Abstract
Cancer cells with mesenchymal attributes potentially display chemoresistance. Cancer stem cells (CSCs), which are intrinsically resistant to most chemotherapy agents, exhibit considerable phenotypic heterogeneity in their epithelial versus mesenchymal states. However, the drug response of CSCs in the epithelial and mesenchymal states has not been completely investigated. In this study, we found that epithelial-type (E-cadherinhigh/CD133high) CSCs displayed a higher sphere formation ability and chemoresistance than mesenchymal-type (E-cadherinlowCD133high) CSCs. Gene expression profiling of the CSC and non-CSC subpopulations with distinct epithelial-to-mesenchymal transition (EMT) states showed that MyoD family inhibitor domain-containing (MDFIC) was selectively upregulated in epithelial-type CSCs. Knockdown of MDFIC sensitized epithelial-type CSCs to chemotherapy agents. Ectopic expression of MDFIC increased the chemoresistance of mesenchymal-type CSCs. In a tissue microarray, high MDFIC expression was associated with poor prognosis of non-small cell lung cancer (NSCLC) patients. A mechanistic study showed that the MDFIC p32 isoform, which is located in the cytoplasm, interacted with the destruction complex, Axin/GSK-3/β-catenin. This interaction stabilized β-catenin by inhibiting β-catenin phosphorylation at S33/37 and increased the nuclear translocation and transcriptional activity of β-catenin. Knockdown of β-catenin decreased MDFIC-enhanced chemoresistance. These results suggested that the upregulation of MDFIC enhanced the chemoresistance of epithelial-type CSCs by elevating β-catenin activity. Thus, targeting MDFIC-regulated β-catenin signaling of epithelial-type CSCs may be a potential strategy to overcome chemoresistance in NSCLC.
Similar content being viewed by others
Introduction
Lung cancer is the most frequently diagnosed malignancy and the leading cause of cancer death worldwide [1, 2]. Platinum-based chemotherapy remains a common first-line treatment for non-small cell lung cancer (NSCLC) patients without treatable oncogenic alterations [3, 4]. Unfortunately, many patients show acquired chemoresistance and tumor relapse [5]. Cancer cells harboring a stem-like phenotype, referred to as cancer stem cells (CSCs), have been characterized as a subpopulation with a tumor-initiating ability, self-renewal, metastatic dissemination, and innate multidrug resistance [6]. The mechanism of innate drug resistance in CSCs has not yet been fully elucidated. Hedgehog, Notch, and Wnt/β-catenin signaling pathways that contribute to the maintenance of the CSC phenotype are also involved in chemoresistance, as well as in epithelial-to-mesenchymal transition (EMT) [7].
EMT is a process of cellular transformation from epithelial phenotypes to mesenchymal attributes. After the first report by Mani et al. [8] that cancer cells undergoing EMT could generate stem-like properties, EMT has been tightly linked to the induction of the CSC phenotype, chemoresistance, and metastasis for the past decade [9]. In general, higher invasiveness, stemness, and drug resistance of cancer cells are commonly associated with a mesenchymal state rather than with an epithelial state [10]. However, this concept of EMT-directed cancer cell behaviors has been challenged recently. First, as cancer cells transform from an epithelial state to a mesenchymal state, they may become resistant to some drugs and sensitive to others [11, 12]. Second, inhibition of some EMT inducers, such as Prrx1, enhances distal metastasis and CSC properties [13]. Third, breast cancer cells in a tumor or circulation exhibit dynamic EMT and show distinct drug responses and redox metabolism [14,15,16]. These diverse studies suggest that EMT may not always couple with metastasis, stemness, and chemoresistance during disease progression. A recent study showed that the Wnt3a-induced CSC phenotype could be achieved only when the cancer cells were at an initially mesenchymal state (low E-cadherin) [17], suggesting that the response of cancer cells to environmental stimuli might depend on their native EMT status. However, the cytotoxic effect of chemotherapy agents on CSCs in an epithelial state or a mesenchymal state is unclear.
In this study, we found that CSCs in an epithelial state were more resistant to conventional chemotherapy agents than CSCs in a mesenchymal state. Among 13 genes that were selectively upregulated in the epithelial-type CSCs, the MyoD family inhibitor domain-containing (MDFIC; also known as human I-mfa domain-containing, HIC) protein contributed to the chemoresistance and self-renewal of epithelial-type CSCs. The MDFIC p32 isoform promoted β-catenin stabilization, nuclear translocation, and transcriptional activity, leading to an increase in chemoresistance. In addition, in a tissue microarray consisting of tumor specimens from 125 NSCLC patients, high MDFIC protein expression was associated with poor disease-free survival and overall survival of the patients.
