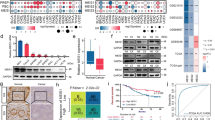
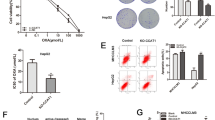
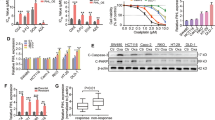
Abstract
A major cause of oxaliplatin chemoresistance in colorectal cancer (CRC) is acquired epithelial-mesenchymal transition (EMT) in cancer cells, making the cancer cells easy to metastasis and recurrence. LncRNA Neighboring Enhancer of FOXA2 (lncRNA-NEF) has been characterized as a tumor suppressor to mediate cancer metastasis in multiple cancer types. However, whether it mediated the drug resistance remains unknown. In the present study, an oxaliplatin-resistant CRC cell line (SW620R) was established and lncRNA-NEF was obviously down-regulated in this resistant cell line. The further loss and gain-of-function studies demonstrated that this lncRNA suppressed oxaliplatin resistance as well as EMT programme in vitro and inhibited metastasis in vivo. Mechanistically, lncRNA-NEF epigenetically promoted the expression of DOK1 (Downstream of Tyrosine kinase 1), a negative regulator of MEK/ERK signaling, by disrupting DNA methyltransferases (DNMTs)-mediated DNA methylation. DOK1, in turn, induced the inactivation of MEK/ERK signaling, forming the lncRNA-NEF/DOK1/MEK/ERK regulatory axis to mediate oxaliplatin resistance in CRC. Collectively, our work reveals the critical function of lncRNA-NEF in mediating the oxaliplatin chemotherapy resistance in CRC, and provides a promising therapeutic strategy for CRC patients with oxaliplatin resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Keum N, Giovannucci E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713–32.
Abraham J, Magee D, Cremolini C, Antoniotti C, Halbert D, Xiu J, et al. Clinical validation of a machine-learning-derived signature predictive of outcomes from first-line oxaliplatin-based chemotherapy in advanced colorectal cancer. Clin Cancer Res. 2021;27:1174–83.
Schmoll HJ, Twelves C, Sun W, O’Connell MJ, Cartwright T, McKenna E, et al. Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post-relapse survival: a pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol. 2014;15:1481–92.
Vogel A, Hofheinz RD, Kubicka S, Arnold D. Treatment decisions in metastatic colorectal cancer - Beyond first and second line combination therapies. Cancer Treat Rev. 2017;59:54–60.
Souglakos J, Kalykaki A, Vamvakas L, Androulakis N, Kalbakis K, Agelaki S, et al. Phase II trial of capecitabine and oxaliplatin (CAPOX) plus cetuximab in patients with metastatic colorectal cancer who progressed after oxaliplatin-based chemotherapy. Ann Oncol: Off J Eur Soc Med Oncol. 2007;18:305–10.
Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–29.
McMillin DW, Negri JM, Mitsiades CS. The role of tumour-stromal interactions in modifying drug response: Challenges and opportunities. Nat Rev Drug Discov. 2013;12:217–28.
Escalante PI, Quiñones LA, Contreras HR. Epithelial-mesenchymal transition and MicroRNAs in colorectal cancer chemoresistance to FOLFOX. Pharmaceutics 2021;13:75.
Chen Y, Deng G, Fu Y, Han Y, Guo C, Yin L, et al. FOXC2 promotes oxaliplatin resistance by inducing epithelial-mesenchymal transition via MAPK/ERK signaling in colorectal cancer. OncoTargets Ther. 2020;13:1625–35.
Chen S, Shen X. Long noncoding RNAs: Functions and mechanisms in colon cancer. Mol cancer. 2020;19:167.
Lu M, Qin X, Zhou Y, Li G, Liu Z, Geng X, et al. Long non-coding RNA LINC00665 promotes gemcitabine resistance of Cholangiocarcinoma cells via regulating EMT and stemness properties through miR-424-5p/BCL9L axis. Cell death Dis. 2021;12:72.
Liu H, Li D, Sun L, Qin H, Fan A, Meng L, et al. Interaction of lncRNA MIR100HG with hnRNPA2B1 facilitates m(6)A-dependent stabilization of TCF7L2 mRNA and colorectal cancer progression. Mol cancer. 2022;21:74.
Liang WC, Ren JL, Wong CW, Chan SO, Waye MM, Fu WM, et al. LncRNA-NEF antagonized epithelial to mesenchymal transition and cancer metastasis via cis-regulating FOXA2 and inactivating Wnt/β-catenin signaling. Oncogene 2018;37:1445–56.
