Abstract
Introduction
The Aurolab aqueous drainage implant (AADI) is a low-cost glaucoma drainage device that is modelled on the Baerveldt glaucoma implant. Studies on AADI have reported absolute success rates of 41.8–93.1% at 1 year. Most studies report on tube placement in the anterior chamber. We report on results of tube insertion in the sulcus/pars plana.
Material and methods
A retrospective chart review of all patients who had undergone AADI implantation (with insertion of tube in the sulcus/posterior segment) between June 2015 and November 2018 was done. Patients were asked to stop anti-glaucoma medications on the 40th post-operative day.
Results
The mean age was 57.4 ± 13.8 years (n = 30). The mean IOP prior to surgery was 34.4 ± 6.1 mmHg which reduced to 15.4 ± 8.6 mmHg on the 45th post-operative day (p < 0.001). The absolute success at last review was 10% and the qualified success was 80%. The complication rate was 26.7%. Three patients had hypotony related complications noted at/after the 45th day review (none before 40th day). The incidence of ocular motility disturbances was 26.7% though none of the patients reported diplopia. One patient had sideways rotation of the scleral patch graft resulting in tube exposure. This complication was not seen after we shifted to using 9–0 nylon sutures to anchor the graft. Six patients had loss of best corrected visual acuity and one patient developed endophthalmitis. The endophthalmitis was preceded by conjunctival retraction and sloughing off of the scleral patch graft.
Discussion
AADI implantation results in a substantial drop in IOP. However, many patients continue to require anti-glaucoma medications. Allowing overlap of scleral/corneal patch graft onto the scleral flap may be effective in preventing peritubular leak. It may be advisable to use 9–0 nylon sutures to secure the scleral patch graft anti-glaucoma medications can be temporarily suspended after the 40th post-operative day to minimize hypotony related complications. Melting of the scleral patch graft may be an early sign of endophthalmitis. It would be prudent to specifically look for ocular motility problems in patients undergoing AADI implantation.
Similar content being viewed by others
Introduction
Glaucoma drainage devices (GDD) are increasingly being used in the treatment of refractory glaucoma. These devices create an alternate pathway for outflow of aqueous from the anterior chamber to an equatorial plate (surrounded by a bleb) through a long tube. The most common devices in use are the Ahmed Glaucoma Valve and the Baerveldt glaucoma implant both of which are very expensive for developing countries. The Aurolab aqueous drainage implant (AADI) is a low-cost (around $50) non-valved glaucoma drainage device that has been modelled on the Baerveldt implant. It differs from the Baerveldt implant in the amount of barium and silicone and in being less stiff. It was introduced in 2013 by Aurolab the manufacturing division of Aravind Eye Hospital, Madurai India and has shown promising results [1, 2]. Most studies on the AADI detail on the placement of the implant in the anterior chamber [1,2,3,4,5,6,7,8]. Two recent studies reported good success rates with pars plana placement of the tube [5, 9]. Placement of tube in the anterior chamber has a learning curve and may result in iris/corneal complications. The posterior chamber sulcus is a potential space for tube implants in patients with a PCIOL in place. Tube placement in the posterior chamber sulcus appears to give comparable results and increases the tube cornea distance [10]. It is the author’s preference to position the tube in the posterior chamber sulcus in pseudophakic patients or in patients needing concurrent cataract surgery (as there is no chance of lens damage). Pars plana placement is preferred in patients with corneal grafts in place or in patients with corneal endothelial compromise. We present the results of posterior chamber/pars plana implantation of the AADI with a modified technique that has the potential to prevent peritubular leak.
Material and methods
Approval of the institutional review board of the hospital was obtained for a retrospective chart review of all patients who had undergone AADI implantation in the posterior chamber/pars plana between June 2015 and November 2018. Patients who had completed at least 2 months of follow-up were included for the study. Patients with keratoprosthesis were excluded from analysis.