Results
The E-cadHCD133H subpopulation has higher proliferation and sphere formation abilities
To study cellular physiology of lung CSCs at different EMT statuses, we first sorted the E-cadherin-high (E-cadH) and E-cadherin-low (E-cadL) subpopulations from PC14 and A549 cell lines, which contain heterogeneous cancer cells with different EMT statuses (Supplementary Figs. S1 and S2). Next, the CD133-high (CD133H) and CD133-low (CD133L) cells were isolated from the E-cadH and E-cadL subpopulations of PC14 and A549 cell lines (Supplementary Fig. S3a and Fig. 1a). The E-cadHCD133H subpopulations had a lower proliferation rate and higher sphere formation ability than the E-cadLCD133H subpopulations (Supplementary Fig. S3b and Fig. 1b). Morphologically, the E-cadHCD133H subpopulations generated compact spheroids, while the E-cadLCD133H subpopulations formed aggregate-like spheroids in the suspension cultures (Fig. 1c). To evaluate the tumor initiation ability of the four subpopulations, we subcutaneously transplanted 100 cells of each subpopulation into nude mice. Only the E-cadHCD133H and E-cadLCD133H subpopulations were capable of generating tumors compared with the E-cadHCD133L and E-cadLCD133L subpopulations, p = 0.043 by Fisher’s exact test (Fig. 1d). However, the tumor initiation ability of CD133H cells was not changed by their native EMT status.
a The western blot images showed the expression patterns of CD133 and EMT biomarkers. b The sphere formation efficiency of the four subpopulations. Scale bar, 100 μm. Statistical analysis showed the sphere number per 100 seeded cells. c The bright-field and fluorescent images showed the different morphology of tumor spheres. Scale bar, 50 μm. d The tumor initiation ability of the four subpopulations of the PC14 cell line was evaluated using subcutaneously xenografted nude mice (n = 8). e The heat maps showed the cell viability of the four subpopulations of PC14 and A549 cell lines treated with different concentrations of chemotherapy agents for 72 h. f The tumor-bearing mice were treated with cisplatin for 2 weeks, and the tumor size was measured. g The E-cadHCD133H or E-cadLCD133H subpopulations were injected into the tail veins of the nude mice (n = 5). Representative images showed metastatic nodules (arrows) in the lungs of mice.
The E-cadHCD133H subpopulation is more resistant to chemotherapy agents
Next, we treated the four subpopulations with conventional chemotherapy agents at different concentrations. The results showed that E-cadHCD133H subpopulations were more resistant to the drug treatments than other subpopulations (Fig. 1e). In a subcutaneous xenograft model, intraperitoneal injection of cisplatin suppressed the growth of E-cadHCD133L- and E-cadLCD133L-derived tumors. However, the E-cadHCD133H- and E-cadLCD133H-derived tumors continued growing during cisplatin treatment (Fig. 1f). To further assess the therapeutic effect of cisplatin on the metastasis of circulating tumor cells, E-cadHCD133H or E-cadLCD133H cells were intravenously injected into the lateral tail veins of the mice followed by cisplatin treatment immediately. Although cisplatin treatment reduced lung metastasis in both groups, more tumor nodules were found in the lungs of mice that received E-cadHCD133H cell transplantation (Fig. 1g). This result suggested that more circulating E-cadHCD133H might survive and completed distal metastasis in the lung. Notably, the E-cadherin expression patterns of the metastatic tumors derived from the four subpopulations were not changed after cisplatin treatment (Supplementary Fig. S3c). Together, these data indicated that the epithelial-type CSCs displayed higher chemoresistance than the mesenchymal-type CSCs.
Drug-resistant signature of the E-cadHCD133H subpopulation
From a gene expression microarray analysis of the four subpopulations, 205 genes that were differentially expressed in the E-cadHCD133H subpopulation (Supplementary Table S1). From gene ontology of gene set enrichment analysis (GSEA), the negative regulation of canonical Wnt signaling, EMT, apoptotic process, and cell mobility-related biological processes were enriched in the E-cadLCD133H subpopulation (Fig. 2a). However, genes associated with lung cell differentiation, lung epithelium development, and cell adhesion were enriched in the E-cadHCD133H subpopulation. In addition, the commonly enriched pathways based on the hallmark gene sets as defined by GSEA were determined. In the E-cadHCD133H subpopulation, the pathways of G2M checkpoint and DNA repair were enriched, while EMT and apoptosis pathways were enriched in the E-cadLCD133H subpopulation (Fig. 2b).
a Gene ontology analysis for the E-cadHCD133H subpopulation of PC14. b The pathways enriched in the E-cadHCD133H and E-cadLCD133H subpopulations were identified by GSEA. c The genes that were concurrently altered in the E-cadHCD133H subpopulation and the drug-resistant-associated microarray datasets were defined as the drug-resistant signature (DRS). d The expression patterns of the DRS in the selected datasets. e The DRS scores of the patients enrolled in the GSE31210 dataset were correlated with tumor relapse. p < 0.001 by Pearson’s Chi-square test. f The Kaplan–Meier survival curve showed that high DRS scores were associated with the poor disease-free survival of the patients in GSE31210. Hazard ratio = 4.2078 [2.5741–6.8783]. g Two-way hierarchical clustering of the 32 genes that individually correlated with the tumor relapse of the patients in GSE31210.
After filtering the genes with three chemoresistance-associated datasets, GSE14231, GSE12791, and GSE15709, 86 genes were putatively associated with chemoresistance (Supplementary Table S2). We therefore defined the expression pattern of these genes as the drug-resistant signature (DRS) (Fig. 2c, d). In a publicly available microarray dataset, GSE31210, which consisted of 226 patients with stage I and II lung cancer, high DRS scores were correlated with tumor relapse (Fig. 2e) and poor disease-free survival of the patients (Fig. 2f, Supplementary Tables S3 and S4). Among the 86 DRS genes, 32 genes were individually correlated with the tumor relapse of the patients (Supplementary Table S5). Indeed, the two-way hierarchical clustering of the 32 genes apparently classified the tumor recurrence of the patients (Fig. 2g).
