Abstract
Study design
Retrospective cohort study with 10 years follow-up.
Objective
To compare the risks of sensorineural hearing loss in patients with and without spinal cord injury, based on a nationally representative sample.
Setting
Taiwan’s Longitudinal Health Insurance Database 2005.
Method
A total of 2006 participants who had been aged between 20 and 69 and who had spinal cord injury as of 2002–06 were enrolled in the spinal cord injury group. The non-spinal cord injury group consisted of 8024 sex- and age-matched, randomly sampled participants without spinal cord injury. Then, their sensorineural hearing loss -cumulative incidence curves were generated using the Kaplan–Meier method. Stratified Cox proportional-hazard regression was employed to estimate the effect of having spinal cord injury on patients’ subsequent risk of sensorineural hearing loss.
Results
During the follow-up, 30 patients in the spinal cord injury group and 87 in the non-spinal cord injury group developed sensorineural hearing loss. As such, the cumulative incidence of sensorineural hearing loss was significantly higher in the spinal cord injury group than the non-spinal cord injury group (2.16 vs. 1.21 per 1000 person-years, p = 0.008). The adjusted hazard ratio of sensorineural hearing loss for the spinal cord injury group was 1.75 times that of the non-spinal cord injury group (95% CI, 1.14–2.68, p = 0.01). The patients with non-cervical SCI appeared to have a higher magnitude of SNHL risk than their cervical SCI counterparts.
Conclusion
Our study showed that patients with spinal cord injury have an increased risk of developing sensorineural hearing loss.
Similar content being viewed by others
Introduction
Sensorineural hearing loss (SNHL) is the most common type of hearing impairment that is characterized by damage to the mechanosensory cells within the cochlea or the auditory nerve. Previous studies have shown that certain cardiovascular risk factors, such as diabetes mellitus [1], hypertension [2], dyslipidemia [3], and cigarette smoking [4] are linked to an increased risk of SNHL, and some researchers have therefore postulated that disrupted vascular blood supply to the inner ear is an important etiology of SNHL [5].
Spinal cord injury (SCI) is associated with a higher risk of cardiovascular diseases. In a cross-sectional community health survey in Canada, for example, SCI was linked to increased odds of heart disease and stroke [6]. Elsewhere, SCI patients were found to have higher coronary-artery calcium scores, and therefore a greater atherosclerotic burden, than their counterparts in a non-SCI control group [7]. However, to our knowledge, no research has hitherto been conducted on the relationship between SCI and SNHL. We therefore carried out this nationwide longitudinal follow-up study to investigate whether patients with SCI are at a higher risk of developing SNHL.
Materials and methods
Data source
Taiwan’s National Health Insurance (NHI), established in 1995, is a compulsory social-insurance program in which ~99% of the country’s citizens are enrolled. The present study drew its data from a subset of the NHI Research Database, the Longitudinal Health Insurance Database 2005 (LHID2005), which includes 1,000,000 persons systematically sampled from all NHI enrollees as of 2005. The LHID2005 has been validated as a representative subset of the NHI Research Database: i.e., there are no significant differences between its members’ age and sex distributions and those of the individuals in the complete NHI Research Database (https://nhird.nhri.org.tw/en/Data_Subsets.html#S3).
Ethics statement
The present study was approved by the Research Ethics Committee of National Taiwan University Hospital. The approval number is 201912207RIND. All participants’ data in LHID2005 are encrypted to ensure personal privacy. Because LHID2005 consists of de-identified data that is released for research purposes, the requirement for informed consent was waived.
Study participants and design
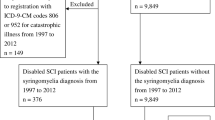
We conducted a retrospective cohort study to investigate the impact of SCI on individuals’ subsequent risk of SNHL. As shown in our flowchart of the enrollment process for research participants (Fig. 1), the inclusion criteria for the initial SCI group were: (1) having at least two outpatient records in LHID2005 showing a diagnosis of SCI (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] codes 806, 952) dating from January 1, 2002 to December 31, 2006, with the date of the first diagnosis of SCI in that period being defined as the index date; and (2) being 20–69 years old on the index date. Based on these criteria, a total of 2385 participants were included in the initial SCI group.