Wan X, Xiang J, Zhang Q, Bian C. Downregulation of lnRNA-NEF is involved in the postoperative cancer distant recurrence in prostate carcinoma patients. J Cell Biochem. 2019;120:9601–7.
Liang Z, Zhu B, Meng D, Shen X, Li X, Wang Z, et al. Down-regulation of lncRNA-NEF indicates poor prognosis in intrahepatic cholangiocarcinoma. Biosci Rep. 2019;39:BSR20181573.
Yang Q, Yu H, Yin Q, Hu X, Zhang C. lncRNA-NEF is downregulated in osteosarcoma and inhibits cancer cell migration and invasion by downregulating miRNA-21. Oncol Lett. 2019;17:5403–8.
Ju W, Luo X, Zhang N. LncRNA NEF inhibits migration and invasion of HPV-negative cervical squamous cell carcinoma by inhibiting TGF-β pathway. Biosci Rep. 2019;39:BSR20180878.
Song X, Liu Z, Yu Z. LncRNA NEF is downregulated in triple negative breast cancer and correlated with poor prognosis. Acta biochimica et biophysica Sin. 2019;51:386–92.
Wu L, Wang P. Long non-coding RNA-neighboring enhancer of FOXA2 inhibits the migration and invasion of small cell lung carcinoma cells by downregulating transforming growth factor-β1. Oncol Lett. 2019;17:4969–75.
Cui X, Fang N, Cui Y, Xiao D, Wang X. Long non-coding RNA NEF inhibits proliferation and promotes apoptosis of laryngeal squamous cell carcinoma cells by inhibiting Wnt/β-catenin signaling. Oncol Lett. 2019;17:4928–34.
Feng L, Shi L, Lu YF, Wang B, Tang T, Fu WM, et al. Linc-ROR promotes osteogenic differentiation of mesenchymal stem cells by functioning as a competing endogenous RNA for miR-138 and miR-145. Molecular therapy. Nucleic acids. 2018;11:345–53.
Shi CJ, Zheng YB, Pan FF, Zhang FW, Zhuang P, Fu WM. Gallic acid suppressed tumorigenesis by an LncRNA MALAT1-Wnt/β-Catenin axis in hepatocellular carcinoma. Front Pharmacol. 2021;12:708967.
Shen J, Peng Y, Wei L, Zhang W, Yang L, Lan L, et al. ARID1A deficiency impairs the DNA damage checkpoint and sensitizes cells to PARP inhibitors. Cancer Discov. 2015;5:752–67.
Tang X, Shi L, Xie N, Liu Z, Qian M, Meng F, et al. SIRT7 antagonizes TGF-β signaling and inhibits breast cancer metastasis. Nat Commun. 2017;8:318.
Liang WC, Wong CW, Liang PP, Shi M, Cao Y, Rao ST, et al. Translation of the circular RNA circβ-catenin promotes liver cancer cell growth through activation of the Wnt pathway. Genome Biol. 2019;20:84.
McCabe EM, Rasmussen TP. lncRNA involvement in cancer stem cell function and epithelial-mesenchymal transitions. Semin cancer Biol. 2021;75:38–48.
Koveitypour Z, Panahi F, Vakilian M, Peymani M, Seyed Forootan F, Nasr Esfahani MH, et al. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019;9:97.
Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: Promises and challenges. Nat Rev Drug Discov. 2014;13:928–42.
Jung G, Hernández-Illán E, Moreira L, Balaguer F, Goel A. Epigenetics of colorectal cancer: Biomarker and therapeutic potential. Nat Rev Gastroenterol Hepatol. 2020;17:111–30.
Zhou Y, Yang Z, Zhang H, Li H, Zhang M, Wang H, et al. DNMT3A facilitates colorectal cancer progression via regulating DAB2IP mediated MEK/ERK activation. Biochimica et biophysica acta Mol basis Dis. 2022;1868:166353.
Zhang K, Zhai Z, Yu S, Tao Y. DNA methylation mediated down-regulation of ANGPTL4 promotes colorectal cancer metastasis by activating the ERK pathway. J Cancer. 2021;12:5473–85.
Shao G, Fan X, Zhang P, Liu X, Huang L, Ji S. Methylation-dependent MCM6 repression induced by LINC00472 inhibits triple-negative breast cancer metastasis by disturbing the MEK/ERK signaling pathway. Aging 2021;13:4962–75.
Wei CH, Wu G, Cai Q, Gao XC, Tong F, Zhou R, et al. MicroRNA-330-3p promotes cell invasion and metastasis in non-small cell lung cancer through GRIA3 by activating MAPK/ERK signaling pathway. J Hematol Oncol. 2017;10:125.
