Abstract
Background
Children with Down syndrome are at risk for significant pulmonary co-morbidities, including recurrent respiratory infections, dysphagia, obstructive sleep apnea, and pulmonary vascular disease. Because the gold standard metric of lung function, spirometry, may not be feasible in children with intellectual disabilities, we sought to assess the feasibility of both airwave oscillometry and spirometry in children with Down syndrome.
Methods
Thirty-four children with Down syndrome aged 5–17 years were recruited. Participants performed airwave oscillometry and spirometry before and 10 min after albuterol. Outcomes include success rates, airway resistance and reactance pre- and post-bronchodilator, and bronchodilator response.
Results
Participants were median age 9.2 years (interquartile range 7.2, 12.0) and 47% male. Airwave oscillometry was successful in 26 participants (76.5%) and 4 (11.8%) were successful with spirometry. No abnormalities in airway resistance were detected, and 16/26 (61.5%) had decreased reactance. A positive bronchodilator response by oscillometry was observed in 5/23 (21.7%) of those with successful pre- and post-bronchodilator testing.
Conclusions
Measures of pulmonary function were successfully obtained using airwave oscillometry in children with Down syndrome, which supports its use in this high-risk population.
Impact
-
Children with Down syndrome are at risk for significant pulmonary co-morbidities, but the gold standard metric of lung function, spirometry, may not be feasible in children with intellectual disabilities. This may limit the population’s enrollment in clinical trials and in standardized clinical care.
-
In this prospective study of lung function in children with Down syndrome, airwave oscillometry was successful in 76% of participants but spirometry was successful in only 12%.
-
This study reinforces that measures of pulmonary function can be obtained successfully using airwave oscillometry in children with Down syndrome, which supports its use in this high-risk population.
Similar content being viewed by others
Introduction
Down syndrome is the most common viable chromosomal disorder, diagnosed in approximately 1 in every 70 live births, or 6000 infants per year, in the United States.1 Significant pulmonary co-morbidities occur throughout the lifespan of people with Down syndrome, including recurrent respiratory infections, dysphagia, obstructive sleep apnea (OSA), and pulmonary vascular disease.1,2,3 Pulmonary disease is the most common cause of death in individuals with Down syndrome; therefore, it is critical to prioritize the diagnosis and management of these co-morbidities.1 Pulmonary function testing is one of the most common clinical and research metrics to monitor patients with pulmonary diseases, including asthma, chronic obstructive pulmonary disease, cystic fibrosis, and others. Objective measures of disease, such as pulmonary function testing, may be useful in screening and monitoring lung disease in people with Down syndrome, but the best method of testing in this population is unclear.
Prior evaluations of lung function in people with Down syndrome have primarily focused on the use of spirometry.4,5,6,7,8,9 Spirometry is an effort-dependent mode of lung function testing, which can be challenging to accomplish in a population with intellectual disabilities and hypotonia. Therefore, generalization from these studies is limited due to their small size and variable success in obtaining acceptable spirometric measures. Some studies describe lower lung function when compared to healthy controls or a reference population,4,5,6,7,8,9 and it is unclear if these baseline differences are related to lung pathology or the ability to perform effort-dependent forced expiratory maneuvers during spirometry. Studies have incorporated repeated spirometry attempts through the duration of an exercise program and show improvements in outcomes from baseline: likely due to improved conditioning and/or additional testing experience.4,5 In addition, some investigations have incorporated practice sessions to train participants in the performance of spirometry, which may be difficult to replicate in the clinical setting.4,7 Due to the challenges in these prior reports, we are proposing the use of airwave oscillometry, a form of the forced oscillation technique, for the evaluation of lung function in children with Down syndrome.