Information about preexisting comorbid conditions was obtained by retrieving all of each participant’s LHID2005 outpatient and inpatient records that dated from before his/her index visit. We identified seven comorbidities as potentially affecting the association between SCI and SNHL: diabetes (ICD-9-CM code 250), dyslipidemia (ICD-9-CM code 272), hypertension (ICD-9-CM codes 401-405), coronary heart disease (ICD-9-CM codes 410-414 and 429.2), stroke (ICD-9-CM codes 430-438), chronic obstructive pulmonary disease (ICD-9-CM codes 491, 492, and 496), and renal disease (ICD-9-CM codes 580-587). Due to the absence of smoking status information from the LHID2005 database, chronic obstructive pulmonary disease was used as a surrogate for smoking exposure [8]. These comorbidities were determined by at least one discharge record or at least two outpatient visits with the relevant diagnosis code(s) in the 12-month period prior to the index date.
We represented the study participants’ socioeconomic status using (1) geographic region of their places of residence, (2) urbanization levels at those places, and (3) monthly income. The first was obtained from the NHI registry, and comprised four regions: Northern, Central, Eastern, and Southern Taiwan. Urbanization was classified into seven levels (with one being the most urbanized and seven the least), according to the system published by the Taiwan National Health Research Institute [9]. However, because that there were only a few participants resided in places with level 5, 6, or 7 urbanization, these three levels were combined into a single group, hereafter referred to as level 5. Lastly, monthly insured payroll amount was used as a proxy for monthly income, and categorized into four levels: new Taiwan dollar (NT$) 0, NT$1–15,840, NT$15,841–25,000, and ≥NT$25,001. We used NT$15,840 as the first cutoff point for income level because this was the government-stipulated minimum wage for full-time employees in Taiwan.
Patients were excluded from the SCI group if they met either of the following criteria: (1) having had a diagnosis of hearing loss (ICD-9-CM code 389) before the index date (n = 30); or (2) having had a diagnosis of SCI (ICD-9-CM codes 806, 952) before the index date (n = 348). This was done to increase the likelihood that the sample consisted of newly diagnosed SCI patients. After 373 SCI patients were excluded due to above exclusion criteria, 2012 remained in the SCI group. Then, six SCI patients were excluded because their records were missing socioeconomic status information, leaving a final total of 2006 patients in the SCI group for analysis.
The initial non-SCI group comprised all the remaining participants in LHID2005 who did not have a diagnosis of SCI during their outpatient visits from January 2002 to December 2006. The dates of these individuals’ first outpatient visits during this period were designated as their index dates. This pool was then limited to participants aged from 20 to 69 years on their respective index dates, yielding a non-SCI group of 631,304 people. The following exclusion criteria were then applied: (1) having had a diagnosis of hearing loss (ICD-9-CM code 389) prior to the index date (n = 2518), or (2) having had a diagnosis of SCI (ICD-9-CM codes 806, 952) prior to the index date (n = 578). Information on comorbid conditions and socioeconomic status was obtained using the same methods described above. After excluding 867 non-SCI participants who were lacking socioeconomic-status information, a total of 627,348 participants remained in the non-SCI pool. We then frequency-matched each SCI patient to four randomly selected non-SCI participants of the same sex and age, leaving 2006 participants in the final matched SCI group and 8024 in the non-SCI group for analysis.
Outcomes
Each member of the SCI and non-SCI groups was tracked from the index date until a new diagnosis of SNHL, his/her death, or the end of 2011 (whichever came first). The occurrence of a new SNHL diagnosis, defined by at least one hospital discharge or two outpatient visits with a diagnosis of SNHL (ICD-9-CM code 389.1), was considered the primary outcome in this study. The date of a person’s withdrawal from the NHI program was assumed to coincide with his/her death, and the cause of death was assumed to be the primary diagnosis of his/her last inpatient or outpatient record in the 3 months preceding death [10].
Statistical analysis
Chi-squared and Student’s t tests were used to examine differences in the baseline characteristics between the SCI and the non-SCI groups. The incidence rate was calculated as the number of SNHL patients divided by the sum of SNHL-free follow-up time (per 1000 person-years). Curves representing the cumulative incidence of SNHL for the two groups were generated using the Kaplan–Meier method, and the differences between these two curves were evaluated using a log-rank test. Cox proportional-hazard regression analyses were then used to evaluate the association between SCI and the occurrence of SNHL, with adjustment made for the participants’ baseline demographic characteristics, socioeconomic status, and comorbidities. Fisher’s exact test was used to compare the distribution of the types of SNHL between the SCI and the non-SCI groups. A p value < 0.05 was considered statistically significant. All analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC).
