Abstract
Introduction
Multiple spinal cord tumors rarely occur without genetic predisposition, and concurrent tumors with discrete pathologies developed at the same spinal level are most rare. Here, we report a case of concurrent dorsal schwannoma and ventral meningioma arising at the same upper cervical level (C1–C2).
Case presentation
A 55-year-old woman presented with neck pain and upper and lower extremity numbness for 1 year. Magnetic resonance imaging of the cervical spine showed a partially circumferential C-shaped intradural extramedullary tumor at C1–C2. The preoperative diagnosis based on imaging was intradural extramedullary meningioma with circumferential development. Surgical resection was performed, and dorsal subpial and ventral tumors were detected. Intraoperative pathological diagnosis was schwannoma for the dorsal tumor and meningioma for the ventral tumor. Both tumors were completely resected, followed by circumferential durotomy and duroplasty (Simpson grade 1 resection). Although symptoms related to cerebrospinal fluid hypovolemia occurred immediately after surgery, they disappeared within several days. At 2 years postoperatively, no local recurrence has been identified with mild kyphotic cervical malalignment.
Discussions
Only nine cases of concurrent multiple spinal tumors with discrete histopathological types at the same cervical level have been reported to date, however, this is the first case of meningioma combined with subpial schwannoma. Furthermore, although the ventral location of meningioma often compelled inadequate resection leaving behind a dura mater from which meningioma originated, a gross total resection including dura mater was achieved accompanied with circumferential duroplasty. Careful and sequential postoperative follow-up is ongoing for this individual.
Similar content being viewed by others
Introduction
Multiple spinal cord tumors rarely occur, except in association with neurofibromatosis (NF) or von Hippel-Lindau disease (vHL) [1,2,3]. The occurrence of heterogeneous multiple spinal cord tumors is even rarer. Multiple spinal cord tumors with discrete histologic type arising at the same cervical level are extremely rare, with only nine cases reported to date [1,2,3,4,5,6,7,8]. Herein, a case of concurrent dorsal subpial schwannoma and ventral meningioma in the same upper cervical (C2) level without any evidence of NF/vHL is reported.
Case presentation
A 55-year-old woman with a 1-year history of neck pain and left upper and lower extremity numbness was referred to our department. Her medical history was unremarkable.
Physical examination revealed hypoesthesia in her left half of the body and saddle area. Although no muscle weakness of the extremities was observed, she complained of mild dizziness while walking. She could walk alone without a cane (modified McCormick scale grade 2 [9]). Her deep tendon reflexes of the extremities were upregulated with positive Babinski reflex bilaterally. She had urinary retention and constipation. The Japanese Orthopedic Association (JOA) score for cervical myelopathy was 10/17 ([3,3] [1,1,1] [1]). The JOA score originally developed in 1975 has been one of the most frequently used outcome measures to evaluate functional status of cervical myelopathy cases [10].
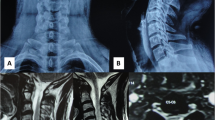
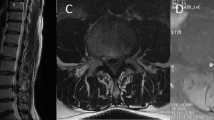
X-ray revealed no structural anomalies. Cervical magnetic resonance imaging (MRI) revealed a C-shaped, well-defined neoplastic lesion with hypo-intensity on T1-weighted image, hyper-intensity on T2-weighted image, and a uniform enhancement by gadolinium located at the C1–C2 level. The mass was located from the left dorsal to ventral side of the intradural space (Fig. 1a–d). Based on these findings, the patient was diagnosed with an intradural extramedullary meningioma with circumferential development on C1–C2 level, and hence, tumor resection with durotomy was planned.
Sagittal T2 (a), T1 pre- (b), and post-gadolinium (c) contrast administration. Axial T2 (d) and T1 post-gadolinium (e) administration. A partially circumferential, C-shaped well-defined neoplastic flat lesion was observed with hypo-intensity on T1-weighted image, hyper-intensity on T2-weighted image, and uniform enhancement by gadolinium located at the C1–C2 level.