A detailed history regarding use of medications, previous ocular surgery, systemic comorbidity was taken and old medical records reviewed. All patients underwent a complete ophthalmic examination including visual acuity testing with the Snellen letter chart, refraction, applanation tonometry, gonioscopy, comprehensive anterior and posterior segment evaluation, ocular motility evaluation, visual fields (with a Humphrey field analyzer using the SITA FAST 30–2 program except in patients with vision <6/60) and optical coherence tomography for optic disc parameters and RNFL thickness (Stratus OCT, Carl Zeiss Meditec, Dublin whenever possible/needed). Whenever visual fields were possible, a mean deviation of <−12 dB was taken as advanced glaucoma, between −6 and −12 dB was taken as moderate glaucoma and <−6 dB was taken as mild glaucoma. When visual fields were not possible because of poor visual acuity, a neuroretinal rim erosion of >180° was taken as severe glaucoma (DDLS 9–10 for average size optic disc, the disc damage likelihood scale—https://doi.org/10.1371/journal.pone.0181428.g001), between 90° and 180° as moderate glaucoma (DDLS 8) and the remainder as mild glaucoma. The RNFL parameters on OCT were also used to corroborate the clinical grading.
Surgical technique
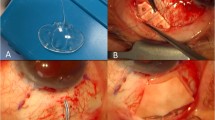
All surgeries were done (Video 1) under local/subtenon’s anaesthesia by a single surgeon (first author). In patients with posterior segment pathology/uveitis, inferior placement of the AADI was preferred in anticipation of future posterior segment surgery. Inferior placement was also done for patients with superior conjunctival scarring such as a failed trabeculectomy. Under surgical asepsis, a limbal peritomy was done and the tenon’s button-holed to facilitate hooking of the extraocular muscles.
For an inferonasal placement of the implant, the medial and inferior recti were hooked and minimal dissection was done to clear the intermuscular septa. The plate of the AADI was first inserted under one rectus muscle and pushed till a minimal kink in the tube was noted. The other end of the plate was then placed under the adjacent rectus muscle. A similar procedure was followed for other quadrants after hooking the appropriate extraocular muscles. The implant was then sutured to the sclera with 9–0 monofilament nylon sutures at 8 mm from the limbus for the inferonasal quadrant and at 10 mm from the limbus for other quadrants. The sutures were rotated to bury the knots. Priming of the AADI was done by injecting balanced salt solution into the tube through a 27 G cannula. The tube was then ligated close to the plate by using 6–0 vicryl sutures and ligation confirmed by injecting saline again. Two ligatures were used in all cases. The tube was trimmed using a Wescott scissors with bevel facing to the side or downward allowing sufficient length of the tube. A horizontal groove was made 5 mm away from the limbus in same quadrant and dissected forward with a crescent blade. The sides of the scleral flap are cut with a Wescott scissors.
Phacoemulsification with IOL implantation was done at this stage when indicated. Viscoelastic was injected into the anterior chamber and under the iris (in the quadrant where the plate was placed). In patients who did not undergo phacoemulsification, anterior chamber entry was done with a 15° angled blade and viscoelastic injected under into the anterior chamber and under the iris (in the quadrant of tube placement). A 23 G needle was used to enter the sulcus under the scleral flap starting 2.5 mm from the limbus and the tube inserted into the sulcus. When difficulty was encountered in getting the tube through the entry site of the needle, a 22 G needle was used to enlarge the entry and the tube inserted into the sulcus. The tube was then inserted under direct visualization. When required, additional viscoelastic was injected under the iris in the quadrant of insertion. Care was taken to keep the needle parallel to the iris plane to avoid damaging the anterior lens capsule. The surgeon directly viewed the tube arising from under the iris into the sulcus. We attempted to have the tube length up to 1 mm of the centre of the pupil.
