carpenter EL, Mossé YP. Targeting ALK in neuroblastoma—preclinical and clinical advancements. Nat Rev Clin Oncol. 2012;9:391–9.
Guan Tucker, Wan Chand, Danielson Ruuth, et al. The ALK inhibitor PF-06463922 is effective as a single agent in neuroblastoma driven by expression of ALK and MYCN. Dis Model Mech Highwire. 2016;9:941–52.
Amin A, Li L, Rajan S, Gokhale V, Groysman M, Pongtornpipat P, et al. TKI sensitivity patterns of novel kinase-domain mutations suggest therapeutic opportunities for patients with resistant ALK+tumors. Oncotarget. 2016;7:23715–29.
Pall G. The next-generation ALK inhibitors. Curr Opin Oncol. 2015;27:118.
Duncan JS, Whittle MC, Nakamura K, Abell AN, Midland AA, Zawistowski JS, et al. Dynamic reprogramming of the kinome in response to targeted MEK Inhibition in triple-negative breast. Cancer Cell. 2012;149:307–21.
Toyokawa Seto. Updated evidence on the mechanisms of resistance to ALK inhibitors and strategies to overcome such resistance: clinical and preclinical data. Oncol Res Treat. 2015;0:291–8.
Bresler SC, Wood AC, Haglund EA, Courtright J, Belcastro LT, Plegaria JS, et al. Differential inhibitor sensitivity of anaplastic lymphoma kinase variants found in neuroblastoma. Sci Transl Med. 2011;3:108ra114.
Bresler SC, Weiser DA, Huwe PJ, Park JH, Krytska K, Ryles H, et al. ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma. Cancer Cell. 2014;26:682–94.
Heuckmann J, Hölzel M, Sos M, Heynck S, Balke-Want H, Koker M, et al. ALK mutations conferring differential resistance to structurally diverse ALK inhibitors. Clin Cancer Res. 2011;17:7394–401.
Yan X, Kennedy CR, Tilkens SB, Wiedemeier O, Guan H, Park J-II, et al. Cooperative cross-talk between neuroblastoma subtypes confers resistance to anaplastic lymphoma kinase inhibition. Genes Cancer. 2011;2:538–49.
Debruyne Bhatnagar, Sharma Luther, Moore CheungN-K, et al. ALK inhibitor resistance in ALK(F1174L)-driven neuroblastoma is associated with AXL activation and induction of EMT. Oncogene. 2016;35:3681–91.
Isozaki H, Ichihara E, Takigawa N, Ohashi K, Ochi N, Yasugi M, et al. Non-small cell lung cancer cells acquire resistance to the ALK inhibitor alectinib by activating alternative receptor tyrosine kinases. Cancer Res Highwire. 2015;76:1506–16.
Lovly CM, Shaw AT. Molecular pathways: resistance to kinase inhibitors and implications for therapeutic strategies. Clin Cancer Res. 2014;20:2249–56.
De Brouwer S, De Preter K, Kumps C, Zabrocki P, Porcu M, Westerhout E, et al. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin Cancer Res. 2010;16:4353–62.
Zhu S, Lee J-S, Guo F, Shin J, Perez-Atayde A, Kutok J, et al. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell. 2012;21:362–73.
Berry T, Luther W, Bhatnagar N, Jamin Y, Poon E, Sanda T, et al. The ALKF1174L mutation potentiates the oncogenic activity of MYCN in neuroblastoma. Cancer Cell. 2012;22:117–30.
Schönherr C, Ruuth K, Kamaraj S, Wang C-L, Yang H-L, Combaret V, et al. Anaplastic lymphoma kinase (ALK) regulates initiation of transcription of MYCN in neuroblastoma cells. Oncogene. 2012;31:5193–200.
Chesler L, Schlieve C, Goldenberg DD, Kenney A, Kim G, McMillan A, et al. Inhibition of phosphatidylinositol 3-kinase destabilizes Mycn protein and blocks malignant progression in neuroblastoma. Cancer Res. 2006;66:8139–46.
Cage TA, Chanthery Y, Chesler L, Grimmer M, Knight Z, Shokat K, et al. Downregulation of MYCN through PI3K inhibition in mouse models of pediatric neural cancer. Front Oncol. 2015;5:111. Jan 4
Vaughan L, Clarke P, Barker K, Chanthery Y, Gustafson C, Tucker E, et al. Inhibition of mTOR-kinase destabilizes MYCN and is a potential therapy for MYCN-dependent tumors. Oncotarget. 2016;7:57525–44.