After a C1–C3 wide laminectomy by applying the muscle-preserving C2–C3 spinous process splitting approach [11], the rostral and caudal edges of tumor were confirmed via ultrasonography. Then, the dura and arachnoid maters were incised microscopically. Contrary to our preoperative imaging analyses, the dorsal part of the tumor was located at the subarachnoid region and even at the subpial space with an orange-colored appearance (Fig. 2a). The dorsal part of the tumor was not integrated to the ventral one. The ventral part of tumor was located at the intradural and extra-arachnoidal space, with dark red-colored appearance, indicating the typical appearance of meningioma (Fig. 2b). After the total resection of the dorsal subpial tumor through a marginal dissection from the normal spinal cord tissue, the size of the ventral tumor was reduced using the cavitational ultrasonic surgical aspirator (CUSA, Integra Life Sciences Corp., Plainsboro, NJ, USA) from the left side, and then the ventral tumor was resected in a piece-by-piece manner. Intraoperatively, a specimen from each (dorsal and ventral) tumor was rapidly examined by a pathologist and revealed the diagnosis of schwannoma for the dorsal tumor and meningioma for the ventral tumor. After reducing the ventral tumor volume, the ventral meningioma was completely resected, including the dura circumferentially, from which the meningioma originated (Fig. 2c). After the tumor excision, duroplasty was performed using an artificial dura mater (made of GORETEX) by wrapping the spinal cord circumferentially as reported previously [12, 13] (Fig. 3a–c). Consequently, gross total resection was achieved with Simpson grade 1 [14].
An orange-colored subpial tumor (a) was found at the subarachnoid space. After the total resection of the dorsal tumor, a dark red-colored mass (b) was found at the subdural extra-arachnoid space, without integrating with the dorsal lesion. After debulking the volume of the ventral tumor, the ventral tumor including the circumferential dura mater was resected (c) achieving Simpson grade 1 resection.
After the total tumor resection, the configured artificial dura mater was discretely inserted behind the exposed cord (a), and then the inserted artificial dura was closed rolling around in a wrapping manner (b). Finally, the inserted rolling artificial dura and remnants of the dura mater were sutured with CV-6 to obtain the watertight condition as much as possible (c).
Histopathologically, the dorsal resected subpial tumor showed a nuclear palisading of spindle-shaped cells and was diagnosed as a schwannoma (Fig. 4a). The resected ventral tumor was diagnosed as a meningioma with a spiral structure of proliferating tumor cells with round nuclei and acidophilic sporangia (Fig. 4e). Immunohistochemistry revealed the dorsal tumor was S-100(+), EMA(−) with MIB-1 index of 5% (Fig. 4b–d), and the ventral tumor was S-100(−), EMA(+) with MIB-1 index of <2% (Fig. 4f–h).
a The dorsal tumor was composed of spindle cells with palisading pattern, compatible with schwannoma, with S-100(+) (b), EMA (–) (c), and MIB-1 index of ~5% (d). e The ventral tumor showed a spiral structure of proliferating tumor cells with a round nuclei and acidophilic sporangia, compatible with meningotherial meningioma, with S-100(–) (f), EMA (+) (g), and MIB-1 index of ~2% (h). (a & e: Hematoxylin & Eosin. All images ×400.).
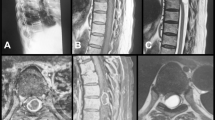
The woman began standing and walking training with fitting a semirigid Philadelphia neck collar (Rehband, Otto Bock Scandinavia AB, Sweden) on the 4th postoperative day. Symptoms related to cerebrospinal fluid hypovolemia occurred after standing up; hence, she underwent total resection of the dura mater circumferentially and subsequent wrapping duroplasty at the C1–C3 level, which resulted in symptom disappearance within several postoperative days. Postoperative muscle strength of the extremities returned to normal; however, dizziness exacerbated, which we believe originated from the posterior column disorder caused by intraoperative manipulations. Although she could not stand and walk alone immediately postoperatively, she had recovered to the same level as preoperatively at 6 months postoperatively after attending the inpatient rehabilitation program in nearby hospital. At 2 years postoperatively, she was independent in ambulation (modified McCormick scale grade 2) and still had urinary retention and constipation. She had mild local kyphosis (C2–C7 kyphotic angel: 5 degrees) of the upper cervical spine on the lateral radiographic view and had no tumor recurrence on MRI (Fig. 5).
Discussion
The incidence of multiple concomitant spinal cord tumors is as rare as 1–<4% of all spinal cord tumors according to previous reports, and those with discrete histopathological types are even rarer at 0.3% [15, 16]. Generally, multiple spinal cord tumors are highly associated with NF/vHL. This individual showed heterogeneous histopathological types of double spinal cord tumors without clinical manifestations of a genetic disorder such as NF/vHL. To the best of our knowledge, only nine cases of different pathological primary spinal cord tumors arising at the same spinal level without genetic manifestation of NF/vHL have been reported (Table 1) [1,2,3,4,5,6,7,8]. The current case presenting with a subpial schwannoma and a ventral located meningioma, who required both dorsal intramedullary tumor resection and total ventral tumor resection including the circumferential dura mater and wrapping duroplasty to amend meningeal defects, required an extremely technically demanding procedure.
