Abstract
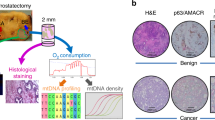
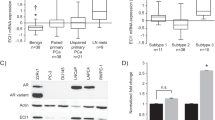
New strategies are needed to predict and overcome metastatic progression and therapy resistance in prostate cancer. One potential clinical target is the stem cell transcription factor SOX2, which has a critical role in prostate development and cancer. We thus investigated the impact of SOX2 expression on patient outcomes and its function within prostate cancer cells. Analyses of SOX2 expression among a case-control cohort of 1028 annotated tumor specimens demonstrated that SOX2 expression confers a more rapid time to metastasis and decreased patient survival after biochemical recurrence. SOX2 ChIP-Seq analyses revealed SOX2-binding sites within prostate cancer cells which differ significantly from canonical embryonic SOX2 gene targets, and prostate-specific SOX2 gene targets are associated with multiple oncogenic pathways. Interestingly, phenotypic and gene expression analyses after CRISPR-mediated deletion of SOX2 in castration-resistant prostate cancer cells, as well as ectopic SOX2 expression in androgen-sensitive prostate cancer cells, demonstrated that SOX2 promotes changes in multiple metabolic pathways and metabolites. SOX2 expression in prostate cancer cell lines confers increased glycolysis and glycolytic capacity, as well as increased basal and maximal oxidative respiration and increased spare respiratory capacity. Further, SOX2 expression was associated with increased quantities of mitochondria, and metabolomic analyses revealed SOX2-associated changes in the metabolism of purines, pyrimidines, amino acids and sugars, and the pentose phosphate pathway. Analyses of SOX2 gene targets with central functions metabolism (CERK, ECHS1, HS6SDT1, LPCAT4, PFKP, SLC16A3, SLC46A1, and TST) document significant expression correlation with SOX2 among RNA-Seq datasets derived from patient tumors and metastases. These data support a key role for SOX2 in metabolic reprogramming of prostate cancer cells and reveal new mechanisms to understand how SOX2 enables metastatic progression, lineage plasticity, and therapy resistance. Further, our data suggest clinical opportunities to exploit SOX2 as a biomarker for staging and imaging, as well as a potential pharmacologic target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
10 February 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41388-022-02228-7
References
Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355:78–83.
Kregel S, Kiriluk KJ, Rosen AM, Cai Y, Reyes EE, Otto KB, et al. Sox2 is an androgen receptor-repressed gene that promotes castration-resistant prostate cancer. PloS one. 2013;8:e53701.
Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355:84–88.
Zhang S, Cui W. Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J Stem Cells. 2014;6:305.
Wegner M. All purpose Sox: The many roles of Sox proteins in gene expression. Int J Biochem Cell Biol. 2010;42:381–90.
Stolzenburg S, Rots MG, Beltran AS, Rivenbark AG, Yuan X, Qian H et al. Targeted silencing of the oncogenic transcription factor SOX2 in breast cancer. Oxford University Press, 2012.
Ji J, Zheng P-S. Expression of Sox2 in human cervical carcinogenesis. Hum Pathol. 2010;41:1438–47.
Jia X, Li X, Xu Y, Zhang S, Mou W, Liu Y, et al. SOX2 promotes tumorigenesis and increases the anti-apoptotic property of human prostate cancer cell. J Mol Cell Biol. 2011;3:230–8.
Hütz K, Mejías-Luque R, Farsakova K, Ogris M, Krebs S, Anton M, et al. The stem cell factor SOX2 regulates the tumorigenic potential in human gastric cancer cells. Carcinogenesis. 2013;35:942–50.
Chen S, Li X, Lu D, Xu Y, Mou W, Wang L, et al. SOX2 regulates apoptosis through MAP4K4-survivin signaling pathway in human lung cancer cells. Carcinogenesis. 2013;35:613–23.
Alonso MM, Diez-Valle R, Manterola L, Rubio A, Liu D, Cortes-Santiago N, et al. Genetic and epigenetic modifications of Sox2 contribute to the invasive phenotype of malignant gliomas. PloS ONE. 2011;6:e26740.
Han X, Fang X, Lou X, Hua D, Ding W, Foltz G, et al. Silencing SOX2 induced mesenchymal-epithelial transition and its expression predicts liver and lymph node metastasis of CRC patients. PloS ONE. 2012;7:e41335.
Sun C, Sun L, Li Y, Kang X, Zhang S, Liu Y. Sox2 expression predicts poor survival of hepatocellular carcinoma patients and it promotes liver cancer cell invasion by activating Slug. Med Oncol. 2013;30:503.