MDFIC contributes to the drug resistance of the E-cadHCD133H subpopulation
The Cox univariate regression analysis showed that 13 out of the 32 tumor relapse-correlated genes potentially predicted the prognosis of the patients (Fig. 3a). MDFIC was at top of the ranked list of the ten genes that were significantly and selectively upregulated in the E-cadHCD133H subpopulation (Fig. 3b). Upregulation of MDFIC mRNA and protein levels in the E-cadHCD133H subpopulations of PC14 and A549 was confirmed by western blot and quantitative real-time PCR analyses (Fig. 3c, d). Protein translation of MDFIC can be initiated from an upstream GUG or an in-frame downstream AUG start codon, leading to the production of p40 and p32 isoforms, respectively [18]. The MDFIC-p40 isoform was mainly located in the nucleus, while the MDFIC p32 isoform was distributed in the cytoplasm (Fig. 3e). Ectopic expression of MDFIC-p32-myc in the E-cadHCD133L subpopulations, which expressed relatively lower levels of endogenous MDFIC and were sensitive to the chemotherapy agents, resulted in drug resistance to conventional chemotherapy agents compared with that of the vector-only control cells (Fig. 3f). However, overexpression of MDFIC-p40-myc did not alter the drug response of the cells to the drug treatments (Supplementary Fig. S4a). In contrast, knockdown of MDFIC sensitized the E-cadHCD133H subpopulations to the chemotherapy agents (Fig. 3g). In a subcutaneous xenograft mouse model, cisplatin treatment significantly inhibited the growth of MDFIC-knockdown tumors compared with that of scramble control tumors (Fig. 3h). In addition to the regulation of drug response, knockdown of MDFIC induced EMT but decreased sphere formation of the E-cadHCD133H subpopulation, while overexpression of MDFIC-p32-myc reversed EMT and increased sphere formation of the E-cadHCD133L subpopulation (Supplementary Fig. S5). These results suggested that the MDFIC p32 isoform contributed to drug resistance and self-renewal of epithelial-type CSCs, and it might also function as an EMT suppressor.
a Cox’s univariate regression analysis of indicated genes was performed for the disease-free survival of the patients in GSE31210. b The fold changes of indicated genes between the E-cadHCD133H and the E-cadLCD133H subpopulations. Western blot (c) and qPCR (d) confirmed that MDFIC was selectively upregulated in the E-cadHCD133H subpopulations of PC14 and A549. e Subcellular localization of MDFIC p32 and p40 isoforms in the E-cadHCD133L subpopulation of PC14. Scale bar, 10 μm. f Ectopic expression of the MDFIC p32 isoform significantly induced the chemoresistance of the E-cadHCD133L subpopulations of PC14 and A549. g Knockdown of MDFIC by two clones of shRNAs sensitized the E-cadHCD133H subpopulations of PC14 and A549. h The mice bearing the tumors of MDFIC-knockdown E-cadHCD133H subpopulation of PC14 were treated with cisplatin for 2 weeks, and then, the tumor size was measured.
High MDFIC expression in tumors is associated with poor prognosis of non-small cell lung cancer patients
Next, we evaluated the association of MDFIC with the prognosis of lung cancer patients in a tissue microarray comprising the clinical specimens of 125 NSCLC patients. The expression of MDFIC protein was evaluated by immunohistochemistry (IHC) using MDFIC antibody. The MDFIC staining signal was scored on a scale from 0 to 3 (Fig. 4a). The patients at the late stage had relatively higher MDFIC expression levels compared with the patients at the early stage (Fig. 4b). In addition, high MDFIC expression was significantly correlated with poor disease-free and overall survival of the patients (Fig. 4c, d and Table 1). Multivariate Cox regression analysis indicated that high MDFIC expression might be a good predictor for the poor outcome of the patients with NSCLC (Table 2). Similar results were also observed in a TCGA database, which consists of 740 lung cancer patients. High MDFIC mRNA levels were associated with poor disease-free and overall survival of the patients (Fig. 4e, f; Supplementary Table S6). Together, these data indicated that upregulation of MDFIC in tumors was correlated with poor prognosis of patients with NSCLC.
a MDFIC expression was examined by IHC in the tissue microarray. Representative images illustrated the IHC scores of MDFIC expression. Scale bar, 100 μm. b Quantification of MDFIC expression by IHC of the NSCLC specimens. The number (n) of samples for each stage is indicated at the top of each column. Kaplan–Meier survival analysis of the disease-free survival (c) and overall (d) survival of the 125 NSCLC patients in the tissue microarray stratified by MDFIC protein levels. The Kaplan–Meier survival analysis showed an association of MDFIC mRNA expression and disease-free survival (e) and overall (f) survival of the 740 NSCLC patients in TCGA database.