For patients who underwent pars plana AADI implantation, inferotemporal placement of the implant was done. The infusion port was made under the scleral flap and the same port used to insert the tube. In all patients, sufficient length of the tube was ensured to permit viewing the tube tip in undilated pupil. After inserting the tube, the edges of the flap were sutured to the sclera using 8–0 vicryl sutures. A figure of eight suture with 10–0 nylon was used to secure the tube to the underlying sclera and prevent retraction. Four tube fenestrations were done using the needle of a 8–0 vicryl suture. A glycerine preserved scleral/corneal patch graft (trimmed to the appropriate size) was used to cover the remainder of the tube till the plate. Uveal tissue if any was scraped off the surface of the graft with a blade breaker. The scleral/corneal patch graft was soaked in 5% povidone iodine solution for 2 min. After one study patient (12th patient in Table 1) developed endophthalmitis, the soak period with 5% povidone iodine was increased to 5 min. It was then washed thoroughly with balanced salt solution and soaked in gentamicin for 5 min and washed again with balanced salt solution.
In all patients who underwent pars plana AADI implantation and in patients where the tube insertion site was enlarged with a 22 G needle, the donor sclera was placed in such a way so as to allow overlap of 1 mm over the scleral flap. The scleral flap was secured to the eye with 10–0 monofilament nylon sutures. After shifting of the scleral flap was noted in patient No. 5 who developed a tube exposure, it was decided to use 9–0 monofilament nylon sutures to anchor the donor sclera for subsequent patients. Viscoelastic was washed off from the anterior chamber and the ports hydrated with balanced salt solution. The conjunctiva was closed with 8–0 vicryl sutures. 0.1 ml of intracameral moxifloxacin was then injected into the anterior chamber. The eye was then bandaged after instilling a drop of 5% povidone iodine solution and prednisolone acetate eye drops.
Postoperatively the patients were placed on a tapering regimen of loteprednol 1% eight times per day (tapered over 10 weeks), topical atropine 1% eye ointment (tapered over 3 weeks), and topical moxifloxacin four times a day (for 1 week). Patients were advised to continue topical (and systemic if any) anti-glaucoma medications in the operated eye till the 40th post-operative day. Patients were reviewed on the 1st post-operative day, 1st post-operative week, 2nd post-operative week, 4th post-operative week and on the 45th post-operative day. At each visit, the visual acuity and applanation tonometry were recorded. Anterior and posterior segment examination was also done. The tube position was noted on the slit lamp. A decision on the anti-glaucoma regime was made on the 45th post-operative day after reviewing the IOP. Further reviews were decided based on the IOP achieved, the extent of glaucomatous damage and fellow eye status. At each visit the patient was asked about diplopia. Ocular motility evaluation was done prior to surgery, at 45 days follow-up and on every subsequent review. Cover, alternate cover and prism cover tests were done in primary, upgaze, downgaze, right and left gazes. When the visual acuity in either eye was <20/80, the deviations in primary position were estimated by the Krimsky method.
Outcome measures
The primary outcome measure was intraocular pressure (IOP). Secondary outcome measures included number of anti-glaucoma medications, best corrected visual acuity and complications. Absolute success was defined as an IOP between 5 and 21 mmHg without the use of anti-glaucoma medications and qualified success was defined as achievement of the same with the use of anti-glaucoma medications. Failure was defined as inability to meet the above criteria, the occurrence of sight threatening complications or re-surgery to lower the IOP.
Analysis
Descriptive analysis was carried out by mean and standard deviation for quantitative variables, frequency and proportion for categorical variables. The key outcome variable considered for the analysis was recurrence free survival rate. If the data were not available on any particular explanatory parameter, they were considered as missing values and were excluded from the analysis, while assessing the association of that factor with disease free survival. The mean/median overall survival and recurrence free survival was compared across various explanatory parameters were using log rank test. The data were presented in Kaplan–Meier survival plots. P value <0.05 was considered statistically significant. IBM SPSS version 22 was used for statistical analysis.