Lambertz I, Kumps C, Claeys S, Lindner S, Beckers A, Janssens E, et al. Upregulation of MAPK negative feedback regulators and RET in mutant ALK neuroblastoma: implications for targeted treatment. Clin Cancer Res. 2015;21:3327–39.
Escamilla-Powers J, Daniel C, Farrell A, Taylor K, Zhang X, Byers S, et al. The tumor suppressor protein HBP1 is a novel c-Myc-binding protein that negatively regulates c-Myc transcriptional activity. J Biol Chem Highwire. 2010;285:4847–58.
Tevosian Shih, Mendelson Sheppard, Paulson Yee. HBP1: a HMG box transcriptional repressor that is targeted by the retinoblastoma family. Genes Dev. 1997;11:383–96.
Coomans de Brachène A, Bollaert E, Eijkelenboom A, de Serra A, van der Vos KE, Burgering BM, et al. The expression of the tumour suppressor HBP1 is down-regulated by growth factors via the PI3K/PKB/FOXO pathway. Biochem J. 2014;460:25–34.
Bollaert E, Johanns M, Herinckx G, Serra A, Vandewalle V, Havelange V, et al. HBP1 phosphorylation by AKT regulates its transcriptional activity and glioblastoma cell proliferation. Cell Signal. 2018;44:158–70.
Mossé Y, Lim M, Voss S, Wilner K, Ruffner K, Laliberte J, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children’s Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14:472–80.
Sakamoto H, Tsukaguchi T, Hiroshima S, Kodama T, Kobayashi T, Fukami T, et al. CH5424802, a Selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell Sci. 2011;19:679–90.
Passoni L, Longo L, Collini P, Coluccia A, Bozzi F, Podda M, et al. Mutation-independent anaplastic lymphoma kinase overexpression in poor prognosis neuroblastoma patients. Cancer Res. 2009;69:7338–46.
Heukamp L, Thor T, Schramm A, Preter K, Kumps C, Wilde B, et al. Targeted expression of mutated ALK induces neuroblastoma in transgenic mice. Sci TranslMedicine. 2012;4:141ra91–141ra91.
Moore NF, Azarova AM, Bhatnagar N, Ross KN, Drake LE, Frumm S, et al. Molecular rationale for the use of PI3K/AKT/mTOR pathway inhibitors in combination with crizotinib in ALK-mutated neuroblastoma. Oncotarget. 2014;5:8737–49.
Santo E, Stroeken P, Sluis P, Koster J, Versteeg R, Westerhout E. FOXO3a is a major target of inactivation by PI3K/AKT signaling in aggressive neuroblastoma. Cancer Res. 2013;73:2189–98.
Gu T-LL, Tothova Z, Scheijen B, Griffin JD, Gilliland DG, Sternberg DW. NPM-ALK fusion kinase of anaplastic large-cell lymphoma regulates survival and proliferative signaling through modulation of FOXO3a. Blood. 2004;103:4622–9.
Li H, Bian C, Liao L, Li J, Zhao R. miR-17-5p promotes human breast cancer cell migration and invasion through suppression of HBP1. Breast Cancer Res Treat. 2011;126:565–75.
Li Y, Choi PS, Casey SC, Dill DL, Felsher DW. MYC through miR-17-92 suppresses specific target genes to maintain survival, autonomous proliferation, and a neoplastic state. Cancer Cell. 2014;26:262–72.
Mestdagh Fredlund, Pattyn Schulte, Muth Vermeulen, et al. MYCN/c-MYC-induced microRNAs repress coding gene networks associated with poor outcome in MYCN/c-MYC-activated tumors. Oncogene. 2009;29:1394–404.
Althoff Beckers, Bell Nortmeyer, Thor Sprüssel, et al. A Cre-conditional MYCN-driven neuroblastoma mouse model as an improved tool for preclinical studies. Oncogene. 2015;34:3357–68.
Subramanian A, Tamayo P, Mootha V, Mukherjee S, Ebert B, Gillette M, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Valentijn LJ, Koster J, Haneveld F, Aissa RA, van Sluis P, Broekmans ME, et al. Functional MYCN signature predicts outcome of neuroblastoma irrespective of MYCN amplification. Proc Natl Acad Sci USA. 2012;109:19190–5.