Results
Table 1 presents the baseline characteristics of the SCI and non-SCI groups. Due to our use of frequency matching, the distributions of sex and age were similar between the two groups, and the mean age of both groups was 47.1 years. The SCI group was more likely than the non-SCI one to have certain comorbid conditions, including diabetes, hypertension, dyslipidemia, coronary heart disease, stroke, and renal disease. There were also significant differences in the two groups’ distributions of monthly income, urbanization level, and geographic region.
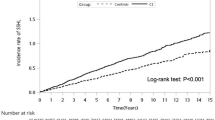
The median follow-up time was 115.0 months, with an interquartile range of 22.4 months. The results of Cox analysis are shown in Table 2. During the follow-up, 30 of the 2006 SCI patients developed SNHL, as against 87 of the 8024 non-SCI participants. The SCI group’s risk of SNHL was significantly higher than the non-SCI group’s (adjusted hazard ratio [HR] = 1.75, 95% confidence interval [CI], 1.14–2.68, p = 0.01). The cumulative incidence of SNHL of the SCI group (2.16 per 1000 person-years) was higher than that of the non-SCI group (1.21 per 1000 person-years, p < 0.01) (Fig. 2).
The subgroup analysis of the crude and adjusted HRs of SNHL is presented in Table 2. The subjects in the SCI group were divided into two subgroups according to the level of SCI: (1) cervical SCI, and (2) non-cervical SCI. The adjusted HR of SNHL for the cervical SCI subgroup was 1.39 (95% CI, 0.63–3.03, p = 0.4133), whereas the adjusted HR of SNHL for the non-cervical SCI subgroup was 1.89 (95% CI, 1.18–3.04, p = 0.008). The non-cervical SCI subgroup appeared to have higher magnitude of SNHL HR than their cervical SCI counterparts. Table 3 presents the distribution of the types of SNHL occurred in the SCI and non-SCI groups. The SNHL cases were classified into two types: (1) bilateral (or asymmetrical) SNHL, and (2) non-bilateral (or uncategorized) SNHL. Most of the SNHL events were non-bilateral. There was lack of significant differences in the two groups’ distributions of SNHL types (Fisher’s exact test, p = 0.2997).
Discussion
Our large-scale longitudinal follow-up study established that its SCI group had a 1.75-fold higher risk of developing SNHL than its non-SCI group did. As such, it is the first study of its kind to demonstrate an increased risk of SNHL in SCI patients. Vascular pathology affecting the blood supply to the inner ear has been suggested as an important etiology for SNHL [11]. The vascular supply to the cochlea is maintained by the labyrinthine artery, and collateral vasculature is largely absent; therefore, the cochlea is highly vulnerable to ischemia-related injury. Thrombosis, embolism, hemorrhage, and vasospasm might all interrupt the inner ear’s blood flow, leading to cochlear injury and dysfunction. Although the exact mechanism underlying the association between SCI and SNHL is uncertain, we can propose the following possible explanations.
Atherosclerosis has been associated with increased risk of hearing impairment [12]. Dyslipidemia, diabetes mellitus, and hypertension are well-recognized risk factors of atherosclerosis. It has also been suggested that SCI patients are prone to metabolic abnormalities such hyperglycemia and hyperlipidemia [13]. In our study, SCI patients also had a significantly higher prevalence of diabetes, hypertension, dyslipidemia, coronary heart disease, and stroke than the non-SCI participants did (Table 1), suggesting that the former likely had an increased risk of developing atherosclerosis disorders. Moreover, individuals with SCI have been found to have impaired vascular control [14] and decreased blood-vessel reactivity [15], which may predispose them to atherosclerosis and thus, further disruption of vascular supply to the cochlea. Nevertheless, after controlling for these comorbidities in our Cox regression analysis, we found that SCI remained an independent predictor of SNHL development. Therefore, the association between SCI and SNHL may also be mediated by other mechanisms.
