Abstract
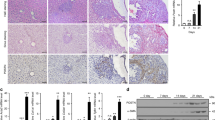
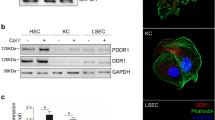
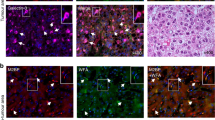
Most cases of hepatocellular carcinoma (HCC) arise with the fibrotic microenvironment where hepatic stellate cells (HSCs) and carcinoma-associated fibroblasts (CAFs) are critical components in HCC progression. Therefore, CAF normalization could be a feasible therapy for HCC. Galectin-1 (Gal-1), a β-galactoside-binding lectin, is critical for HSC activation and liver fibrosis. However, few studies has evaluated the pathological role of Gal-1 in HCC stroma and its role in hepatic CAF is unclear. Here we showed that Gal-1 mainly expressed in HCC stroma, but not cancer cells. High expression of Gal-1 is correlated with CAF markers and poor prognoses of HCC patients. In co-culture systems, targeting Gal-1 in CAFs or HSCs, using small hairpin (sh)RNAs or an therapeutic inhibitor (LLS30), downregulated plasminogen activator inhibitor-2 (PAI-2) production which suppressed cancer stem-like cell properties and invasion ability of HCC in a paracrine manner. The Gal-1-targeting effect was mediated by increased a disintegrin and metalloprotease 17 (ADAM17)-dependent TNF-receptor 1 (TNFR1) shedding/cleavage which inhibited the TNF-α → JNK → c-Jun/ATF2 signaling axis of pro-inflammatory gene transcription. Silencing Gal-1 in CAFs inhibited CAF-augmented HCC progression and reprogrammed the CAF-mediated inflammatory responses in a co-injection xenograft model. Taken together, the findings uncover a crucial role of Gal-1 in CAFs that orchestrates an inflammatory CSC niche supporting HCC progression and demonstrate that targeting Gal-1 could be a potential therapy for fibrosis-related HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RMF, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174–86.
Biffi G, Tuveson DA. Diversity and biology of cancer-associated fibroblasts. Physiol Rev. 2021;101:147–76.
Feig C, Jones JO, Kraman M, Wells RJB, Deonarine A, Chan DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti–PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:20212–7.
Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93.
Martinez-Bosch N, Navarro P. Galectins in the tumor microenvironment: focus on galectin-1. Adv Exp Med Biol. 2020;1259:17–38.
Cousin JM, Cloninger MJ. The role of galectin-1 in cancer progression, and synthetic multivalent systems for the study of galectin-1. Int J Mol Sci. 2016;17:1566.
Gao Y, Li X, Shu Z, Zhang K, Xue X, Li W, et al. Nuclear galectin-1-FOXP3 interaction dampens the tumor-suppressive properties of FOXP3 in breast cancer. Cell Death Dis. 2018;9:416.
Wu M-H, Chen Y-L, Lee K-H, Chang C-C, Cheng T-M, Wu S-Y, et al. Glycosylation-dependent galectin-1/neuropilin-1 interactions promote liver fibrosis through activation of TGF-β- and PDGF-like signals in hepatic stellate cells. Sci Rep. 2017;7:11006.
Wu H, Chen P, Liao R, Li YW, Yi Y, Wang JX, et al. Overexpression of galectin-1 is associated with poor prognosis in human hepatocellular carcinoma following resection. J Gastroenterol Hepatol. 2012;27:1312–9.
Spano D, Russo R, Di Maso V, Rosso N, Terracciano LM, Roncalli M, et al. Galectin-1 and its involvement in hepatocellular carcinoma aggressiveness. Mol Med. 2010;16:102–15.
Zhang PF, Li KS, Shen YH, Gao PT, Dong ZR, Cai JB, et al. Galectin-1 induces hepatocellular carcinoma EMT and sorafenib resistance by activating FAK/PI3K/AKT signaling. Cell Death Dis. 2016;7:e2201.
Nurmik M, Ullmann P, Rodriguez F, Haan S, Letellier E. In search of definitions: cancer-associated fibroblasts and their markers. Int J Cancer. 2020;146:895–905.