Clark SJ, Molloy PL. Early insights into cancer epigenetics: Gene promoter hypermethylation emerges as a potential biomarker for cancer detection. Cancer Res. 2022;82:1461–3.
Erin N, Grahovac J, Brozovic A, Efferth T. antimicrobial ci, chemotherapy a. Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resist Updat. 2020;53:100715.
Boumahdi S, de Sauvage FJ. The great escape: Tumour cell plasticity in resistance to targeted therapy. Nat Rev Drug Discov. 2020;19:39–56.
Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412.
Williams E, Gao D, Redfern A, Thompson EW. Controversies around epithelial-mesenchymal plasticity in cancer metastasis. Nat Rev Cancer. 2019;19:716–32.
Ma J, Zeng S, Zhang Y, Deng G, Qu Y, Guo C, et al. BMP4 promotes oxaliplatin resistance by an induction of epithelial-mesenchymal transition via MEK1/ERK/ELK1 signaling in hepatocellular carcinoma. Cancer Lett. 2017;411:117–29.
Ren H, Wang Z, Chen Y, Liu Y, Zhang S, Zhang T, et al. SMYD2-OE promotes oxaliplatin resistance in colon cancer through MDR1/P-glycoprotein via MEK/ERK/AP1 pathway. OncoTargets Ther. 2019;12:2585–94.
Liu W, Zhang Z, Zhang Y, Chen X, Guo S, Lei Y, et al. HMGB1-mediated autophagy modulates sensitivity of colorectal cancer cells to oxaliplatin via MEK/ERK signaling pathway. Cancer Biol Ther. 2015;16:511–7.
Hadji F, Boulanger MC, Guay SP, Gaudreault N, Amellah S, Mkannez G, et al. Altered DNA Methylation of Long Noncoding RNA H19 in Calcific Aortic Valve Disease Promotes Mineralization by Silencing NOTCH1. Circulation 2016;134:1848–62.
Cheng D, Deng J, Zhang B, He X, Meng Z, Li G, et al. LncRNA HOTAIR epigenetically suppresses miR-122 expression in hepatocellular carcinoma via DNA methylation. EBioMedicine 2018;36:159–70.
Yamanashi Y, Tamura T, Kanamori T, Yamane H, Nariuchi H, Yamamoto T, et al. Role of the rasGAP-associated docking protein p62(dok) in negative regulation of B cell receptor-mediated signaling. Genes Dev. 2000;14:11–6.
Berger AH, Niki M, Morotti A, Taylor BS, Socci ND, Viale A, et al. Identification of DOK genes as lung tumor suppressors. Nat Genet. 2010;42:216–23.
Saulnier A, Vaissière T, Yue J, Siouda M, Malfroy M, Accardi R, et al. Inactivation of the putative suppressor gene DOK1 by promoter hypermethylation in primary human cancers. Int J cancer. 2012;130:2484–94.
He PF, Xu ZJ, Zhou JD, Li XX, Zhang W, Wu DH, et al. Methylation-associated DOK1 and DOK2 down-regulation: Potential biomarkers for predicting adverse prognosis in acute myeloid leukemia. J Cell Physiol. 2018;233:6604–14.
Gutting T, Merkel A, Fink DJ, Hetjens S, Weidner P, Yu Y, et al. Expression of the EGFR-RAS Inhibitory Proteins DOK1 and MTMR7 and its Significance in Colorectal Adenoma and Adenoma Recurrence. J Gastrointest liver Dis: JGLD. 2021;30:446–55.
Friedrich T, Söhn M, Gutting T, Janssen KP, Behrens HM, Röcken C, et al. Subcellular compartmentalization of docking protein-1 contributes to progression in colorectal cancer. EBioMedicine 2016;8:159–72.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81773066, 81772404) and Natural Science Foundation of Guangdong Province (2020A1515010961; 2021A1515012111).
Author information
Authors and Affiliations
Contributions
JFZ and WMF conceptualized and designed all the experiments. CJS, ZHX, WQZ, LQD, FXP, and FWZ performed the experiments and analyzed the data. CJS, JFZ, and WMF prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The usage and treatment of animals were approved by Institutional Animal Care and Use Committee (IACUC) of SMU (Guangzhou, China, Approval No. L2019140).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, CJ., Xue, ZH., Zeng, WQ. et al. LncRNA-NEF suppressed oxaliplatin resistance and epithelial-mesenchymal transition in colorectal cancer through epigenetically inactivating MEK/ERK signaling. Cancer Gene Ther 30, 855–865 (2023). https://doi.org/10.1038/s41417-023-00595-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-023-00595-1