Zeller K, Jegga A, Aronow B, O’Donnell K, Dang C. An integrated database of genes responsive to the Myc oncogenic transcription factor: identification of direct genomic targets. Genome Biol. 2003;4:1–10.
Bracken A, Dietrich N, Pasini D, Hansen K, Helin K. Genome-wide mapping of polycomb target genes unravels their roles in cell fate transitions. Genes Dev Highwire. 2006;20:1123–36.
Janky R, Verfaillie A, Imrichová H, Sande B, Standaert L, Christiaens V, et al. iRegulon: from a gene list to a gene regulatory network using large motif and track collections. PLoS Comput Biol. 2014;10:e1003731.
Henrich K-O, Bender S, Saadati M, Dreidax D, Gartlgruber M, Shao C, et al. Integrative genome-scale analysis identifies epigenetic mechanisms of transcriptional deregulation in unfavorable neuroblastomas. Cancer Res. 2016;76:5523–37.
Watanabe N, Kageyama R, Ohtsuka T. Hbp1 regulates the timing of neuronal differentiation during cortical development by controlling cell cycle progression. Development. 2015;142:2278–90.
Yao Works, Romagnoli Austin. Effects of overexpression of HBP1 upon growth and differentiation of leukemic myeloid cells. Leukemia. 2005;19:1958–68.
Frumm S, Fan Z, Ross K, Duvall J, Gupta S, VerPlank L, et al. Selective HDAC1/HDAC2 inhibitors induce neuroblastoma differentiation. Chem Biol. 2013;20:713–25.
Kim J, Zhang X, Rieger-Christ KM, Summerhayes IC, Wazer DE, Paulson KE.Yee AS, Suppression of Wnt signaling by the green tea compound (-)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. J Biol Chem. 2006;281:10865–75. https://www.ncbi.nlm.nih.gov/pubmed/16495219.
Puissant A, Frumm S, Alexe G, Bassil C, Qi J, Chanthery Y, et al. Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Discov. 2013;3:308–23.
Schubbert S, Cardenas A, Chen H, Garcia C, Guo W, Bradner J, et al. Targeting the MYC and PI3K pathways eliminates leukemia-initiating cells in T-cell acute lymphoblastic leukemia. Cancer Res. 2014;74:7048–59.
Stratikopoulos EE, Dendy M, Szabolcs M, Khaykin AJ, Lefebvre C, Zhou M-M, et al. Kinase and BET inhibitors together clamp inhibition of PI3K signaling and overcome resistance to therapy. Cancer Cell. 2015;27:837–51.
Stratikopoulos E, Parsons R. Molecular pathways: targeting the PI3K pathway in cancer—BET inhibitors to the rescue. Clin Cancer Res. 2016;22:2605–10.
Cortés C, Kozma S, Tauler A, Ambrosio S. MYCN concurrence with SAHA-induced cell death in human neuroblastoma cells. Cell Oncol. 2015;38:341–52.
Braekeveldt N, Wigerup C, Gisselsson D, Mohlin S, Merselius M, Beckman S, et al. Neuroblastoma patient‐derived orthotopic xenografts retain metastatic patterns and geno‐ and phenotypes of patient tumours. Int J Cancer. 2015;136:E252–E261.
Persson CU, von Stedingk K, Bexell D, Merselius M, Braekeveldt N, Gisselsson D, et al. Neuroblastoma patient-derived xenograft cells cultured in stem-cell promoting medium retain tumorigenic and metastatic capacities but differentiate in serum. Sci Rep. 2017;7:10274.
Pei Y, Liu K-W, Wang J, Garancher A, Tao R, Esparza LA, et al. HDAC and PI3K antagonists cooperate to inhibit growth of MYC-driven medulloblastoma. Cancer Cell. 2016;29:311–23.
Umapathy G, Wakil A, Witek B, Chesler L, Danielson L, Deng X, et al. The kinase ALK stimulates the kinase ERK5 to promote the expression of the oncogene MYCN in neuroblastoma. Sci Signal. 2014;7:ra102–ra102.
Duffy D, Krstic A, Schwarzl T, Higgins D, Kolch W. GSK3 inhibitors regulate MYCN mRNA levels and reduce neuroblastoma cell viability through multiple mechanisms, including p53 and Wnt signaling. Mol Cancer Ther. 2014;13:454–67.