Shih TC, Liu R, Wu CT, Li X, Xiao W, Deng X, et al. Targeting galectin-1 impairs castration-resistant prostate cancer progression and invasion. Clin Cancer Res. 2018;24:4319–31.
Castello-Cros R, Bonuccelli G, Molchansky A, Capozza F, Witkiewicz AK, Birbe RC, et al. Matrix remodeling stimulates stromal autophagy, “fueling” cancer cell mitochondrial metabolism and metastasis. Cell Cycle. 2011;10:2021–34.
Sakimoto T, Yamada A, Sawa M. Release of soluble tumor necrosis factor receptor 1 from corneal epithelium by TNF-α–converting enzyme-dependent ectodomain shedding. Invest Ophthalmol Vis Sci. 2009;50:4618–21.
Deng M, Loughran PA, Zhang L, Scott MJ, Billiar TR. Shedding of the tumor necrosis factor (TNF) receptor from the surface of hepatocytes during sepsis limits inflammation through cGMP signaling. Sci Signal. 2015;8:ra11.
Ludwig A, Hundhausen C, Lambert MH, Broadway N, Andrews RC, Bickett DM, et al. Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules. Comb Chem High Throughput Screen. 2005;8:161–71.
Chen Z, Quan L, Huang A, Zhao Q, Yuan Y, Yuan X, et al. seq-ImmuCC: cell-centric view of tissue transcriptome measuring cellular compositions of immune microenvironment from mouse RNA-Seq data. Front Immunol. 2018;9:1286.
Leung Z, Ko FCF, Tey SK, Kwong EML, Mao X, Liu BHM, et al. Galectin-1 promotes hepatocellular carcinoma and the combined therapeutic effect of OTX008 galectin-1 inhibitor and sorafenib in tumor cells. J Exp Clin Cancer Res. 2019;38:423.20.
Yeh CC, Hsu CH, Shao YY, Ho WC, Tsai MH, Feng WC, et al. Integrated stable isotope labeling by amino acids in cell culture (SILAC) and Isobaric tags for relative and absolute quantitation (iTRAQ) quantitative proteomic analysis identifies galectin-1 as a potential biomarker for predicting sorafenib resistance in liver cancer. Mol Cell Proteom. 2015;14:1527–45.
Toegel S, Weinmann D, André S, Walzer SM, Bilban M, Schmidt S, et al. Galectin-1 couples glycobiology to inflammation in osteoarthritis through the activation of an NF-κB–regulated gene network. J Immunol. 2016;196:1910–21.
Lei T, Moos S, Klug J, Aslani F, Bhushan S, Wahle E, et al. Galectin-1 enhances TNFα-induced inflammatory responses in Sertoli cells through activation of MAPK signalling. Sci Rep. 2018;8:3741.
Potikha T, Pappo O, Mizrahi L, Olam D, Maller SM, Rabinovich GA, et al. Lack of galectin-1 exacerbates chronic hepatitis, liver fibrosis, and carcinogenesis in murine hepatocellular carcinoma model. FASEB J. 2019;33:7995–8007.
Lockett AD, Kimani S, Ddungu G, Wrenger S, Tuder RM, Janciauskiene SM, et al. α1-Antitrypsin modulates lung endothelial cell inflammatory responses to TNF-α. Am J Respir Cell Mol Biol. 2013;49:143–50.
Bernot D, Stalin J, Stocker P, Bonardo B, Scroyen I, Alessi M-C, et al. Plasminogen activator inhibitor 1 is an intracellular inhibitor of furin proprotein convertase. J Cell Sci. 2011;124:1224–30.
Izaguirre G, Arciniega M, Quezada AG. Specific and selective inhibitors of proprotein convertases engineered by transferring serpin B8 reactive-site and exosite determinants of reactivity to the serpin α1PDX. Biochemistry. 2019;58:1679–88.
Boulaftali Y, François D, Venisse L, Jandrot-Perrus M, Arocas V, Bouton MC. Endothelial protease nexin-1 is a novel regulator of A disintegrin and metalloproteinase 17 maturation and endothelial protein C receptor shedding via furin inhibition. Arterioscler Thromb Vasc Biol. 2013;33:1647–54.
Monteran L, Erez N. The dark side of fibroblasts: cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front Immunol. 2019;10:1835.
Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Disco. 2019;9:1102–23.
Chen P-C, Kuo Y-C, Chuong C-M, Huang Y-H. Niche modulation of IGF-1R signaling: its role in stem cell pluripotency, cancer reprogramming, and therapeutic applications. Front Cell Dev Biol. 2021;8:625943.
Ngo MT, Jeng HY. The role of IGF/IGF-1R signaling in hepatocellular carcinomas: stemness-related properties and drug resistance. Int J Mol Sci. 2021;22:1931.
Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33:463–79. e410
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl J Med. 2008;359:378–90.
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl J Med. 2020;382:1894–905.
Abolarinwa BA, Ibrahim RB, Huang YH. Conceptual development of immunotherapeutic approaches to gastrointestinal cancer. Int J Mol Sci. 2019;20:4624.
Donisi C, Puzzoni M, Ziranu P, Lai E, Mariani S, Saba G, et al. Immune checkpoint inhibitors in the treatment of HCC. Front Oncol. 2021;10:601240.
Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–8.
Dominguez CX, Müller S, Keerthivasan S, Koeppen H, Hung J, Gierke S, et al. Single-cell RNA sequencing reveals stromal evolution into LRRC15+ myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Disco. 2020;10:232–53.
Tarrats N, Moles A, Morales A, García-Ruiz C, Fernández-Checa JC, Marí M. Critical role of tumor necrosis factor receptor 1, but not 2, in hepatic stellate cell proliferation, extracellular matrix remodeling, and liver fibrogenesis. Hepatology. 2011;54:319–27.
Lin ZY, Chuang YH, Chuang WL. Cancer-associated fibroblasts up-regulate CCL2, CCL26, IL6 and LOXL2 genes related to promotion of cancer progression in hepatocellular carcinoma cells. Biomed Pharmacother. 2012;66:525–9.
Acknowledgements
We thank the National RNAi Core Facility at Academia Sinica, Taiwan for providing shRNA reagents and related services. We thank the Image and Bioinformatics Core Facility of Taipei Medical University for confocal microscopy and the microarray analysis. Human samples were from the Human Tumor Tissue Bank, Chang Gung Memorial Hospital (Chiayi, Taiwan). This work was financially supported by the “TMU Research Center of Cancer Translational Medicine” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Funding
This work was supported by grants MOST 107-2314-B-038-062, MOST 107-2320-B-038-062, MOST 108-2314-B-038-007, MOST 109-2314-B-038-134, MOST 110-2320-B-038-018, and MOST 110-2314-B-038-133 from the Ministry of Science and Technology, Taiwan; grants DP2-110-21121-01-T-03-01, DP2-109-21121-01-T-03-01, DP2-108-21121-01-T-02-04, and DP2-107-21121-T-02 from the Higher Education Sprout Project by the Ministry of Education (MOE), Taiwan; grants NHRI-EX109-10918BI, NHRI-EX110-10918BI and NHRI-EX111-10918BI from the National Health Research Institutes, Taiwan; and SATU Joint Research Grant ST039-2020.
Author information
Authors and Affiliations
Contributions
Conception and design: MHW. Development of methodology: MHW, YTT, and CYL. Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): YHH, TSC, CYL, ZYL, and YLC. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): CHC, CYW, THC, SYS, KHY, WLT, GA, CVY, and MHW. Writing, review, and/or revision of the manuscript: IC, KHL, CCC and MHW. Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): ZYL, YLC, and TCS. Study supervision: MHW.
Corresponding author
Ethics declarations
Competing interests
TCS is an inventor of Galectin-1 inhibitor LLS30, and a scientific founder of Kibio Inc. which plans to develop the LLS30.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tsai, YT., Li, CY., Huang, YH. et al. Galectin-1 orchestrates an inflammatory tumor-stroma crosstalk in hepatoma by enhancing TNFR1 protein stability and signaling in carcinoma-associated fibroblasts. Oncogene 41, 3011–3023 (2022). https://doi.org/10.1038/s41388-022-02309-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02309-7
This article is cited by
-
P38 MAPK activated ADAM17 mediates ACE2 shedding and promotes cardiac remodeling and heart failure after myocardial infarction
Cell Communication and Signaling (2023)