The MDFIC p32 isoform interacts with the Axin-GSK-3−β-catenin complex and stabilizes β-catenin
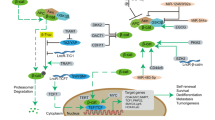
To study the molecular mechanism of MDFIC-mediated drug resistance, the potential binding proteins of MDFIC were predicted by using STRING [19, 20]. The protein network suggested that MDFIC might interact with the proteins involved in the Hippo and Wnt signaling pathways, including β-catenin (CTNNB1) (Fig. 5a and Supplementary Table S7). Immunoprecipitation assays showed that MDFIC p32-GFP, but not MDFIC p40-GFP, interacted with β-catenin, GSK-3, and Axin (Supplementary Fig. S4b, c). In parallel, immunoprecipitation of the endogenous MDFIC in the E-cadHCD133H cells by using anti-MDFIC or anti-β-catenin antibody confirmed the interaction of MDFIC with the Axin/GSK-3/β-catenin complex (Fig. 5b, c). The ectopic expression of MDFIC p32-GFP increased the total β-catenin but decreased the phosphorylated β-catenin at serine 33/37 (S33/37) and threonine 41 (T41) residues, which are all involved in β-catenin degradation (Fig. 5d). Treatment with MG132, a specific proteasome inhibitor, increased the β-catenin level, which was similar to the effect of MDFIC p32 overexpression on β-catenin (Fig. 5e). These results indicated that cytosolic MDFIC might interact with the Axin/GSK-3/β-catenin complex and stabilize β-catenin.
a MDFIC-interacting proteins were predicted by STRING. b Immunoprecipitation of MDFIC was performed by using anti-MDFIC antibody in the cell lysate of E-cadHCD133H subpopulation of PC14 and A549. c Immunoprecipitation of β-catenin was performed by using anti-β-catenin antibody in the cell lysate of E-cadHCD133H subpopulation of PC14 and A549. d The pLEX-GFP or MDFIC-p32-GFP plasmid was ectopically expressed in E-cadHCD133L subpopulation of PC14 and A549, and the expression of GFP, total β-catenin, and phosphorylated β-catenin at Ser33, Ser37, and Thr41 (S33/37/T41) was examined by western blots. e The pLEX-MCS vector or MDFIC-p32-GFP plasmid was ectopically expressed in E-cadHCD133L subpopulation of PC14 and A549, and the cells were treated with vehicle or MG132 (10 μg/ml) for 6 h. The expression of β-catenin was examined by western blot.
The MDFIC p32 isoform promotes the nuclear translocation of β-catenin, leading to drug resistance
To study whether MDFIC-mediated β-catenin stabilization can functionally contribute to drug resistance, we first analyzed nuclear localization of β-catenin in the four subpopulations. A high level of nuclear β-catenin was detected in the E-cadHCD133H subpopulations, which exhibited the highest drug resistance and MDFIC expression (Fig. 6a). The downstream target genes of β-catenin, Axin2, CD44, Cyclin D1, and Survivin, were upregulated in the E-cadHCD133H subpopulation compared with other subpopulations (Fig. 6b). The enforced expression of MDFIC p32 isoform increased the total and nuclear β-catenin (Fig. 6c and Supplementary Fig. S6a). Next, the transcriptional activity of β-catenin was evaluated by a TOP/FOP reporter assay. Overexpression of MDFIC p32 isoform significantly increased the luciferase activity, indicating the enhancement of the transcriptional activity of β-catenin (Supplementary Fig. S6b). Consistently, overexpression of MDFIC p32 isoform elevated the expression of downstream genes of β-catenin (Fig. 6d). Knockdown of β-catenin in the MDFIC p32 isoform-overexpressing E-cadHCD133L subpopulations increased the drug sensitivity of cells to the cisplatin and paclitaxol treatments compared to the scramble control group (Fig. 6e, f). In a xenograft animal study, overexpression of MDFIC-p32-myc significantly inhibited the therapeutic effect of cisplatin, while knockdown of β-catenin significantly sensitized the MDFIC-p32-myc-expressing tumors to cisplatin treatment (Fig. 6g). To confirm the role of MDFIC p32 isoform in nuclear translocation of β-catenin in clinical samples, IHC for the expression of MDFIC, and β-catenin was performed in the tumor samples from 25 NSCLC patients. The staining intensity of MDFIC and cytosolic and nuclear β-catenin was analyzed by using HistoQuest image analysis system. The results showed that the elevation of MDFIC levels was correlated with the increase of nuclear β-catenin staining signal (Fig. 6h). These results indicated that the MDFIC p32 isoform promoted the drug resistance of epithelial-type CSCs by enhancing the nuclear translocation and transcriptional activity of β-catenin.
a The expression of the indicated proteins in the nuclear and cytoplasmic fractions of the four subpopulations of PC14 and A549. b The mRNA expression of the β-catenin-downstream genes in the four subpopulations of PC14 and A549. c The expression of the indicated proteins in the nuclear and cytoplasmic fractions of vector- or MDFIC-p32-myc-expressed of E-cadHCD133L subpopulation of PC14 and A549. d MDFIC-p32-myc was enforced to be expressed in the E-cadHCD133L subpopulation. The mRNA levels of the indicated genes were analyzed by qPCR. e The β-catenin was knocked down by two clones of shRNA in the stable clone of the MDFIC-p32-myc-expressed E-cadHCD133L subpopulation of PC14 and A549. The protein levels of β-catenin and MDFIC-p32-myc were analyzed by western blots. f Cell viability was analyzed after paclitaxol or cisplatin treatment for 72 h. g The four groups of tumor-bearing mice were treated with cisplatin for 4 weeks (n = 5). The tumor size was measured weekly. h The IHC staining signal of MDFIC and nuclear β-catenin was examined and plotted.