Previous studies have reported that patients with thrombophilic risk factors, and especially those with strong family histories of thromboembolic events, are at a greater risk of developing sudden SNHL [16, 17]. The three major factors composing Virchow’s triad—i.e., venous stasis, hypercoagulability, and endothelial injury—increase one’s propensity for thromboembolism. As such, patients with SCI are more likely to have thromboembolism due to the presence of two major risk factors, stasis and hypercoagulability [18]; and this leads these individuals to have significantly higher risk of thromboembolism events, such as deep-vein thrombosis and pulmonary embolism, than the general population [19]. Therefore, the higher risk of SNHL in SCI patients observed in our study might also be explained by SCI-related thrombophilic states.
It has been reported that SNHL can be associated with various systemic autoimmune diseases such as rheumatoid arthritis [20], multiple sclerosis [21], and systemic lupus erythematosus [22]. In 1979, McCabe proposed the term autoimmune SNHL after a patient with idiopathic bilateral progressive SNHL had been effectively treated with steroids [23]. Autoimmune SNHL remains a diagnosis of exclusion, supported by clinical presentation with bilateral progressive SNHL, and by responsiveness to corticosteroid treatment. Autoimmune damage to the inner ear can be mediated by both humoral and cell-mediated mechanisms. SCI, meanwhile, has been associated with trauma-induced autoimmune reactions: with experimental research showing that it is related to chronic B lymphocyte activation with increased synthesis of anti-DNA antibodies [24]. There is also evidence that autoreactive T lymphocytes are activated after SCI [25, 26]. Therefore, we hypothesize that SCI-related alterations to systemic immunity may also contribute to SCI patients’ observed higher risk of developing SNHL.
Previous studies have suggested that cervical spine disorders is associated with hearing impairment [27]. It has also been reported that manipulation of the cervical spine may cause damage to the vertebrobasilar arterial system, resulting in sudden SNHL [28]. However, the subgroup analysis showed that the magnitude of hazard ratio for SNHL was not higher in the cervical SCI subgroup (Table 2). Accordingly, the association between SCI and SNHL seen in our study may be less likely to be directly caused by local cervical spine injury. We therefore speculate that SCI-related systemic effects (e.g., atherosclerosis, thrombophilic states, and alterations in systemic immunity) may play roles in the link between SCI and SNHL.
The majority of SNHL cases are unilateral, and bilateral condition is rare [29]. In our study, we also found most of the SNHL cases were non-bilateral in both the SCI and non-SCI groups (Table 3), and there was no significant difference in these two groups’ distribution of SNHL types. It is common for SCI patients to suffer from balance problems, gait disturbances, and fall related injuries [30]. Previous research also suggests that hearing loss is associated with postural instability [31]. Therefore, SNHL superimposed on SCI may synergistically increase the risk of falling in patients with SCI. Moreover, hearing impairment is associated with reduced quality of life and impaired activity of daily living [32]. Therefore, our findings suggest that healthcare professionals need to be vigilant about the increased risk of SNHL after SCI, and to educate SCI patients about this potential risk. If SNHL occurs, medical services such as hearing aids, assistive listening devices, and auditory rehabilitation, should be provided. Such interventions may have a positive impact on the quality of life, and may possibly reduce the risk of falls.
In the present study, there was significant differences in socioeconomic variables (e.g., urbanization level) between the SCI and non-SCI groups (Table 1). The SCI group appeared to have a slightly higher proportion of patients living in less urbanized areas (i.e., urbanization level 2, 3, 4, and 5) than the non-SCI group. Lien et al. examined the association between socioeconomic factors and the incidence of SCI in Taiwan [33], and found that urbanization was associated with lower occurrence of SCI, possibly due to improvements in transportation infrastructure and accessibility of public transportation in urban areas [33].
Our findings suggest that there is a temporal association between SCI and SNHL. The key strength of the present study is its use of a nationally representative database with longitudinal follow-up, which increases the generalizability of its results. Nevertheless, several limitations of this research should be acknowledged. First, the diagnoses of SCI, SNHL, and medical comorbidities in the present study were determined using ICD-9-CM codes in the LHID2005 database, which may prompt concerns about the accuracy of the diagnosis. However, medical experts on the NHI Bureau’s professional peer review committee have reviewed randomly sampled medical records from each hospital to validate the recorded diagnoses, as well as the quality of care. Therefore, the NHI database is highly regarded as a source of research data by scholars in various biomedical fields [34]. Second, due to the limitation of the LHID2005 database, there is lack of information regarding the severity of SCI (e.g., American Spinal Injury Association Impairment Scale) and SNHL. Therefore, the relationship between the severity of SCI and the risk of SNHL could not be assessed in this study. Third, information about some potential lifestyle risk factors for SNHL, such as smoking and alcohol consumption, was not available in the LHID2005 and not included in our analyses, apart from our use of COPD as a surrogate for smoking. It is therefore possible that residual confounding may have been present.
