Abstract
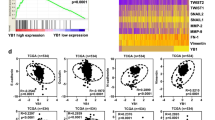
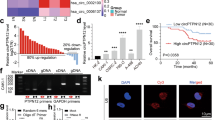
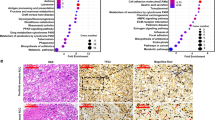
Despite the promise of targeted tyrosine kinase inhibitors (TKIs), such as sunitinib, in the extension of survival time in patients with clear cell renal cell carcinoma (ccRCC) progression or metastasis, the patients eventually succumb to inevitable drug resistance. Protein degradation executed by the ubiquitin-dependent proteasome system played an important role in determining the sensitivity of ccRCC to sunitinib. Here, we applied the bioinformatic analysis to identify that E3 ligase RBCK1 was elevated in the sunitinib-resistant renal cancer cell lines or patient specimens. The subsequent in vitro or in vivo studies demonstrated that RBCK1 contributed to decreasing the sensitivity of ccRCC to sunitinib. Then, we showed that inhibition of RBCK1 inactivated the AKT and MAPK signaling pathways, which might be one of the main reasons why RBCK1 induces sunitinib resistance in ccRCC cells. Mechanistically, our results indicated that RBCK1 promotes the degradation of ANKRD35 and that ANKRD35 destabilizes MITD1 by binding with SUMO2 in ccRCC cells. In addition, we showed that the RBCK1-ANKRD35-MITD1-ANXA1 axis regulates the phosphorylation of AKT and ERK and contributes to the dysregulation of sunitinib in ccRCC cells. Therefore, we identified a novel mechanism for regulating the sensitivity of sunitinib in ccRCC. Therefore, we elucidated a novel mechanism by which RBCK1 regulates sunitinib sensitivity in ccRCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request. The original western blot data has been submitted to the figshare repository (https://doi.org/10.6084/m9.figshare.21557733). The sequencing data was deposited in the GEO public dataset (GSE214635).
References
Wettersten HI, Aboud OA, Lara PN Jr, Weiss RH. Metabolic reprogramming in clear cell renal cell carcinoma. Nat Rev Nephrol. 2017;13:410–9.
Pontes O, Oliveira-Pinto S, Baltazar F, Costa M. Renal cell carcinoma therapy: current and new drug candidates. Drug Discov Today. 2022;27:304–14.
Xie Y, Shangguan W, Chen Z, Zheng Z, Chen Y, Zhong Q, et al. Establishment of sunitinib-resistant xenograft model of renal cell carcinoma and the identification of drug-resistant hub genes and pathways. Drug Des Dev Ther. 2021;15:5061–74.
Rizzo M, Porta C. Sunitinib in the treatment of renal cell carcinoma: an update on recent evidence. Ther Adv Urol. 2017;9:195–207.
Aeppli S, Schmaus M, Eisen T, Escudier B, Grunwald V, Larkin J, et al. First-line treatment of metastatic clear cell renal cell carcinoma: a decision-making analysis among experts. ESMO Open. 2021;6:100030.
Atkins MB, Tannir NM. Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev. 2018;70:127–37.
Xiang Y, Zheng G, Zhong J, Sheng J, Qin H. Advances in renal cell carcinoma drug resistance models. Front Oncol. 2022;12:870396.
Vu LD, Gevaert K, De Smet I. Protein language: post-translational modifications talking to each other. Trends Plant Sci. 2018;23:1068–80.
Gajjala PR, Fliser D, Speer T, Jankowski V, Jankowski J. Emerging role of post-translational modifications in chronic kidney disease and cardiovascular disease. Nephrol Dial Transplant. 2015;30:1814–24.
Czuba LC, Hillgren KM, Swaan PW. Post-translational modifications of transporters. Pharmcol Ther. 2018;192:88–99.
Hanna J, Guerra-Moreno A, Ang J, Micoogullari Y. Protein degradation and the pathologic basis of disease. Am J Pathol. 2019;189:94–103.
Sun Y, Li H. Functional characterization of SAG/RBX2/ROC2/RNF7, an antioxidant protein and an E3 ubiquitin ligase. Protein Cell. 2013;4:103–16.
Ling Q, Broad W, Trosch R, Topel M, Demiral Sert T, Lymperopoulos P, et al. Ubiquitin-dependent chloroplast-associated protein degradation in plants. Science. 2019;363:eaav4467.
Yang L, Chen J, Huang X, Zhang E, He J, Cai Z. Novel Insights Into E3 Ubiquitin Ligase in Cancer Chemoresistance. Am J Med Sci. 2018;355:368–76.
Beasley SA, Safadi SS, Barber KR, Shaw GS. Solution structure of the E3 ligase HOIL-1 Ubl domain. Protein Sci. 2012;21:1085–92.
Liu ML, Zang F, Zhang SJ. RBCK1 contributes to chemoresistance and stemness in colorectal cancer (CRC). Biomed Pharmacother. 2019;118:109250.
Yu S, Dai J, Ma M, Xu T, Kong Y, Cui C, et al. RBCK1 promotes p53 degradation via ubiquitination in renal cell carcinoma. Cell Death Dis. 2019;10:254.
Xiong W, Zhang B, Yu H, Zhu L, Yi L, Jin X. RRM2 regulates sensitivity to sunitinib and PD-1 blockade in renal cancer by stabilizing ANXA1 and activating the AKT pathway. Adv Sci. 2021;8:e2100881.