Discussion
In the past decade, CSCs have been considered to display a mesenchymal phenotype, representing higher cell mobility, invasiveness, and drug resistance. However, a growing number of studies indicate that cancer cells with a stem-like phenotype may not always be in a mesenchymal state [13, 17, 21]. Here, we showed that CSCs (CD133H) could be isolated from both the E-cadH and E-cadL subpopulations of PC14 and A549 cell lines, suggesting heterogeneity of the EMT status of CSCs. The epithelial-type CSCs and mesenchymal-type CSCs had similar tumor-initiating and distal metastasis abilities. Nevertheless, the epithelial-type CSCs had higher self-renewal ability, tumor growth, and chemoresistance compared with the mesenchymal-type CSCs. Consistently, Tiran et al. reported that the CSCs isolated from a patient-derived lung adenocarcinoma cell culture showed a stronger association with the epithelial phenotype and were more resistant to cisplatin [21]. In triple-negative breast cancer, GSK-3 inhibitors selectively killed CD44+/CD24− cells with mesenchymal attributes and spared the cells with epithelial properties [16]. Pore et al. found that small-cell lung cancer with a mesenchymal phenotype (c-MET-high/E-cadherin-low) is associated with better survival and showed a trend toward lower circulating tumor cells [22]. In agreement with these reported data, our results revealed that epithelial-type CSCs might be a unique subpopulation driving chemoresistance, suggesting that targeting CSCs with epithelial attributes might be a potential strategy to prevent tumor relapse in NSCLC.
The heterogeneity of tumor is one of the reasons for drug resistance and relapse. Our study demonstrated the heterogeneous EMT and CSC phenotypes of cancer cells. It is worth to note that, in addition to CD133, more biomarkers for isolating CSCs in NSCLC have been identified in the past decade [23]. Although our data showed that the CD133H subpopulation harbored higher tumor-initiating ability compared with the CD133L subpopulations, we cannot exclude that the CD133L subpopulations may also contain tumor-initiating cells without CD133 expression. In addition, a little number of CD133H cells might escape from the FACS and contaminated the CD133L subpopulations. Thus, the cancer cell-derived tumors might remain heterogeneous.
Activation of canonical Wnt signaling by binding of Wnt ligands to Frizzled receptors leads to initiation of EMT, invasiveness, stemness, and drug resistance [24,25,26]. In the absence of Wnt ligands, β-catenin associates with the destruction complex consisting of adenomatous polyposis coli protein and Axin, which induce the phosphorylation of β-catenin at S45 and S33/37 by casein kinase 1 and GSK-3, respectively [27]. In epithelial cells, β-catenin binds to E-cadherin on the plasma membrane, while cytoplasmic β-catenin proteins associate with the destruction complex, leading to β-catenin degradation [28]. In our data, MDFIC interacted with the destruction complex and decreased the phosphorylation of β-catenin at Ser33/37, leading to the stabilization and nuclear translocation of β-catenin. Because MDFIC was selectively upregulated in the epithelial-type CSCs, the E-cadHCD133H subpopulation, the total β-catenin in the epithelial CSCs was dramatically higher than in the mesenchymal CSCs. Moreover, by comparing the epithelial-type CSCs and non-CSCs, nuclear β-catenin was significantly increased in the epithelial-type CSCs. These results confirmed that MDFIC contributes to β-catenin stabilization and nuclear translocation in epithelial CSCs, even in the absence of Wnt ligands.
MDFIC contains a cysteine-rich C-terminal domain, which shows a high degree of homology to the C-terminal domain of I-mfa [29, 30]. MDFIC interacts with several mammalian proteins and modulates their biological functions, although the regulatory mechanism of these MDFIC-mediated modulations is still unclear. Oakley et al. showed that MDFIC interacted with a glucocorticoid receptor and increased its phosphorylation at S211 and transcriptional activity [31]. Wang et al. reported that MDFIC bound to a regulatory histidine-rich region of cyclin T1 and a lysine and arginine-rich motif of cyclin T1 and T2 through its I-mfa domain [32]. Importantly, Kusano and Raab-Traub demonstrated that MDFIC directly bound to Axin through its I-mfa domain, and this interaction of MDFIC and Axin did not affect the binding of Axin to GSK-3 [33]. However, a Xenopus ortholog of MDFIC, XIC, inhibited the DNA binding of T cell factor 3 (TCF3) and the ability of β-catenin to activate the lymphoid enhancer factor/T cell factor (LEF/TCF) reporter constructs in early Xenopus embryos [34]. These studies indicate that MDFIC may be involved in the Wnt signaling pathway at different levels. Further research is required to understand the detailed mechanism of the MDFIC-regulated Wnt signaling pathway.