Airwave oscillometry provides estimates of airway resistance and reactance by superimposing pressure oscillations during tidal breathing.10 This technique requires passive cooperation and is considered effort-independent because the subject is instructed to breathe normally throughout testing without forced maneuvers. Airwave oscillometry has been successfully used to measure lung function in preschool children, who are typically unable to perform spirometry,10 and pilot acceptability data have been reported in people with Down syndrome.11 In this investigation, our primary aim was to determine the proportion of airwave oscillometry and spirometry tests that met acceptability criteria, and our secondary aim was to describe airway resistance and lung reactance in a small cohort of children with Down syndrome. We hypothesized that airwave oscillometry is a feasible and reproducible measure of pulmonary function in children with Down syndrome, which may provide a widely available and non-invasive method of screening, monitoring, and studying pulmonary disease in this high-risk population.
Methods
Study participants
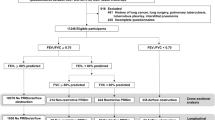
Children with Down syndrome between 5 and 17 years old with an existing clinical relationship with the Sie Center for Down Syndrome at Children’s Hospital Colorado were eligible for inclusion. Study enrollment and testing occurred between May 2019 and January 2020. Children were excluded if they were diagnosed with autism spectrum disorder that may impact their testing abilities, or if they had a tracheostomy or continuous oxygen requirement. Children with an acute respiratory illness were temporarily excluded and able to complete testing 2 weeks after symptom resolution. Acute respiratory illnesses were defined as a new onset or increase from baseline in lower respiratory tract symptoms, along with the need for one of the following: antibiotics, systemic steroids, increase in oxygen from baseline, or hospitalization.
Study procedures
This study was approved by the Institutional Review Board at the University of Colorado School of Medicine. All study data were entered and secured in the University of Colorado Research Electronic Data Capture (REDCap) database, a HIPAA-compliant web-based application designed for data collection.12 Informed consent was obtained from the parent or legal guardian before study procedures and assent was obtained from children aged 7 years or older, if developmentally able.
All participants underwent baseline measures of airwave oscillometry and spirometry, with specific procedures described below. Airwave oscillometry was performed prior to spirometry to avoid the potential effect of spirometry-induced bronchospasm on oscillometry outcomes.10 Post-bronchodilator airwave oscillometry and spirometry were performed at least 10 min after administration of albuterol; 90 μg meter-dose inhaler, 3 puffs delivered via LiteAire® valve holding chamber by Thayer Medical (Tucson, AZ).
Airwave oscillometry
Airwave oscillometry was performed using the tremoflo® c-100 Airwave Oscillometry SystemTM (Thorasys, Montreal, QC, Canada). The device utilizes vibrating mesh technology to generate multi-frequency oscillations that are superimposed on tidal breathing. The oscillations comprise multiple frequencies from 5 to 37 Hz, allowing for measures of airway resistance (Rrs) and reactance (Xrs) from the central to the distal airways. Resistance at 5 Hz (R5) estimates overall airway resistance, while resistance at 20 Hz (R20) estimates resistance in the central, or conducting, airways. Small airway disease is assessed by the difference between the overall and conducting airways resistance (R5–R20). Reactance at 5 Hz (X5), the resonant frequency (Fres), and the reactance area (AX) estimate the elastance of the lungs.
Verification of the device was performed daily using a 2 cm H2O s/L reference test load. Testing occurred with participants in a seated position, with nose clips used to minimize air leak through the nasal passages and use of the child’s or parent’s hands to firmly support the cheeks and minimize upper airway vibrations.13 Participants were coached to maintain a seal around the mouthpiece and to breathe normally through the mouthpiece during the 20-s test (Fig. 1). Brief breaks were given in between testing attempts and participants were allowed up to eight attempts.