Conclusion
The present study has shown that SCI patients were at an increased long-term risk of developing SNHL, and that the magnitude of this extra SNHL risk appears to be higher in patients with non-cervical SCI. Further studies should explore the mechanism(s) underlying this association.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to person’s health information privacy but are available from the corresponding author on reasonable request.
References
Lin SW, Lin YS, Weng SF, Chou CW. Risk of developing sudden sensorineural hearing loss in diabetic patients: a population-based cohort study. Otol Neurotol: Off Publ Am Otological Soc, Am Neurotol Soc Eur Acad Otol Neurotol 2012;33:1482–8.
Reed NS, Huddle MG, Betz J, Power MC, Pankow JS, Gottesman R, et al. Association of Midlife Hypertension with Late-Life Hearing Loss. Otolaryngol-Head Neck Surg: Off J Am Acad Otolaryngol-Head Neck Surg 2019;161:996–1003.
Hong JW, Jeon JH, Ku CR, Noh JH, Yoo HJ, Kim DJ. The prevalence and factors associated with hearing impairment in the Korean adults: the 2010-2012 Korea National Health and Nutrition Examination Survey (observational study). Medicine. 2015;94:e611.
Cruickshanks KJ, Klein R, Klein BE, Wiley TL, Nondahl DM, Tweed TS. Cigarette smoking and hearing loss: the epidemiology of hearing loss study. Jama. 1998;279:1715–9.
Hsu YH, Hu HY, Chiu YC, Lee FP, Huang HM. Association of Sudden Sensorineural Hearing Loss With Vertebrobasilar Insufficiency. JAMA Otolaryngol- Head Neck Surg. 2016;142:672–5.
Cragg JJ, Noonan VK, Krassioukov A, Borisoff J. Cardiovascular disease and spinal cord injury: results from a national population health survey. Neurology. 2013;81:723–8.
Orakzai SH, Orakzai RH, Ahmadi N, Agrawal N, Bauman WA, Yee F, et al. Measurement of coronary artery calcification by electron beam computerized tomography in persons with chronic spinal cord injury: evidence for increased atherosclerotic burden. Spinal Cord. 2007;45:775–9.
Stang P, Lydick E, Silberman C, Kempel A, Keating ET. The prevalence of COPD: using smoking rates to estimate disease frequency in the general population. Chest. 2000;117:354s–9s.
Liu CY, Hung YT, Chuang YL, Chen YJ, Weng WS, Liu JS, et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manag. 2006;4:1–22.
Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, et al. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. Jama. 2012;308:1906–14.
Jorgensen MB. Changes of aging in the inner ear. Histological studies. Arch Otolaryngol (Chic, Ill: 1960) 1961;74:164–70.
Fischer ME, Schubert CR, Nondahl DM, Dalton DS, Huang GH, Keating BJ, et al. Subclinical atherosclerosis and increased risk of hearing impairment. Atherosclerosis. 2015;238:344–9.
Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR. Effects of spinal cord injury on body composition and metabolic profile - part I. J Spinal Cord Med. 2014;37:693–702.
Olive JL, McCully KK, Dudley GA. Blood flow response in individuals with incomplete spinal cord injuries. Spinal Cord. 2002;40:639–45.
Olive JL, Dudley GA, McCully KK. Vascular remodeling after spinal cord injury. Med Sci Sports Exerc. 2003;35:901–7.
Capaccio P, Ottaviani F, Cuccarini V, Bottero A, Schindler A, Cesana BM, et al. Genetic and acquired prothrombotic risk factors and sudden hearing loss. Laryngoscope. 2007;117:547–51.
Yildiz Z, Ulu A, Incesulu A, Ozkaptan Y, Akar N. The importance of thrombotic risk factors in the development of idiopathic sudden hearing loss. Clin Appl Thrombosis/Hemost: Off J Int Acad Clin Appl Thrombosis/Hemost. 2008;14:356–9.