Lou X, Han X, Jin C, Tian W, Yu W, Ding D, et al. SOX2 targets fibronectin 1 to promote cell migration and invasion in ovarian cancer: new molecular leads for therapeutic intervention. Omics: A J Integr Biol. 2013;17:510–8.
Bareiss PM, Paczulla A, Wang H, Schairer R, Wiehr S, Kohlhofer U, et al. SOX2 expression associates with stem cell state in human ovarian carcinoma. Cancer Res. 2013;73:5544–55.
Chen S, Xu Y, Chen Y, Li X, Mou W, Wang L, et al. SOX2 gene regulates the transcriptional network of oncogenes and affects tumorigenesis of human lung cancer cells. PloS ONE. 2012;7:e36326.
Herreros-Villanueva M, Zhang J, Koenig A, Abel E, Smyrk TC, Bamlet W, et al. SOX2 promotes dedifferentiation and imparts stem cell-like features to pancreatic cancer cells. Oncogenesis. 2013;2:e61.
Vazquez-Martin A, Cufí S, Lopez-Bonet E, Corominas-Faja B, Cuyàs E, Vellon L, et al. Reprogramming of non-genomic estrogen signaling by the stemness factor SOX2 enhances the tumor-initiating capacity of breast cancer cells. Cell Cycle. 2013;12:3471–7.
Yu X, Cates JM, Morrissey C, You C, Grabowska MM, Zhang J, et al. SOX2 expression in the developing, adult, as well as, diseased prostate. Prostate Cancer Prostatic Dis. 2014;17:301–9.
Ugolkov AV, Eisengart LJ, Luan C, Yang XJ. Expression analysis of putative stem cell markers in human benign and malignant prostate. Prostate. 2011;71:18–25.
Matsika A, Srinivasan B, Day C, Mader SA, Kiernan DM, Broomfield A, et al. Cancer stem cell markers in prostate cancer: an immunohistochemical study of ALDH1, SOX2 and EZH2. Pathology. 2015;47:622–8.
Russo MV, Esposito S, Tupone MG, Manzoli L, Airoldi I, Pompa P, et al. SOX2 boosts major tumor progression genes in prostate cancer and is a functional biomarker of lymph node metastasis. Oncotarget. 2016;7:12372–85.
Hempel HA, Cuka NS, Kulac I, Barber JR, Cornish TC, Platz EA, et al. Low Intratumoral Mast Cells Are Associated With a Higher Risk of Prostate Cancer Recurrence. Prostate. 2017;77:412–24.
Toubaji A, Albadine R, Meeker AK, Isaacs WB, Lotan T, Haffner MC, et al. Increased gene copy number of ERG on chromosome 21 but not TMPRSS2-ERG fusion predicts outcome in prostatic adenocarcinomas. Mod Pathol. 2011;24:1511–20.
McAuley E, Moline D, VanOpstall C, Lamperis S, Brown R, Vander Griend DJ. Sox2 Expression Marks Castration-Resistant Progenitor Cells in the Adult Murine Prostate. Stem Cells. 2019;37:690–700.
Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56.
Sarkar A, Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12:15–30.
Liu P, Sanalkumar R, Bresnick EH, Keleş S, Dewey CN. Integrative analysis with ChIP-seq advances the limits of transcript quantification from RNA-seq. Genome Res. 2016;26:1124–33.
Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl J Med. 2014;371:1028–38.
Cancer Genome Atlas Research N. The Molecular Taxonomy of Primary Prostate Cancer. Cell. 2015;163:1011–25.
Bhanvadia RR, VanOpstall C, Brechka H, Barashi NS, Gillard M, McAuley EM, et al. MEIS1 and MEIS2 Expression and Prostate Cancer Progression: A Role For HOXB13 Binding Partners in Metastatic Disease. Clin Cancer Res. 2018;24:3668–80.
Pflueger D, Terry S, Sboner A, Habegger L, Esgueva R, Lin PC, et al. Discovery of non-ETS gene fusions in human prostate cancer using next-generation RNA sequencing. Genome Res. 2011;21:56–67.
Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–28.
Cutruzzolà F, Giardina G, Marani M, Macone A, Paiardini A, Rinaldo S, et al. Glucose metabolism in the progression of prostate cancer. Front Physiol. 2017;8:97.
Russo MV, Esposito S, Tupone MG, Manzoli L, Airoldi I, Pompa P, et al. SOX2 boosts major tumor progression genes in prostate cancer and is a functional biomarker of lymph node metastasis. Oncotarget. 2016;7:12372.
Lowrance WT, Breau RH, Chou R, Chapin BF, Crispino T, Dreicer R, et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART I. J Urol. 2021;205:14–21.