Acceptable testing was defined by three attempts with a coefficient of variation ≤15% for resistance measures at 5 Hz (R5).14 Reference equations from a healthy population of children were used for interpretation and account for the participant’s sex and height.15 Selection of the reference equation was based on the age distribution of the cohort. Bronchodilator response was defined a priori by a decrease in R5 by 40%, an increase in X5 by 50%, and/or a decrease in AX by 80% from baseline measurements.14
Spirometry
Spirometry was performed using the KoKo® Legend II (nSpire Health, Longmont, CO). Outcomes from spirometry included forced expiratory volume in 1 s (FEV1, in liters), forced vital capacity (FVC, in liters), the ratio of FEV1/FVC, and the forced expiratory flow in the mid-expiratory phase (FEF25–75, in L/s).
Calibration checks using a 3-L syringe were performed daily and room temperature, barometric pressure, and relative humidity were inputted for the purpose of BTPS correction. Participants completed testing while seated and wearing nose clips. Participants were coached to inhale to total lung capacity followed by forced and complete expiration with the use of verbal and visual prompts. Participants were allowed a maximum of eight attempts.13
Acceptability was determined based on spirometry criteria outlined in a technical statement by the American Thoracic Society, with allowance for attempts that met preschool criteria.13,16 Grading for spirometry quality, ranging from “A” through “F,” were assigned based on the number of attempts that were considered acceptable and reproducible per the aforementioned criteria.16 Testing with grades A, B, and C are considered clinically usable, whereas grades D and E should be interpreted cautiously, and grade F is uninterpretable.16 Initial spirometry grading was performed by study member MLV and then reviewed by study member MAB. Reference equations were used for interpretation using the participant’s sex, age, race/ethnicity, and height.17 Bronchodilator response was defined by an increase in FEV1 by 12%.18
Questionnaire and clinical information
The parent or caregiver completed a questionnaire to assess baseline respiratory symptoms, which incorporated the International Study of Asthma and Allergies in Children (ISAAC) core questionnaire for wheezing to understand the prevalence of wheezing, prior diagnoses of asthma, and persistent asthma symptoms.19 Relevant cardiopulmonary co-morbidities were assessed through chart review, including history of premature birth and congenital heart disease. If completed within 2 years of the study visit, results from videofluoroscopic swallow studies to evaluate dysphagia and polysomnograms to evaluate for OSA were included.
Data analysis
The study was designed to primarily assess the feasibility of lung function testing in this population, so there was not a targeted sample size. Categorical variables were reported as frequencies and percentages. Continuous variables were reported as median with first and third quartiles, or mean values with standard deviations using R studio. Lung function results are presented as percent-predicted and z-scores compared to referent populations.
Results
Demographics and clinical characteristics
Thirty-four children with Down syndrome, with a median age of 9.2 years (interquartile range 7.2, 12.0), participated in the study. Of those, 47.1% were male and 67.6% were Caucasian (Table 1). Additional cardiopulmonary co-morbidities are described in Table 1. Of note, nearly half of the cohort (47.1%) was born prematurely, predominantly in the late-preterm period, 76.4% were diagnosed with congenital heart disease, and 64.7% were diagnosed with OSA (Table 1).
Responses to the ISAAC core questionnaire for wheezing revealed that 22 (64.7%) children had a prior history of wheezing or whistling in the chest, and 5 (14.7%) had been previously diagnosed with asthma. In the past 12 months, wheezing during exercise was reported in 5 (14.7%) children and dry nocturnal cough in 8 (23.5%) children.
Pulmonary function testing feasibility
All 34 subjects attempted both airwave oscillometry and spirometry. Twenty-six (76.5%) children had three or more tests with airwave oscillometry that met acceptability and reproducibility criteria. Higher rates of success were observed in participants aged 8–12 (86.7%) and 13–17 years (85.7%) compared to children aged 5–7 years (58.3%). Older children also demonstrated superiority in the quality of testing as measured by between- and within-test reliability. Between-test variability, reported as the coefficient of variation, decreased across the age groups (5–7 years = 10.5%, 8–12 years = 6.2%, 13–17 years = 5.9%). Similarly, coherence values, a representation of within-test reliability, increased across the age groups (5–7 years = 0.74, 8–12 years = 0.80, 13–17 years = 0.84). In those with successful testing, participants completed an average of 6.5 testing attempts within an 8.5-min time period.