Merli GJ, Crabbe S, Paluzzi RG, Fritz D. Etiology, incidence, and prevention of deep vein thrombosis in acute spinal cord injury. Arch Phys Med Rehabil. 1993;74:1199–205.
Teasell RW, Hsieh JT, Aubut JA, Eng JJ, Krassioukov A, Tu L. Venous thromboembolism after spinal cord injury. Arch Phys Med Rehabil. 2009;90:232–45.
Melikoglu MA, Senel K. Sudden hearing loss in a patient with rheumatoid arthritis; a case report and review of the literature. Acta Reumatologica Portuguesa. 2013;38:138–9.
Hellmann MA, Steiner I, Mosberg-Galili R. Sudden sensorineural hearing loss in multiple sclerosis: clinical course and possible pathogenesis. Acta Neurologica Scandinavica. 2011;124:245–9.
Bowman CA, Linthicum FH Jr., Nelson RA, Mikami K, Quismorio F. Sensorineural hearing loss associated with systemic lupus erythematosus. Otolaryngol-Head Neck Surg: Off J Am Acad Otolaryngol-Head Neck Surg. 1986;94:197–204.
McCabe BF. Autoimmune sensorineural hearing loss. Ann Otol, Rhinol, Laryngol. 1979;88:585–9.
Ankeny DP, Lucin KM, Sanders VM, McGaughy VM, Popovich PG. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J Neurochem. 2006;99:1073–87.
Popovich PG, Stokes BT, Whitacre CC. Concept of autoimmunity following spinal cord injury: possible roles for T lymphocytes in the traumatized central nervous system. J Neurosci Res. 1996;45:349–63.
Kil K, Zang YC, Yang D, Markowski J, Fuoco GS, Vendetti GC, et al. T cell responses to myelin basic protein in patients with spinal cord injury and multiple sclerosis. J Neuroimmunol. 1999;98:201–7.
Vasaghi-Gharamaleki B, Naser Z. Predicting the Risk of Hearing Impairment Following the Cervical Spine Diseases by Measuring the Cervical Range of Movements: a pilot study. Basic Clin Neurosci. 2017;8:413–8.
Brownson RJ, Zollinger WK, Madeira T, Fell D. Sudden sensorineural hearing loss following manipulation of the cervical spine. Laryngoscope. 1986;96:166–70.
Oh JH, Park K, Lee SJ, Shin YR, Choung YH. Bilateral versus unilateral sudden sensorineural hearing loss. Otolaryngol-Head Neck Surg: Off J Am Acad Otolaryngol-Head Neck Surg. 2007;136:87–91.
Wirz M, van Hedel HJA. Balance, gait, and falls in spinal cord injury. Handb Clin Neurol. 2018;159:367–84.
Bang SH, Jeon JM, Lee JG, Choi J, Song JJ, Chae SW. Association Between Hearing Loss and Postural Instability in Older Korean Adults. JAMA Otolaryngol- Head Neck Surg. 2020;146:530–4.
Ciorba A, Bianchini C, Pelucchi S, Pastore A. The impact of hearing loss on the quality of life of elderly adults. Clin Interventions Aging. 2012;7:159–63.
Lien W-C, Wang W-M, Wang J-D, Wang F. The association between economic indicators and the incidence of tetraplegia from traumatic spinal cord injury in Taiwan. BMC Neurol. 2021;21:117.
Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20:236–42.
Acknowledgements
This work was supported by grant MOST 109-2314-B-002-117 from the Ministry of Science and Technology, Executive Yuan, Republic of China.
Author information
Authors and Affiliations
Contributions
SMY participated in Concept and design, acquisition of data, analysis and interpretation of data, drafting the paper or revising it critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. KCY participated in concept and design, acquisition of data, analysis and interpretation of data, drafting the paper or revising it critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. SLP participated in concept and design, acquisition of data, analysis and interpretation of data, drafting the paper or revising it critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the National Taiwan University Hospital Research Ethics Committee. (Reference number:202005045W).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, SM., Yeh, KC. & Pan, SL. Increased risk of sensorineural hearing loss in patients with spinal cord injury: a nationwide longitudinal follow-up study. Spinal Cord 59, 1200–1205 (2021). https://doi.org/10.1038/s41393-021-00697-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-021-00697-3