After nuclear translocation, β-catenin acts as a transcriptional coactivator with LEF/TCF transcription factors to stimulate the expression of Wnt downstream target genes [35]. Since the binding of MDFIC to Axin, mainly distributed in the cytoplasm, and LEF/TCF members, mainly localized in the nucleus, has been examined in other studies, the subcellular localization of MDFIC may ultimately affect its regulatory function in the Wnt signaling pathway [36]. The MDFIC p32 isoform lacks an N-terminal nuclear localization signaling and is distributed in the cytoplasm, while the MDFIC p40 isoform is mainly localized in the nucleus [30]. In our study, the MDFIC p32 isoform was associated with the destruction complex and contributed to β-catenin stabilization and nuclear translocation, while the MDFIC p40 isoform did not interact with β-catenin or other destruction complex members. Furthermore, our data showed that ectopic expression of the MDFIC p40 isoform did not alter the drug response of the cells to chemotherapy agents, suggesting that cytoplasmic MDFIC, mainly the MDFIC p32 isoform, is required for the β-catenin-mediated chemoresistance.
In our data, ectopic expression of MDFIC significantly increased the chemoresistance and sphere formation ability. Unexpectedly, we found that overexpression of MDFIC reversed EMT, while knockdown of MDFIC induced EMT, suggesting that MDFIC may function as an EMT inhibitor. These results suggest that the high level of MDFIC may maintain the epithelial phenotype of the E-cadHCD133H subpopulation, which exhibited higher chemoresistance and stemness. A recent study showed that E-cadherin, β-catenin, and SOX15 form a complex that binds to the promoter of caspase 3, leading to Twist1 cleavage and degradation in the selected epithelial-type subpopulation of the A549 cell line [17]. Accordingly, upregulation of MDFIC in the E-cadHCD133H subpopulation may contribute to the stabilization of the E-cadherin/β-catenin/SOX15 complex and subsequently maintain the epithelial phenotype of the cells by inhibiting Twist1 transcriptional activity. However, the role of SOX15 in cancer progression is still under debate [37,38,39]. Thus, future studies are required to explore the association of SOX15, β-catenin, and E-cadherin.
From an evolutionary perspective of neoplasia, EMT results in greater tumor initiation, metastatic potential, and resistance to therapies of cancer cells [40]. Many clinical studies indicate that high expression of mesenchymal or CSC biomarkers correlates with poor prognosis in lung cancer [41, 42]. However, many studies indicate that cancer cells with a stem-like phenotype may not always be in a mesenchymal state [13, 17, 21]. Here, we demonstrated that CSCs in the epithelial state were more resistant to chemotherapy agents. This phenomenon might be regulated through MDFIC-promoted β-catenin activity. Our study showed that targeting epithelial-type CSCs by inhibiting MDFIC and β-catenin activity may be a potential strategy to overcome chemoresistance in NSCLC.
Materials and methods
Human subjects
The tissue microarray (TMA-38AB), which was provided by Dr Michael Hsiao (Genomics Research Center, Academia Sinica, Taiwan) consists of tumor samples from 125 NSCLC patients enrolled in Kaohsiung Medical University Hospital from 1991 to 2007. All specimen collection procedures and analyses were approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-E(I)-20170048). Informed consent was obtained from all enrolled patients.
For studying the correlation between MDFIC and β-catenin nuclear translocation, the tumor samples from 25 NSCLC patients were collected from the Biobank (KMUH-BLOCK-108-0805) in Kaohsiung Medical University Hospital, Kaohsiung Taiwan with the approval of Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-E(I)-20190261). The histologic diagnosis of the specific type of lung cancer was made according to the recommendations of the World Health Organization classification, and the tumor size, local invasion, lymph node involvement, distal metastasis, and final disease stage were determined according to the 7th edition of the TNM staging system for lung cancer by the International Union Against Cancer and the American Joint Committee on Cancer [43]. The specimens were paraffin-embedded, sectioned, and used for IHC.
Cell lines and cell culture
For the maintenance of CSCs, the CD133H cells were cultured in ultralow adhesion culture dishes (Corning, New York, NY, USA) containing DMEM/F-12 with N2 supplement (Invitrogen), 20 ng/mL epidermal growth factor (EGF), and 20 ng/mL basic fibroblast growth factor (referred as stem-cell medium) (PeproTech, Rocky Hill, NJ, USA). The cell lines were kept under a humidified incubator containing 5% CO2 at 37 °C. Only early passage (<20 passage) CD133H cells were used in this study. All cells were routinely tested for mycoplasma contamination.
Fluorescence-activated cell sorting (FACS) and isolation of the subpopulations
For isolating the epithelial (E-cadH)- and mesenchymal (E-cadL)-type NSCLC cells, 1 × 106 PC14 and A549 cells were incubated with the PE-conjugated anti-E-cadherin antibody (BD Biosciences, San Jose, CA, USA) on ice for 40 min. After washing, the E-cadH (top 2%) and E-cadL (bottom 2%) cells were sorted by using a FACSAria cell sorter (BD Biosciences). Two rounds of FACS were performed to ensure the purity of the sorted subpopulations (>95%). For isolating the CD133H subpopulations, the E-cadH and E-cadL subpopulations were infected by a CD133 P1 promoter-driven GFP reporter lentivirus [44]. The GFP-positive cells were sorted and recovered in the stem-cell medium in ultralow adhesion culture dishes, which allowed the growth of tumor spheres. To evaluate the sphere formation efficiency of each subpopulation, 500 cells were cultured in six-well ultralow adhesion culture plates containing stem-cell medium. After 1 week, ten photos of nonoverlapping areas were randomly taken, and the number of tumor spheres was counted.