There were eight children (23.5%) who were not successful in airwave oscillometry testing. Three (8.8%) of these children completed two acceptable tests but did not complete a third attempt that met acceptability criteria. The remaining five (14.7%) children did not have any acceptable attempts due to their inability to maintain a seal around the mouthpiece for the duration of the testing. Four of these participants were between the ages of 5 and 7 years.
Spirometry was unsuccessful in the majority (88.2%) of children in this study, with no clear distinction between the age groups. Of those with interpretable spirometry (n = 4, 11.8%), one was grade A, one was grade B, and two were grade E based on ATS criteria.16 All participants with successful spirometry were also successful in completing airwave oscillometry. The most commonly encountered errors noted during testing and/or interpretation included submaximal blast, cough within the first second of exhalation, and early termination.
Airwave oscillometry results
The results for participants who successfully completed airwave oscillometry are outlined in Table 2 and are reported for both pre- and post-bronchodilator testing. Measures of resistance (R5, R20, R5–R20) were within normal limits for both pre- and post-bronchodilator testing in all participants, with z-scores that did not exceed 1.64 (Table 2). Decreased reactance (X5), defined by a z-score below −1.64, was observed in 61.5% (16/26). Of those with successful testing, 13% (3/23) had sustained low reactance after bronchodilator administration (Table 2).
Within-breath analysis shows the difference between expiratory and inspiratory reactance values (X5Ex–In) for those with normal and abnormal reactance at baseline (Fig. 2). Children with abnormal baseline reactance had a greater difference between their expiratory and inspiratory reactance values, with a more negative reactance during expiration compared to inspiration. This difference was less pronounced after bronchodilator administration and in those with normal reactance at baseline (Fig. 2).
Negative values for X5Ex–In indicate that the expiratory reactance was more negative (i.e. more abnormal) than the inspiratory reactance. Positive values for X5Ex–In indicate that the expiratory reactance was less negative (i.e. more normal) than the inspiratory reactance. For each box plot, the central line represents the median, the box ranges from the 25th to the 75th percentiles, and the whiskers extend to extreme values that are not considered outliers.
Bronchodilator response
The percent-predicted values for measures of airway resistance and reactance all decreased post-bronchodilator. A positive bronchodilator response, defined a priori as a decrease in R5 by 40%, an increase in X5 by 50%, and/or a decrease in AX by 80%, was observed in 21.7% (5/23) of those with successful pre- and post-bronchodilator airwave oscillometry. There were no trends observed in responses to the ISAAC core questionnaire for wheezing when comparing those with and without a bronchodilator response.
Discussion
This prospective study demonstrates that airwave oscillometry was a feasible method of pulmonary function testing in this cohort of children with Down syndrome and supports further investigation of its use in the clinical setting to aid in the management of this high-risk population. We attribute the success of oscillometry to the effort-independent nature of the technique, with measures that are obtained at normal tidal breathing. This contrasts with the effort-dependent forced expiratory maneuvers that are required for spirometry, which can be challenging in a population of children with intellectual disability and hypotonia.
Feasibility of the forced oscillation technique
Our study is now the second cohort to demonstrate the successful application of the forced oscillation technique in people with Down syndrome. A cohort of children and adults with Down syndrome in Mexico City underwent a panel of pulmonary function testing, including impulse oscillometry, spirometry, 6-min walk test, and lung diffusing capacity for carbon monoxide.11 The highest rates of success were in impulse oscillometry, with achievement demonstrated in 58.5% of children 5–11 years of age and 68.8% in children 12–18 years old. There were also low rates of success for spirometry, similar to those in our cohort.11
In both our cohort and as reported by Fernandéz-Plata et al.,11 older children have higher success rates. Over 85% of the children aged 8 or older were successful with airwave oscillometry in our cohort. Most testing failures occurred in children aged 5–7 years old, in which the children were unable to maintain a seal around the mouthpiece for the duration of testing. Additionally, testing failures for three children aged 8 or older were characterized by their inability to perform three testing attempts meeting acceptability criteria based on the between-test variability.