Van den Broeck T, van den Bergh RCN, Arfi N, Gross T, Moris L, Briers E, et al. Prognostic Value of Biochemical Recurrence Following Treatment with Curative Intent for Prostate Cancer: A Systematic Review. Eur Urol. 2019;75:967–87.
Metz EP, Wilder PJ, Dong J, Datta K, Rizzino A. Elevating SOX2 in prostate tumor cells upregulates expression of neuroendocrine genes, but does not reduce the inhibitory effects of enzalutamide. J Cell Physiol. 2020;235:3731–40.
Li H, Wang L, Li Z, Geng X, Li M, Tang Q, et al. SOX2 has dual functions as a regulator in the progression of neuroendocrine prostate cancer. Lab Invest. 2020;100:570–82.
Kwon OJ, Zhang L, Jia D, Zhou Z, Li Z, Haffner M, et al. De novo induction of lineage plasticity from human prostate luminal epithelial cells by activated AKT1 and c-Myc. Oncogene. 2020;39:7142–51.
Yoo YA, Vatapalli R, Lysy B, Mok H, Desouki MM, Abdulkadir SA. The Role of Castration-Resistant Bmi1 + Sox2+ Cells in Driving Recurrence in Prostate Cancer. J Natl Cancer Inst. 2019;111:311–21.
Kar S, Sengupta D, Deb M, Pradhan N, Patra SK. SOX2 function and Hedgehog signaling pathway are co-conspirators in promoting androgen independent prostate cancer. Biochim Biophys Acta Mol Basis Dis. 2017;1863:253–65.
Zhang Z, Zhou C, Li X, Barnes SD, Deng S, Hoover E, et al. Loss of CHD1 Promotes Heterogeneous Mechanisms of Resistance to AR-Targeted Therapy via Chromatin Dysregulation. Cancer Cell. 2020;37:584–98 e511.
Metz EP, Wuebben EL, Wilder PJ, Cox JL, Datta K, Coulter D, et al. Tumor quiescence: elevating SOX2 in diverse tumor cell types downregulates a broad spectrum of the cell cycle machinery and inhibits tumor growth. BMC Cancer. 2020;20:941.
Hussenet T, Dali S, Exinger J, Monga B, Jost B, Dembelé D, et al. SOX2 is an oncogene activated by recurrent 3q26. 3 amplifications in human lung squamous cell carcinomas. PloS ONE. 2010;5:e8960.
Annovazzi L, Mellai M, Caldera V, Valente G, Schiffer D. SOX2 expression and amplification in gliomas and glioma cell lines. Cancer Genomics-Proteom. 2011;8:139–47.
Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238.
Maier S, Wilbertz T, Braun M, Scheble V, Reischl M, Mikut R, et al. SOX2 amplification is a common event in squamous cell carcinomas of different organ sites. Hum Pathol. 2011;42:1078–88.
Wuebben EL, Rizzino A. The dark side of SOX2: cancer - a comprehensive overview. Oncotarget. 2017;8:44917–43.
Varum S, Rodrigues AS, Moura MB, Momcilovic O. Easley CAt, Ramalho-Santos J et al. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One. 2011;6:e20914.
Bergers G, Fendt SM. The metabolism of cancer cells during metastasis. Nat Rev Cancer. 2021;21:162–80.
Elia I, Doglioni G, Fendt SM. Metabolic Hallmarks of Metastasis Formation. Trends Cell Biol. 2018;28:673–84.
Whitburn J, Edwards CM. Metabolism in the Tumour-Bone Microenvironment. Curr Osteoporos Rep. 2021;19:494–9.
Zong WX, Rabinowitz JD, White E. Mitochondria and Cancer. Mol Cell. 2016;61:667–76.
Fraum TJ, Ludwig DR, Kim EH, Schroeder P, Hope TA, Ippolito JE. Prostate cancer PET tracers: essentials for the urologist. Can J Urol. 2018;25:9371–83.
Jadvar H. Is There Use for FDG-PET in Prostate Cancer? Semin Nucl Med. 2016;46:502–6.
Vander Griend DJ, Karthaus WL, Dalrymple S, Meeker A, DeMarzo AM, Isaacs JT. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 2008;68:9703–11.
Wang MH, Shugart YY, Cole SR, Platz EA. A simulation study of control sampling methods for nested case-control studies of genetic and molecular biomarkers and prostate cancer progression. Cancer Epidemiol Biomark Prev. 2009;18:706–11.
Kregel S, Chen JL, Tom W, Krishnan V, Kach J, Brechka H, et al. Acquired resistance to the second-generation androgen receptor antagonist enzalutamide in castration-resistant prostate cancer. Oncotarget. 2016;7:26259–74.