Gene expression microarray
Total RNA was isolated from the four subpopulations by using RNeasy Mini Kits (QIAGEN, Valencia, CA, USA). The Affymetrix cDNA microarray analysis was performed according to the user manual. The cDNA samples were purified and labeled with biotin-conjugated ribonucleotides using an IVT labeling kit (Affymetrix, Inc., Santa Clara, CA, USA). The labeled cDNA was subsequently fragmented and hybridized overnight with the Affymetrix human U133 2.0 plus arrays (Affymetrix). After hybridization, the chips were washed and stained. The chips were scanned using an Affymetrix Gene Chips canner 3000. The .DAT files were processed using the Affymetrix Gene Chip Operating System to generate the .CEL files. The raw intensities in the .CEL files were normalized by robust multichip analysis, and fold-change analysis was performed using GeneSpring GX software (Agilent Technologies, Santa Clara, CA, USA). The microarray data were uploaded, and the accession number is GSE93586 in the Gene Expression Omnibus database of National Center for Biotechnology Information.
Statistical analysis
All observations were confirmed by at least three independent experiments, and the data are presented as the mean ± SD. A two-tailed Student’s t test was used for the comparison between two groups. A 1-way ANOVA followed by Dunnett’s post hoc test was used for multiple comparisons. The variations within each group were considered in 1-way ANOVA. The similarity of variance between groups for each comparison was tested. The Fisher’s exact test was used for the tumor initiation animal study. The log-rank test was applied to evaluate the statistical significance of the Kaplan–Meier survival curve. Cox proportional hazards regression analysis was used to test the prognostic significance of the factors in the univariate and multivariate models. The association of DRS and tumor relapse was evaluated by Pearson’s Chi-square test. For all statistical tests, *p < 0.05 or **p < 0.01 was considered significant. Statistical analysis was performed using SPSS (Statistical Package for the Social Sciences, version 14.0) software.
For the primer sequences used in real-time PCR (Supplementary Table 8) and other detail information please see Supplementary information.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med. 2017;377:849–61.
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92.
Wu DM, Zhang T, Liu YB, Deng SH, Han R, Liu T, et al. The PAX6-ZEB2 axis promotes metastasis and cisplatin resistance in non-small cell lung cancer through PI3K/AKT signaling. Cell Death Dis. 2019;10:349.
Yadav AK, Desai NS. Cancer stem cells: acquisition, characteristics, therapeutic implications, targeting strategies and future prospects. Stem Cell Rev Rep. 2019;15:331–55.
Heng WS, Gosens R, Kruyt FAE. Lung cancer stem cells: origin, features, maintenance mechanisms and therapeutic targeting. Biochem Pharmacol. 2019;160:121–33.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15.
Gupta PB, Pastushenko I, Skibinski A, Blanpain C, Kuperwasser C. Phenotypic plasticity: driver of cancer initiation, progression, and therapy resistance. Cell Stem Cell. 2019;24:65–78.
Mittal V. Epithelial mesenchymal transition in aggressive lung cancers. Adv Exp Med Biol. 2016;890:37–56.
Beck TN, Korobeynikov VA, Kudinov AE, Georgopoulos R, Solanki NR, Andrews-Hoke M, et al. Anti-Mullerian hormone signaling regulates epithelial plasticity and chemoresistance in lung cancer. Cell Rep. 2016;16:657–71.
Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–3.
Ocana OH, Corcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S, et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22:709–24.
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–4.
Luo M, Shang L, Brooks MD, Jiagge E, Zhu Y, Buschhaus JM, et al. Targeting breast cancer stem cell state equilibrium through modulation of redox signaling. Cell Metab. 2018;28:69–86.e6.
Vijay GV, Zhao N, Den Hollander P, Toneff MJ, Joseph R, Pietila M, et al. GSK3beta regulates epithelial-mesenchymal transition and cancer stem cell properties in triple-negative breast cancer. Breast Cancer Res. 2019;21:37.
Chang YW, Su YJ, Hsiao M, Wei KC, Lin WH, Liang CL, et al. Diverse targets of beta-catenin during the epithelial-mesenchymal transition define cancer stem cells and predict disease relapse. Cancer Res. 2015;75:3398–410.
Song C, Lu R, Bienzle D, Liu HC, Yoo D. Interaction of the porcine reproductive and respiratory syndrome virus nucleocapsid protein with the inhibitor of MyoD family-a domain-containing protein. Biol Chem. 2009;390:215–23.
Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–8.
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–13.
Tiran V, Lindenmann J, Brcic L, Heitzer E, Stanzer S, Tabrizi-Wizsy NG, et al. Primary patient-derived lung adenocarcinoma cell culture challenges the association of cancer stem cells with epithelial-to-mesenchymal transition. Sci Rep. 2017;7:10040.
Pore M, Meijer C, de Bock GH, Boersma-van EkW, Terstappen LW, Groen HJ, et al. Cancer stem cells, epithelial to mesenchymal markers, and circulating tumor cells in small cell lung cancer. Clin Lung Cancer. 2016;17:535–42.
Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, Gao B, et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 2010;70:9937–48.