Despite the increasing success with age, the results from our cohort promote using the forced oscillatory technique to measure lung function in children aged 5 years or older with Down syndrome. As seen in other evaluations of pulmonary function testing, results may improve with repeated attempts of the technique.4,7 Therefore, if unsuccessful with initial testing attempts, it would be appropriate to allow for home practice, particularly in maintaining a mouth seal, and then returning to the clinic for repeat testing. Expansion of airwave oscillometry could introduce an objective measure to aid in the diagnosis and monitoring of lung disease, similar to management of asthma and cystic fibrosis-related lung disease. Although airwave oscillometry is not as widespread as spirometry, implementation of airwave oscillometry longitudinally could facilitate decision-making in the individual management of pulmonary co-morbidities in Down syndrome, such as dysphagia or recurrent respiratory infections.
Airwave oscillometry findings of normal lung resistance and abnormal reactance
Interestingly, resistance measurements were not abnormal in our cohort when compared to the reference values. This finding was unexpected given that there are many sites of airway obstruction reported in the Down syndrome population, such as upper airway collapse, tracheobronchomalacia,20,21 and a smaller caliber trachea,22 which may result in an increased resistance within the central airways.
The most striking abnormality was a low reactance at 5 hertz (X5), which was observed in over 60% of those with successful testing. This finding is typically described in pediatric conditions such as asthma or bronchopulmonary dysplasia where there is decreased reactance of the airways and lung parenchyma.23,24,25 However, in our cohort, there were not corresponding increases in airway resistance typically seen in asthma and bronchopulmonary dysplasia. A decreased reactance pattern can also be observed in those with restrictive lung physiology, including certain interstitial lung diseases, but these diagnoses were not present in our cohort.
The underlying reason for the finding of low reactance in our cohort is unclear. One potential explanation for decreased reactance includes abnormal lung structure or alveolar simplification, which is known to occur in children with Down syndrome and may be further impacted by the rates of congenital heart disease and late-preterm births observed in our cohort.
Another possible explanation for this finding is small airway obstruction either driven by an inflammatory process or due to low airway tone with early closing volumes during exhalation. The within-breath analysis comparing expiratory and inspiratory reactance values may align with the latter explanation, with early airway closure leading to more negative expiratory reactance values. A difference between expiratory and inspiratory reactance values has been described in adults with expiratory dynamic airway collapse.26 This is an area for future investigation given the high rates of tracheobronchomalacia that have been reported in children with Down syndrome.20,21
We are limited in our ability to generalize these findings due to the small size of our study and the lack of reference equations that are specific to airwave oscillometry. Currently used reference equations were developed in healthy children using impulse oscillometry,15 and have been applied to airwave oscillometry without validation in this newer form of the forced oscillation technique. Additionally, there is evidence that airwave oscillometry may be a more sensitive technique for the detection of reactance abnormalities. Kuo and colleagues compared airwave and impulse oscillometry in adults with chronic obstructive pulmonary disease, and found that reactance abnormalities (X5, AX, and Fres) were significantly larger in magnitude in airwave oscillometry and that resistance measures were similar across devices.27 Large longitudinal studies are needed to evaluate if lung reactance may be appropriate as an early and subtle finding of lung disease.