Cheng G, Liu Y, Liu L, Ruan H, Cao Q, Song Z, et al. LINC00160 mediates sunitinib resistance in renal cell carcinoma via SAA1 that is implicated in STAT3 activation and compound transportation. Aging. 2020;12:17459–79.
Dufies M, Verbiest A, Cooley LS, Ndiaye PD, He X, Nottet N, et al. Plk1, upregulated by HIF-2, mediates metastasis and drug resistance of clear cell renal cell carcinoma. Commun Biol. 2021;4:166.
Roskoski R Jr. Vascular endothelial growth factor (VEGF) and VEGF receptor inhibitors in the treatment of renal cell carcinomas. Pharmacol Res. 2017;120:116–32.
Kelsall IR, Zhang J, Knebel A, Arthur JSC, Cohen P. The E3 ligase HOIL-1 catalyses ester bond formation between ubiquitin and components of the Myddosome in mammalian cells. Proc Natl Acad Sci USA. 2019;116:13293–8.
Hayward D, Kouznetsova VL, Pierson HE, Hasan NM, Guzman ER, Tsigelny IF, et al. ANKRD9 is a metabolically-controlled regulator of IMPDH2 abundance and macro-assembly. J Biol Chem. 2019;294:14454–66.
Chen C, Sheng Y. Prognostic impact of MITD1 and associates with immune infiltration in kidney renal clear cell carcinoma. Technol Cancer Res Treat. 2021;20:15330338211036233.
Mo Y, Wang Y, Zhang S, Xiong F, Yan Q, Jiang X, et al. Circular RNA circRNF13 inhibits proliferation and metastasis of nasopharyngeal carcinoma via SUMO2. Mol Cancer. 2021;20:112.
Li W, Ye K, Li X, Liu X, Peng M, Chen F, et al. YTHDC1 is downregulated by the YY1/HDAC2 complex and controls the sensitivity of ccRCC to sunitinib by targeting the ANXA1-MAPK pathway. J Exp Clin Cancer Res. 2022;41:250.
Ding J, Kuang P. Regulation of ERalpha stability and estrogen signaling in breast cancer by HOIL-1. Front Oncol. 2021;11:664689.
Wang G, Zhuang Z, Shen S, Yang F, Jiang Z, Liu Z, et al. Regulation of PTEN and ovarian cancer progression by an E3 ubiquitin ligase RBCK1. Hum Cell. 2022;35:896–908.
Cong M, Wang Y, Yang Y, Lian C, Zhuang X, Li X, et al. MTSS1 suppresses mammary tumor-initiating cells by enhancing RBCK1-mediated p65 ubiquitination. Nat Cancer. 2020;1:222–34.
Ruiz-Arenas C, Caceres A, Lopez M, Pelegri-Siso D, Gonzalez J, Gonzalez JR. Identifying chromosomal subpopulations based on their recombination histories advances the study of the genetic basis of phenotypic traits. Genome Res. 2020;30:1802–14.
Lee S, Chang J, Renvoise B, Tipirneni A, Yang S, Blackstone C. MITD1 is recruited to midbodies by ESCRT-III and participates in cytokinesis. Mol Biol Cell. 2012;23:4347–61.
Agromayor M, Martin-Serrano J. Knowing when to cut and run: mechanisms that control cytokinetic abscission. Trends Cell Biol. 2013;23:433–41.
Abu-Ghanem Y, van Thienen JV, Blank C, Aarts MJB, Jewett M, de Jong IJ, et al. Cytoreductive nephrectomy and exposure to sunitinib - a post hoc analysis of the Immediate Surgery or Surgery After Sunitinib Malate in Treating Patients With Metastatic Kidney Cancer (SURTIME) trial. BJU Int. 2022;130:68–75.
Liu W, Ren D, Xiong W, Jin X, Zhu L. A novel FBW7/NFAT1 axis regulates cancer immunity in sunitinib-resistant renal cancer by inducing PD-L1 expression. J Exp Clin Cancer Res. 2022;41:38.
Funding
This work was supported by grants from the Chinese National Natural Science Foundation Grant No. 82073321 (XJ), 82272910 (XJ) and 82103341 (WX). Excellent Youth Foundation of Hunan Scientific Committee (Grant No. 2022JJ10092, XJ). Hunan leading program for science and technology innovation of high technology industries (Grant No. 2022GK4020, XJ). Central South University Innovation-Driven Research Programme (Grant No. 2023CXQD059, XJ). Natural Science Foundation of Hunan Province of China (Grant No. 2022JJ30870 (LZ), 2022JJ40719 (WX)).
Author information
Authors and Affiliations
Contributions
YW: Methodology; MP: Methodology; YZ: Methodology; WX: Investigation, conceptualization; LZ: Investigation, project administration; XJ: Methodology, project administration, investigation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in accordance with the principles of the Declaration of Helsinki principles (Approved no. 2021068). It was approved by the Animal Use and Care Committees at the Second Xiangya hospital, Central South University.
Consent for publication
All subjects have written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Peng, M., Zhong, Y. et al. The E3 ligase RBCK1 reduces the sensitivity of ccRCC to sunitinib through the ANKRD35-MITD1-ANXA1 axis. Oncogene 42, 952–966 (2023). https://doi.org/10.1038/s41388-023-02613-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02613-w