Results
Baseline characteristics
Thirty eyes of twenty nine patients (18 males, 11 females) underwent implantation of AADI between November 2015 and December 2018. The mean age was 57.4 ± 13.8 years (23–78 years). Patient characteristics are detailed in Table 1. Five patients had glaucoma post vitrectomy (with silicone oil injection, oil removal had been done in all cases, one patient had undergone penetrating keratoplasty), eight patients had neovascular glaucoma, three patients had post uveitic glaucoma (of which one patient had undergone vitrectomy with silicone oil injection followed by removal), one had angle recession glaucoma with persistent low grade inflammation, three patients had undergone corneal transplant (of which one mentioned earlier had also undergone pars plana vitrectomy), one patient had secondary angle closure glaucoma with aphakic bullous keratopathy due to a complicated cataract surgery (aphakic) done elsewhere and the remainder had failed trabeculectomy. Twenty eyes had advanced glaucomatous optic nerve damage at baseline, four eyes had moderate glaucomatous damage and the remainder had mild damage. Visual fields could not be done in 9 out of 30 eyes because of poor visual acuity. The grading was done based on the optic disc as described earlier. OCT of the optic disc was used to corroborate the grading. Good quality OCT images could not be obtained in four eyes. The OCT parameters were normal in three eyes classified as having mild glaucomatous optic disc damage based on the DDLS. In patient No. 28, the OCT showed advanced damage based on the optic nerve head parameters, but the retinal nerve fibre thickness was normal (Table 2).
Six eyes had pars plana implantation of the tube and the rest had the tube implanted in the ciliary sulcus. Overall twenty one eyes had inferonasal implantation of AADI, two had superotemporal implantation and the remaining had inferotemporal placement of the implant. Patient No. 27 was phakic and a sulcus implantation of the tube was possible because of traumatic subluxation of the crystalline lens in the inferonasal quadrant. The crystalline lens remained clear during follow-up (Supplementary Fig. 1). Six eyes had combined phacoemulsification with IOL implantation in addition to AADI placement. All other patients were pseudophakic or had pars plana implantation of the tube. A 22 G needle was used to enlarge the entry site in 12 eyes. The median follow-up was 12 months (Range: 2–43 months).
Intraocular pressure (IOP)
The mean IOP prior to surgery was 34.4 ± 6.1 mmHg. All patients were on maximal permissible topical anti-glaucoma medications. Topical brimonidine and brinzolamide was used in all patients. Topical prostaglandin analogues were avoided in neovascular glaucoma and in the presence of active uveitis. Topical beta blockers were avoided in patients with cardiac comorbidity (failure/bradyarrhythmias/heart block) and severe bronchial asthma. In addition, seven patients were prescribed oral acetazolamide and glycerine syrup prior to surgery. Details of preoperative medication can be found in Table 1. No patient had shallowing of the anterior chamber or IOP < 6 mmHg in the first 2 weeks after surgery. The IOP drop in the first 2 weeks was statistically significant (p < 0.001). Ten eyes had IOP > 21 mmHg 1 week after surgery and 15 eyes had IOP > 21 mmHg 2 weeks after surgery. The mean IOP 45 days after AADI implantation was 15.4 ± 8.6 mmHg. After excluding patient No. 11 (Table 1) who had vitreous blocking the tube the mean IOP at the 45th day was 14.4 ± 6.7 mmHg. The reduction in IOP was 19.4 ± 9.1 mmHg (56.5%) and this was statistically significant (P < 0.001). Supplementary Fig. 2 shows the IOP at different follow ups and Supplementary Fig. 3 shows the scatter plot diagram of correlation between baseline IOP and IOP at 1 year review. The Kaplan–Meier survival curve showing the cumulative probability of overall success is depicted in Supplementary Fig. 4. Supplementary Fig. 5 depicts the Hazard function curve showing the cumulative probability of failure. The complete success at the last review was 10% and the qualified success was 80.0%.
The mean number of anti-glaucoma medications dropped from 3.5 ± 0.7 preoperatively to 2.2 ± 1.3 at last review (p < 0.001).
Complications and re-surgeries
Eight out of thirty eyes (26.7%) had a complication directly attributable to the surgery.
In patient No. 1 and 16 some difficulty was encountered in inserting the tube into the sulcus as the initial passage went behind the IOL into the vitreous cavity. The original entry site was closed with 8–0 vicryl and another opening made some distance away for tube insertion. No vitreous prolapse was noted at the entry site. It was unlikely that the lens zonules had been damaged during needle entry as the needle passed through into the sulcus effortlessly. These patients were not included in calculating the complication rate.