Spinal schwannoma, which originates from the Schwann cells, often develops from the dorsal rootlet in the subarachnoid space [17]. Spinal schwannomas account for ~30% of all spinal cord tumors. Most of them are intradural-extramedullary with or without a dumbbell-shaped extension, and intramedullary schwannomas are extremely rare, constituting ~1.1% of spinal schwannomas and 0.3% of intraspinal tumors [18,19,20]. Approximately 70 cases of spinal subpial schwannomas have been reported since the first case report in 1931 [19, 21, 22]. According to the diagnostic imaging of spinal subpial schwannoma, low- to iso-intensity on T1-weighted images, high-intensity on T2-weighted images, and homogenous gadolinium-enhancement are typically observed with fine margins. Furthermore, edematous signal changes within the spinal cord surrounding the tumor might also be considered [23]. The dorsal tumor of this patient also exhibited T1-low, T2-high-intensity signal with homogenous gadolinium enhancement (Fig. 1), which was totally resected via the posterior approach.
Gross total resection including the dura mater (Simpson grade 1 resection) is recommended for the treatment of spinal meningioma with recurrence prevention during long-term follow-up. Nakamura et al. reported the long-term (mean, 12-year follow-up) surgical outcomes of spinal meningioma and revealed that 6 out of 19 patients who underwent Simpson grade 2 resection for ventral located tumors showed tumor recurrence requiring re-operation, whereas 0 out of 43 patients with Simpson grade 1 resection exhibited tumor recurrence [24]. Ventrally located spinal meningiomas make Simpson grade 1 resection harder because of its technically high degree of difficulty [24, 25]. Based on these findings, we would try to achieve Simpson grade 1 resection, even for ventrally located meningiomas, as often as possible. Thus, preoperative elaborate imaging analysis is crucial as the surgical treatment strategy differs depending on the histopathological type of the tumor.
In this case, wide C1–C3 laminectomy was performed to access the tumors and execute “wrapping” duroplasty via the posterior approach. Inoue et al. reported the risk of progression of kyphotic deformity after a C2 laminectomy for spinal cord tumor resection [26]. Asazuma et al. analyzed the degree of progression of kyphotic deformity according to three types of posterior approaches (laminectomy, open-door laminoplasty, and hemilaminectomy) and showed that range-of-motion restriction and cervical curvature worsening involving the C2 lamina were prone to occur postoperatively [27]. To prevent and minimize the deterioration of a postoperative cervical kyphotic deformity, the muscle-preserving C2-3 spinous process splitting technique was adopted, and, consequently, the progression of cervical kyphosis was only five degrees and well controlled at 2 years postoperatively (Fig. 5c, d).
In conclusion, a case of concurrent dorsal subpial schwannoma and ventral meningioma at the C1–C2 level is reported requiring a gross total resection of both tumors, followed by circumferential durotomy and duroplasty. A good postoperative course has been maintained at 2 years with mild cervical kyphotic changes. However, careful and serial follow-up in the outpatient setting is essential.
References
Chen KY, Wu JC, Lin SC, Huang WC, Cheng H. Coexistence of neurofibroma and meningioma at exactly the same level of the cervical spine. J Chin Med Assoc. 2014;77:594–7.
Nakamizo A, Suzuki SO, Shimogawa T, Amano T, Mizoguchi M, Yoshimoto K, et al. Concurrent spinal nerve root Schwannoma and meningioma mimicking single-component Schwannoma. Neuropathology 2012;32:190–5.
Liebelt BD, Haider AS, Steele WJ, Krishna C, Blacklock JB. Spinal Schwannoma and Meningioma mimicking a single mass at the craniocervical junction subsequent to remote radiation therapy for acne vulgaris. World Neurosurg 2016;93:484.e13–6.
Hokari M, Hida K, Ishii N, Seki T, Iwasaki Y, Nakamura N. Associated meningioma and neurofibroma at the same cervical level without clinical signs of neurofibromatosis: case report. No Shinkei Geka. 2002;30:953–7.
Ogihara S, Seichi A, Iwasaki M, Kawaguchi H, Kitagawa T, Tajiri Y. et al. Concurrent spinal schwannomas and meningiomas. Case illustration. J Neurosurg. 2003;98(Suppl 3):300.
Oichi T, Chikuda H, Morikawa T, Mori H, Kitamura D, Higuchi J, et al. Concurrent spinal schwannoma and meningioma mimicking a single cervical dumbbell-shaped tumor: case report. J Neurosurg Spine 2015;23:784–7.
Matsuda S, Kajihara Y, Abiko M, Mitsuhara T, Takeda M, Karlowee V. et al. Concurrent Schwannoma and Meningioma arising in the same spinal level: a report of two cases. NMC Case Rep. J. 2018;5:105–9.