Huang X, Zhu H, Gao Z, Li J, Zhuang J, Dong Y, et al. Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by miR-370-3p. J Biol Chem. 2018;293:6693–706.
Li G, Su Q, Liu H, Wang D, Zhang W, Lu Z, et al. Frizzled7 promotes epithelial-to-mesenchymal transition and stemness via activating canonical Wnt/beta-catenin pathway in gastric cancer. Int J Biol Sci. 2018;14:280–93.
Yakisich JS, Azad N, Kaushik V, O’Doherty GA, Iyer AK. Nigericin decreases the viability of multidrug-resistant cancer cells and lung tumorspheres and potentiates the effects of cardiac glycosides. Tumour Biol. 2017;39:1010428317694310.
Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–47.
MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26.
Chen CM, Kraut N, Groudine M, Weintraub H. I-mf, a novel myogenic repressor, interacts with members of the MyoD family. Cell. 1996;86:731–41.
Thebault S, Gachon F, Lemasson I, Devaux C, Mesnard JM. Molecular cloning of a novel human I-mfa domain-containing protein that differently regulates human T-cell leukemia virus type I and HIV-1 expression. J Biol Chem. 2000;275:4848–57.
Oakley RH, Busillo JM, Cidlowski JA. Cross-talk between the glucocorticoid receptor and MyoD family inhibitor domain-containing protein provides a new mechanism for generating tissue-specific responses to glucocorticoids. J Biol Chem. 2017;292:5825–44.
Wang Q, Young TM, Mathews MB, Pe’ery T. Developmental regulators containing the I-mfa domain interact with T cyclins and Tat and modulate transcription. J Mol Biol. 2007;367:630–46.
Kusano S, Raab-Traub N. I-mfa domain proteins interact with Axin and affect its regulation of the Wnt and c-Jun N-terminal kinase signaling pathways. Mol Cell Biol. 2002;22:6393–405.
Snider L, Thirlwell H, Miller JR, Moon RT, Groudine M, Tapscott SJ. Inhibition of Tcf3 binding by I-mfa domain proteins. Mol Cell Biol. 2001;21:1866–73.
Novak A, Dedhar S. Signaling through beta-catenin and Lef/Tcf. Cell Mol Life Sci. 1999;56:523–37.
Thebault S, Mesnard JM. How the sequestration of a protein interferes with its mechanism of action: example of a new family of proteins characterized by a particular cysteine-rich carboxy-terminal domain involved in gene expression regulation. Curr Protein Pept Sci. 2001;2:155–67.
Thu KL, Becker-Santos DD, Radulovich N, Pikor LA, Lam WL, Tsao MS. SOX15 and other SOX family members are important mediators of tumorigenesis in multiple cancer types. Oncoscience 2014;1:326–35.
Xu YR, Yang WX. SOX-mediated molecular crosstalk during the progression of tumorigenesis. Semin Cell Dev Biol. 2017;63:23–34.
Zhang M, Wang J, Gao T, Chen X, Xu Y, Yu X, et al. Inhibition of SOX15 sensitizes esophageal squamous carcinoma cells to paclitaxel. Curr Mol Med. 2019;19:349–56.
Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84.
Tsoukalas N, Aravantinou-Fatorou E, Tolia M, Giaginis C, Galanopoulos M, Kiakou M, et al. Epithelial-mesenchymal transition in non small-cell lung cancer. Anticancer Res. 2017;37:1773–8.
Wang D, Wen GM, Hou W, Xia P. The roles of CD133 expression in the patients with non-small cell lung cancer. Cancer Biomark. 2018;22:385–94.
Sculier JP, Chansky K, Crowley JJ, Van Meerbeeck J, Goldstraw P, International Staging C, et al. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the proposals for the 7th edition. J Thorac Oncol. 2008;3:457–66.
Liu YP, Yang CJ, Huang MS, Yeh CT, Wu AT, Lee YC, et al. Cisplatin selects for multidrug-resistant CD133+ cells in lung adenocarcinoma by activating Notch signaling. Cancer Res. 2013;73:406–16.
Acknowledgements
This study was financially supported by the Ministry of Science and Technology of Taiwan (MOST 107-2320-B-037-025-) and Kaohsiung Medical University Hospital (KMUH106-6M63). This work was also financially supported by the Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. We thank Dr Michael Hsiao for providing the tissue microarray chips. We thank the assistance of the Department of Pathology and the Biobank in Kaohsiung Medical University Hospital, Kaohsiung, Taiwan for the clinical sample collection. We thank the Center for Research Resources and Development in Kaohsiung Medical University for the instrumental support for the confocal microscope and TissueFAX system. The authors thank the Immunobiology core facility of Clinical Medicine Research Center in National Cheng Kung University Hospital for assisting with the fluorescence-activated cell sorting.
Funding
This study was financially supported by the Ministry of Science and Technology of Taiwan (MOST 107-2320-B-037-025-) and Kaohsiung Medical University Hospital (KMUH105-5M58).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, CJ., Yang, CJ., Yang, SF. et al. The MyoD family inhibitor domain-containing protein enhances the chemoresistance of cancer stem cells in the epithelial state by increasing β-catenin activity. Oncogene 39, 2377–2390 (2020). https://doi.org/10.1038/s41388-019-1152-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-1152-4