Patient No. 2 had progressive glaucomatous damage because of poor IOP control in the post-operative period. Patient No. 5 developed exposure of the tube 9 months after surgery (inferonasal implantation). It was noted that the scleral patch graft had rotated sideward (Supplementary Fig. 6). The patient was taken to the operating room and under surgical asepsis, the conjunctiva over the exposed tube was incised and a fresh scleral patch graft placed to cover the tube. It was noted that the old graft still had two 10–0 nylon sutures on one edge (partial thickness bites through the donor sclera that were covered by the graft) though none was noted on preoperative slit lamp exam. No further complications were noted during review. 9–0 nylon was used to anchor the scleral patch graft from the patient No. 9 onwards. None of these patients were noted to have shifting of the patch graft.
Patient No. 11 presented with eye pain and was noted to have vitreous blocking the tube with an IOP of 42 mmHg on the 40th post-operative day. He was treated with pars plana vitrectomy and clearing of tube in the operating room. Postoperatively the IOP was noted to be 12 mmHg.
Patient No. 12 had conjunctival retraction with sloughing of the corneal patch graft at 3 weeks after inferonasal implantation of the AADI (Supplementary Fig. 7a). A conjunctival swab from the area over the tube did not reveal any growth of micro-organisms. The corneal patch graft was obtained from eye bank tissue after the corneal button was used for penetrating keratoplasty. The donor was a 50-year-old male who had died of suicide by hanging. A blood sample taken by the eye bank had tested negative for HIV, HBsAg, syphilis and HCV. Two patients had undergone optical penetrating keratoplasty with the donor corneas and did not have any infective complications in the post-operative period. The remaining tissue in the corneal patch graft was also sent for bacterial culture and no growth was noted after 7 days.
The exposed area was covered with a scleral patch graft (after soaking in povidone iodine and gentamicin as per the protocol mentioned above) and the conjunctiva was sutured back with 10–0 monofilament nylon sutures. The patient presented with a drop in vision 6 weeks after the surgery. He was noted to have tunnel (the phacoemulsification wound) infiltrates with hypopyon on anterior chamber examination (Supplementary Fig. 7b) and vitreous exudates on ultrasonogram. The scleral patch graft had sloughed off and the conjunctiva had retracted. Under aseptic precautions and retrobulbar block, a diagnostic vitreous tap and anterior chamber tap was done in the operating room. A 26 G needle was used to scrap off the tunnel infiltrates for culture sensitivity. The tunnel infiltrates and the hypopyon were washed off with a Symcoe cannula. The scleral patch graft (remainder) and the AADI were explanted and sent for culture sensitivity. A vitrectomy was done followed by intracameral and intravitreal injection of vancomycin and amikacin. Postoperatively the patient was put on fortified topical vancomycin and amikacin drops half hourly. None of the culture samples revealed any growth. Gram staining of the aqueous tap showed many pus cells. A recurrence of tunnel infiltrates was noted 2 weeks later. Under aseptic precautions, the cornea overlying the infiltrates was excised in the operating room and a free cut donor corneal graft used to cover the defect. The patient was noted to have no perception of light in the eye a month later and later developed phthisis bulbi.
Patient No. 17 had IOP of 40 mmHg in the first 3 weeks after surgery and underwent a trabeculectomy for interim IOP control. This patient was not included in calculating the complication rate.