Hasan K, Nafady A, Takatori A, Kishida S, Ohira M, Suenaga Y, et al. ALK is a MYCN target gene and regulates cell migration and invasion in neuroblastoma. Sci Rep Nat. 2013;3:3450.
Corvetta D, Chayka O, Gherardi S, D’Acunto C, Cantilena S, Valli E, et al. Physical interaction between MYCN oncogene and polycomb repressive complex 2 (PRC2) in neuroblastoma functional and therapeutic implications. J Biol Chem. 2013;288:8332–41.
Ferreira A, de-Freitas-Junior J, Morgado-Díaz J, Ridley A, Klumb C. Dual inhibition of histone deacetylases and phosphoinositide 3-kinases: effects on Burkitt lymphoma cell growth and migration. J Leukoc Biol. 2016;99:569–78.
Chou T-C, Talalay P. Analysis of combined drug effects: a new look at a very old problem. Trends Pharmacol Sci Sci. 1983;4:450–4.
Hellemans J, Mortier G, Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:1–14.
Coller H, Grandori C, Tamayo P, Colbert T, Lander E, Eisenman R, et al. Expression analysis with oligonucleotide microarrays reveals that MYC regulates genes involved in growth, cell cycle, signaling, and adhesion. Proc Natl Acad Sci. 2000;97:3260–5.
Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–38.
Fredlund E, Ringnér M, Maris JM, Påhlman S. High Myc pathway activity and low stage of neuronal differentiation associate with poor outcome in neuroblastoma. Proc Natl Acad Sci USA. 2008;105:14094–9.
Yu D, Cozma D, Park A, Thomas-Tikhonenko A. Functional validation of genes implicated in lymphomagenesis: an in vivo selection assay using a Myc-induced B-cell tumor. Ann N Y Acad Sci. 2005;1059:145–59.
Kim Y, Girard L, Giacomini C, Wang P, Hernandez-Boussard T, Tibshirani R, et al. Combined microarray analysis of small cell lung cancer reveals altered apoptotic balance and distinct expression signatures of MYC family gene amplification. Oncogene. 2005;25:130–8.
Acknowledgements
We thank Jeroen Schacht, Jolien Van Laere, Els De Smet, Fanny De Vloed, Givani Dewyn, and Aline Eggermont from our lab as well as Glenn Wagemans from the LECR for their outstanding technical assistance. Furthermore, we thank Els Janssens, Annelies Fieuw, Sara De Brouwer, and Irina Lambertz for their guidance during this project.
Funding
S.C. is supported by a pre-doctoral fellowship of the Research Foundation—Flanders (FWO; 11J8313N) and an Emmanuel van der Schueren grant (‘Kom op tegen Kanker’). S.V. is funded by the VLK (Flemish League against cancer) and ‘Stichting Villa Joep’. B.D. and S.L. are supported by a pre-doctoral fellowship of the FWO Research Foundation—Flanders (FWO). K.D. is supported by Ghent University (BOF; BOF16/PDO/043). C.V., B.D.W. and T.V.M. are senior clinical investigators of the Research Foundation—Flanders (FWO; 18B1716N (B.D.W.), 12N6917N (C.V.), 1803115 N (T.V.M.)). We would further like to thank the following funding agencies: the Belgian Foundation against Cancer (project 2014–175) to F.S., Ghent University (BOF10/GOA/019, BOF16/GOA/23) to F.S., the Belgian Program of Interuniversity Poles of Attraction (IUAP Phase VII–P7/03) to F.S., the fund for Scientific Research Flanders (Research projects G053012N, G050712N, G051516N to F.S) and ‘Stichting Villa Joep’ to F.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Claeys, S., Denecker, G., Durinck, K. et al. ALK positively regulates MYCN activity through repression of HBP1 expression. Oncogene 38, 2690–2705 (2019). https://doi.org/10.1038/s41388-018-0595-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0595-3
This article is cited by
-
Methylation of HBP1 by PRMT1 promotes tumor progression by regulating actin cytoskeleton remodeling
Oncogenesis (2022)
-
The ETS transcription factor ETV5 is a target of activated ALK in neuroblastoma contributing to increased tumour aggressiveness
Scientific Reports (2020)
-
The HMG box transcription factor HBP1: a cell cycle inhibitor at the crossroads of cancer signaling pathways
Cellular and Molecular Life Sciences (2019)
-
The targetable kinase PIM1 drives ALK inhibitor resistance in high-risk neuroblastoma independent of MYCN status
Nature Communications (2019)