Bronchodilator responsiveness
A positive bronchodilator response was observed in 21.7% of the participants who underwent successful pre- and post-bronchodilator testing, but these results were not correlated to parental report of prior asthma diagnosis or history of persistent asthma symptoms in our cohort. It is unclear if these findings are suggestive of an underlying asthma phenotype in this population, or if the bronchodilator response is related to increased resting smooth muscle tone of another etiology. Prior assessments of asthma in children with Down syndrome have been limited to questionnaires, in which parents have reported higher rates of wheezing than observed in healthy children, while physician-confirmed diagnoses of asthma are much lower.28 The inability to obtain pulmonary function testing in this population has been a barrier to assessing for bronchodilator responsiveness, but airwave oscillometry may be helpful for the diagnosis and management of children with recurrent wheeze and/or chronic respiratory symptoms.
This investigation is limited by a small sample size and the results from airwave oscillometry testing should be interpreted cautiously due to a lack of established reference equations using airwave oscillometry, as discussed above. The reference equations were developed in healthy pediatric populations using impulse oscillometry, another form of the forced oscillation technique, and have been applied to populations tested using airwave oscillometry without validation in a control population. Additionally, our investigation was limited by a single evaluation of participants without repeat testing to assess for reproducibility and/or intra-subject variability over time. This cohort includes “all-comers” with Down syndrome who volunteered for the study. Further evaluations are needed to assess the utility of airwave oscillometry as a surrogate of lung function with specific pulmonary co-morbidities and in those with more significant delays and/or behavioral challenges that may occur with a dual diagnosis such as autism spectrum disorder.
In conclusion, there are significant pulmonary risk factors in the pediatric population with Down syndrome, and there is a paucity of data to understand how these co-morbidities impact lung function. The availability of a simple, reproducible, and objective measure of lung function, such as airwave oscillometry, greatly enhances our ability to identify and trend the impact of these co-morbidities on pulmonary functional outcomes. Further work is needed to characterize the lung function abnormalities that accompany these co-morbidities and to determine if there are changes observed with treatment of the conditions.
References
Colvin, K. L. & Yeager, M. E. What people with Down syndrome can teach us about cardiopulmonary disease. Eur. Respir. Rev. 26, 160098 (2017).
McDowell, K. M. & Craven, D. I. Pulmonary complications of Down syndrome during childhood. J. Pediatr. 158, 319–325 (2011).
Pandit, C. & Fitzgerald, D. A. Respiratory problems in children with Down syndrome. J. Paediatr. Child Health 48, E147–E152 (2012).
Khalili, M. A. & Elkins, M. R. Aerobic exercise improves lung function in children with intellectual disability: a randomised trial. Aust. J. Physiother. 55, 171–175 (2009).
El Kafy, E. M. & Helal, O. F. Effect of rowing on pulmonary functions in children with Down syndrome. Pediatr. Phys. Ther. 26, 437–445 (2014).
Pastore, E. et al. Clinical and cardiorespiratory assessment in children with Down syndrome without congenital heart disease. Arch. Pediatr. Adolesc. Med. 154, 408–410 (2000).
Dichter, C. C., Darbee, J. C., Effgen, S. K. & Palisano, R. J. Assessment of pulmonary function and physical fitness in children with Down syndrome. Pediatr. Phys. Ther. 5, 3–8 (1993).
Salgueirinho, C., Venâncio, J., Martin-Nogueras, A. M. & Ribeiro, F. Pulmonary function in young adults with Down syndrome: a cross-sectional study. Int. Med. Rev. Down Syndr. 20, 17–20 (2015).
Laibsirinon, S., Jarusurin, N., Kokoi, C. & Manakiatichai, T. Pulmonary function and chest expansion in Thai boys with Down syndrome. Thammasat Med. J. 12, 269–275 (2012).
Komarow, H. D. et al. A study of the use of impulse oscillometry in the evaluation of children with asthma: analysis of lung parameters, order effect, and utility compared with spirometry. Pediatr. Pulmonol. 47, 18–26 (2012).
Fernandez-Plata, R. et al. Quality of pulmonary function tests in participants with Down syndrome. Arch. Bronconeumol. 55, 513–518 (2019).
Harris, P. A. et al. Research Electronic Data Capture (Redcap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381 (2009).