Patient No. 22 (poorly controlled diabetic) presented with a sudden drop in vision at 6 weeks in the operated left eye. He was noted to have a flat anterior chamber. The patient had not stopped anti-glaucoma medications on the 40th day as advised. An attempt to reform the anterior chamber with 1% sodium hyaluronate did not succeed. Tube re-ligation was done under aseptic precautions in the operating room with 6–0 vicryl and the anterior chamber filled with 1% sodium hyaluronate. Anti-glaucoma medications (Travatan® and Simbrinza®) were resumed and the patient was reviewed two-weekly. He was placed on a tapering regime of topical loteprednol starting 8 times/d, atropine 1% ointment tds and oral vitamin C. He was advised to stop anti-glaucoma medications and to start topical atropine drops on the 39th post-operative day (after tube re-ligation). At 42 days, post tube re-ligation, the IOP was recorded as 10 mmHg in the left eye. The anterior chamber was noted to be well formed. During subsequent reviews he developed a persistent corneal epithelial defect that did not resolve with lubricants and soft bandage contact lens (14.0 mm diameter). He also developed tube exposure 4.5 months post AADI implantation and was treated with tube repositioning and amniotic membrane graft under retrobulbar block with surgical asepsis in the operating room. A bandage contact lens was placed. As the patient had advanced glaucomatous damage (and due to inability to measure IOP because of the amniotic membrane graft and bandage contact lens), topical anti-glaucoma medications (Travatan® and Simbrinza®) were resumed. The epithelial defect healed and the IOP was noted to be 8 mmHg at the last review.
Patient No. 28 was lost to follow-up after the 2nd post-operative week. Because of some family issues, the patient reviewed with us 52 days after surgery. She complained of a drop in vision in the operated eye for the past 10 days and had been continuing to instil anti-glaucoma medications all along. Visual acuity was noted to be perception of light. The IOP was 0 mmHg with a shallow anterior chamber and kissing choroidal detachment. The patient however declined further surgical intervention and was lost to follow-up.
Patient No. 29 had a fibrinous anterior chamber reaction that subsided in 4 days with a increase in frequency of topical steroids.
Patient No. 30 presented with shallow anterior chamber and corneal oedema in the operated eye on the 45th post-operative day. The anti-glaucoma medications (which the patient had continued) were stopped and the anterior chamber was filled with Healon® (under topical anaesthesia in the operating room with surgical asepsis) and the patient was started on a tapering regime of topical loteprednol, atropine eye drops and oral Vitamin C. The anterior chamber formed well, and the patient was noted to have an IOP of 18 mmHg at the third month review without any anti-glaucoma medications. The cornea however was noted to be oedematous with few epithelial bullae in the periphery and the patient was started on hypertonic saline drops.
The complications are summed up in Table 1. Eighteen eyes had an uneventful post-operative period.
Visual acuity outcomes
Six eyes had loss of best corrected visual acuity during follow-up. In two patients it was judged to be due to progression of the disease. One patient was noted to have severe macular ischaemia. One had a persistent corneal epithelial defect and another had pseudophakic bullous keratopathy. Patient No. 12 lost vision due to endophthalmitis (Table 1). Patient No. 28 had poor vision due to severe hypotony and serous choroidal detachment. Thus, the vision loss could be attributed to the surgery in four patients.
Hypertensive phase
Two patients (6.7%) were judged to have hypertensive phase during the review. In both patients the IOP was adequately controlled with topical medications.
Ocular motility disturbance
None of the patients had preoperative ocular motility disturbances. A total of 8/30 (26.7%) eyes were noted to have ocular motility disturbance. Of these seven eyes had inferonasal implantation of the device. Four patients had elevation restriction of 1- in the operated eye. Three had adduction restriction of 1- and another patient had depression restriction 2- (on a scale of 1–4). Seven patients were orthophoric in primary position and none complained of diplopia. Patient No. 14 had no perception of light vision in the right eye and had a preoperative sensory exotropia. The angle of strabismus as measured by Krimsky did not change after AADI implantation.