Michiel Sedelaar JP, Dalrymple SS, Isaacs JT. Of mice and men-warning: intact versus castrated adult male mice as xenograft hosts are equivalent to hypogonadal versus abiraterone treated aging human males, respectively. Prostate. 2013;73:1316–25.
Dracopoli NC Current protocols in human genetics. Wiley: New York, NY, 1994, pp 2 volumes (loose-leaf).
Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–56.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20.
Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60.
Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008;9:R137.
Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–08.
Grant CE, Bailey TL, Noble WS. FIMO: scanning for occurrences of a given motif. Bioinformatics. 2011;27:1017–8.
Mathelier A, Fornes O, Arenillas DJ, Chen CY, Denay G, Lee J, et al. JASPAR 2016: a major expansion and update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2016;44:D110–15.
Wang L, Wang S, Li W. RSeQC: quality control of RNA-seq experiments. Bioinformatics. 2012;28:2184–5.
Acknowledgements
We acknowledge Vander Griend and Szmulewitz lab members for their input; Dr. Brendan Looyenga at Calvin College for his gift of vectors used to generate knockout cell lines using CRISPR; support of the University of Illinois at Chicago Department of Pathology led by Dr. Fred Behm, as well as the University of Illinois at Chicago Research Histology and Tissue Imaging Core led by Dr. Peter Gann; Drs. Bruce Trock and Karen Sfanos of the Prostate Cancer Biorepository Network (PCBN) for help obtaining annotated tumor specimens and data; expert technical assistance of the Human Tissue Resource Center core facility led by Dr. Mark Lingen, and the assistance of Mary Jo Fekete; the Immunohistochemistry Core Facility run by Terri Li; the Northwestern University Metabolomics Core Facility for their assistance and service; the University of Chicago Genomics Facility led by Dr. Pieter Faber; support of the University of Chicago Committee on Cancer Biology, led by Dr. Kay Macleod and Stephen Kron; and support of Drs. Alan Diamond, Larisa Nonn, and Gail Prins at the University of Illinois at Chicago Departments of Pathology and Urology.
Funding
R01CA178431 (DJ Vander Griend), R00CA218885-04 (P.M.); University of Chicago Comprehensive Cancer Center Support Grant (P30CA014599); The Brinson Foundation; Alvin Baum Family Fund; The Pierce Foundation; and National Center for Advancing Translational Sciences (UL1TR002003). The Prostate Cancer Biorepository Network is funded by the Department of Defense Prostate Cancer Research Program Award No. W81XWH-14-2-0182, W81XWH-14-2-0183, W81XWH-14-2-0185, W81XWH-14-2-0186, and W81XWH-15-2-0062, and W81XWH-18-1-0411 (P.M.). L. de Wet was supported by the Goldblatt Foundation Fellowship; S. Kregel was supported by a Cancer Biology Training Grant (T32 CA 009594). P.M. is also supported by CPRIT (RR170050), Welch Foundation (I-2005-20190330) and Prostate Cancer Foundation (17YOUN12).
Author information
Authors and Affiliations
Contributions
Conceptualization: DJVG, LDW, SK, JC, RZS. Methodology: DJVG, LDW, MG, SK, SL. Formal Analysis: DJVG, LDW, AW, MG, SK, GP, HW. Investigations: DJVG, LDW, AW, MG, SK, SL, LG, JV, RB, KC. Resource: EP, AMD, PM. Data Curation: DJVG, LDW, AW, GP. Writing and Editing: DJVG, LDW. Supervision: DJVG.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Wet, L., Williams, A., Gillard, M. et al. SOX2 mediates metabolic reprogramming of prostate cancer cells. Oncogene 41, 1190–1202 (2022). https://doi.org/10.1038/s41388-021-02157-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-02157-x
This article is cited by
-
Glioblastoma stem cell metabolism and immunity
Cancer and Metastasis Reviews (2024)
-
Therapeutic importance and diagnostic function of circRNAs in urological cancers: from metastasis to drug resistance
Cancer and Metastasis Reviews (2024)
-
SOX2 promotes vasculogenic mimicry by accelerating glycolysis via the lncRNA AC005392.2-GLUT1 axis in colorectal cancer
Cell Death & Disease (2023)
-
Coupled fibromodulin and SOX2 signaling as a critical regulator of metastatic outgrowth in melanoma
Cellular and Molecular Life Sciences (2022)
-
Ectopic JAK–STAT activation enables the transition to a stem-like and multilineage state conferring AR-targeted therapy resistance
Nature Cancer (2022)