Beydon, N. et al. An official American Thoracic Society/European Respiratory Society Statement: pulmonary function testing in preschool children. Am. J. Respir. Crit. Care Med. 175, 1304–1345 (2007).
King, G. G. et al. Technical standards for respiratory oscillometry. Eur. Respir. J. 55, 1900753 (2020).
Nowowiejska, B. et al. Transient reference values for impulse oscillometry for children aged 3–18 years. Pediatr. Pulmonol. 43, 1193–1197 (2008).
Culver, B. H. et al. Recommendations for a standardized pulmonary function report. An Official American Thoracic Society Technical Statement. Am. J. Respir. Crit. Care Med. 196, 1463–1472 (2017).
Quanjer, P. H. et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur. Respir. J. 40, 1324–1343 (2012).
Pellegrino, R. et al. Interpretative strategies for lung function tests. Eur. Respir. J. 26, 948–968 (2005).
Asher, M. I. et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur. Respir. J. 8, 483–491 (1995).
Bertrand, P., Navarro, H., Caussade, S., Holmgren, N. & Sanchez, I. Airway anomalies in children with Down syndrome: endoscopic findings. Pediatr. Pulmonol. 36, 137–141 (2003).
De Lausnay, M. et al. The prevalence of lower airway anomalies in children with Down syndrome compared to controls. Pediatr. Pulmonol. 55, 1259–1263 (2020).
Fockens, M. M., Holscher, M., Limpens, J. & Dikkers, F. G. Tracheal anomalies associated with Down syndrome: a systematic review. Pediatr. Pulmonol. 56, 814–822 (2021).
Malmberg, L. P. et al. Lung function measured by the oscillometric method in prematurely born children with chronic lung disease. Eur. Respir. J. 16, 598–603 (2000).
Brostrom, E. B., Thunqvist, P., Adenfelt, G., Borling, E. & Katz-Salamon, M. Obstructive lung disease in children with mild to severe Bpd. Respir. Med. 104, 362–370 (2010).
Sol, I. S. et al. Assessment of within-breath impulse oscillometry parameters in children with asthma. Pediatr. Pulmonol. 54, 117–124 (2019).
Fielding, D. I. et al. Expiratory reactance abnormalities in patients with expiratory dynamic airway collapse: a new application of impulse oscillometry. ERJ Open Res. 4, 00080–2018 (2018).
Kuo, C. R., Jabbal, S. & Lipworth, B. I say Ios you say Aos: comparative bias in respiratory impedance measurements. Lung 197, 473–481 (2019).
Weijerman, M. E., Brand, P. L., van Furth, M. A., Broers, C. J. & Gemke, R. J. Recurrent wheeze in children with Down syndrome: is it asthma? Acta Paediatr. 100, e194–e197 (2011).
Acknowledgements
This publication is supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535, allowing the use of REDCap for data storage and R21 HL151261-01 (to E.D.B.). Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. We would also like to thank the Global Down Syndrome Foundation and the Anna and John J. Sie Foundation for their financial support of research conducted at the Sie Center for Down Syndrome at Children’s Hospital Colorado.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
E.D.B. is a consultant of EvoEndoscopy (formerly TripleEndoscopy Inc.) and is listed as a co-inventor on patents related to endoscopy. E.D.B. also receives funding from the Cystic Fibrosis Foundation and NIH, and is a consultant for Boehringer Ingelheim, not related to this project. The remaining authors declare no competing interests.
Consent statement
Informed consent was obtained from the parent or legal guardian before study procedures and assent was obtained from children aged 7 years or older, if developmentally able.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Vielkind, M.L., Hamlington, K.L., Wolter-Warmerdam, K. et al. Airwave oscillometry to measure lung function in children with Down syndrome. Pediatr Res 91, 1775–1780 (2022). https://doi.org/10.1038/s41390-021-01664-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01664-7