Zhan Z, Yan X, Nie W, Ding Y, Xu W, Huang H. Neurofibroma and meningioma within a single dumbbell-shaped tumor at the same cervical level without neurofibromatosis: a case report and literature review. World Neurosurg. 2019;130:1–6.
Matsuyama Y, Sakai Y, Katayama Y, Imagama S, Ito Z, Wakao N. et al. Surgical results of intramedullary spinal cord tumor with spinal cord monitoring guide extent resection. J Neurosurg Spine. 2009;10:404–13.
Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K. Interobserver and intraobserver reliability of the Japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine (Philos Pa 1976). 2001;26:1890–94. discussion 1895.
Shiraishi T. A new technique for exposure of the cervical spine laminae. Technical note. J Neurosurg. 2002;96(Suppl 1):122–6.
Horn EM, Deshmukh VR, Lekovic GP, Dickman CA. Durectomy and reconstruction for the treatment of a recurrent spinal meningioma. Case report. J Neurosurg Spine. 2006;5:76–8.
Elwy R, Pinckard-Dover H, McCarthy R, Cai R. Circumferential dural reconstruction after excision of recurrent intradural extra medullary spinal meningioma. Interdiscip Neurosurg 2019;15:22–6.
Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22–39.
Svien HJ, Camp JD, Adson AW. Multiple primary tumors of the spinal cord; report of case. Surg Clin North Am. 1949;29:1223–31.
Lombardi G, Passerini A. Multiple lesions of the spinal cord. Am J Roentgenol Radium Ther Nucl Med. 1964;92:1298–300.
Lenzi J, Anichini G, Landi A, Piciocchi A, Passacantilli E, Pedace F, et al. Spinal nerves Schwannomas: experience on 367 cases-historic overview on how clinical, radiological, and surgical practices have changed over a course of 60 years. Neurol Res Int 2017;2017:3568359.
Ross DA, Edwards MS, Wilson CB. Intramedullary neurilemomas of the spinal cord: report of two cases and review of the literature. Neurosurgery 1986;19:458–64.
Yang T, Wu L, Deng X, Yang C, Xu Y. Clinical features and surgical outcomes of intramedullary schwannomas. Acta Neurochir (Wien). 2014;156:1789–97.
Lee SE, Chung CK, Kim HJ. Intramedullary Schwannomas: long-term outcomes of ten operated cases. J Neurooncol 2013;113:75–81.
Nicacio JM, Rodrigues JC, Galles MH, Faquini IV, de Brito Pereira CA, Ganau M. Cervical intramedullary schwannoma: a case report and review of the literature. Rare Tumors 2009;1:e44.
Lyle CA, Malicki D, Senac MO, Levy ML, Crawford JR. Congenital giant intramedullary spinal cord schwannoma. Neurology 2010;75:1752.
Ozawa N, Tashiro T, Okamura T, Koyama K, Ohata K, Inoue Y. Subpial schwannoma of the cervical spinal cord mimicking an intramedullary tumor. Radiat Med 2006;24:690–4.
Nakamura M, Tsuji O, Fujiyoshi K, Hosogane N, Watanabe K, Tsuji T, et al. Long-term surgical outcomes of spinal meningiomas. Spine (Philos Pa 1976). 2012;37:E617–23.
Kim CH, Chung CK, Lee SH, Jahng TA, Hyun SJ, Kim KJ, et al. Long-term recurrence rates after the removal of spinal meningiomas in relation to Simpson grades. Eur Spine J. 2016;25:4025–32.
Inoue A, Ikata T, Katoh S. Spinal deformity following surgery for spinal cord tumors and tumorous lesions: analysis based on an assessment of the spinal functional curve. Spinal Cord 1996;34:536–42.
Asazuma T, Nakamura M, Matsumoto M, Chiba K, Toyama Y. Postoperative changes of spinal curvature and range of motion in adult patients with cervical spinal cord tumors: analysis of 51 cases and review of the literature. J Spinal Disord Tech. 2004;17:178–82.
Author information
Authors and Affiliations
Contributions
Writing – original draft: YS and OT. Writing – review and editing: NN, SN, SS, EO, MY, NF, and KW. Data curation: YS, OT, and MN. OT was responsible for all working related to this submission as corresponding author. Also, all authors approved the final version manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Suematsu, Y., Tsuji, O., Nagoshi, N. et al. Concurrent dorsal subpial schwannoma and ventral meningioma arising at the same upper cervical level: a case report. Spinal Cord Ser Cases 6, 64 (2020). https://doi.org/10.1038/s41394-020-0308-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-020-0308-3