Discussion
The design of the AADI has been authorized by Professor George Baerveldt. This device has a great potential to break cost barriers in the developing world. The absolute success reported for the AADI varies from 41.8% to 93.1% at 1 year and the qualified success rates have exceeded 90%. Some studies did not elaborate on the reported success rates as absolute or qualified. Most studies published on the AADI report on its placement in the anterior chamber [1,2,3,4,5,6,7,8]. Many studies have shown progressive endothelial loss after GDD implantation in the anterior chamber over the first 2 years after surgery. The regional endothelial loss is maximum in the quadrant of GDD implantation. The mechanism of corneal endothelial damage is unknown and likely multifactorial. Various theories proposed include jet flow around the tube, intermittent tube corneal touch, tube uveal touch, foreign body reaction, pre-existing endothelial damage, IOP rise and change in composition of the aqueous humour [11]. Some studies have found that a shorter tube cornea length correlates with a greater loss of endothelial cells, but other studies have found no association. A negative correlation has been noted between the tube cornea angle and endothelial cell loss. No association has been noted between tube iris distance and intracameral length of the tube [12, 13]. Sulcus/posterior segment placement potentially avoids corneal complications of surgery [10]. While pars plana placement of the tube has been noted to have minimum impact on the corneal endothelial density [14], there are no studies on endothelial cell loss after sulcus placement to the best of our knowledge.
We report a relatively low absolute success rate of 10% and qualified success rate of 80%. Our success rates are similar to those reported in The Ahmed Versus Baerveldt Study for the Baerveldt glaucoma drainage device (absolute success—17%, qualified success—56% at 1 year) [15]. One reason for the reduced success rate could be the high proportion of secondary and difficult to control glaucoma. Our IOP drop of 56.5% at 6 weeks was comparable to other studies. GDD are generally implanted in refractory glaucoma and it is difficult to compare results across studies on different patient groups.
Our post-operative complication rate of 26.7% for AADI implantation compares well with other studies (19–51%) [1,2,3,4,5,6,7,8,9]. Patient No. 22, 28 and 30 developed complications related to hypotony because of failure to stop anti-glaucoma medications at 40th day (around the time when the tube ligature is expected to open). We believe that it may be prudent to stop anti-glaucoma medications (in patients who undergo AADI implantation) between the 40–45th day. A decision to resume anti-glaucoma medications may be taken thereafter. We did not have any incidences of hypotony in the early post-operative period though a larger bore needle was used in many patients. We believe that this was because of tight suturing of the scleral flap and an overlap of the scleral/corneal patch graft and the scleral flap which may be protective in the event of a peritubular leak. Our technique thus has the potential to prevent peritubular leak. Re-ligation of the tube in the event of hypotony at 6 weeks is a viable option as in patient No. 22. This presumably allows more time for the capsule to form around the implant. Tube re-ligation was one of the most common interventions in the report on AADI by George et al. [1, 2]. Scleral patch grafts are generally reported to be safe and effective in covering the tube [16]. Shifting of the scleral flap caused the tube exposure as in patient No. 5. Breakage/weakening of sutures may be a possible cause. This has not been reported earlier to the best of our knowledge. We have not noted this complication after we shifted to using 9–0 nylon sutures for anchoring the donor scleral flap.
It is likely that melting of the donor scleral flap as in Patient no. 12 was an early sign of infection, though no organisms were isolated on a conjunctival swab or on culture of remaining donor tissue. The donor had died due to suicide. No infective complications were noted in other patients receiving the donor cornea. We therefore consider it unlikely that donor cornea was the source of infection. A decision to increase the soak time in povidone iodine and adding a gentamicin soak was taken as a precautionary measure. It is difficult to assess the impact of this measure though. We are not aware of any reports of scleral melting being reported as an early sign of endophthalmitis in patients undergoing glaucoma drainage device implantation.
Two patients (Patient No. 19 and 30) in our series developed progressive corneal decompensation. One patient had pre-existing pseudophakic bullous keratopathy and the second patient had shallow anterior chamber with tube corneal touch at 6 weeks after surgery.
There is a chance of damaging the lens zonules during tube insertion into the sulcus. We recommend repeated injection of viscoelastic under the iris in the quadrant of insertion and to check the tube position in the sulcus to prevent inappropriate positioning of the tube. We had inserted the tube after IOL implantation in most of our cases and did not notice any damage to the lens capsule. While the sole phakic patient in our series maintained a clear crystalline lens during follow-up, we admit the possibility of lens capsule damage in phakic patients with sulcus implantation of the tube. Caution is advisable with sulcus implantation in these patients.
There is limited literature on the restrictions of ocular motility caused by the AADI implant [5, 8]. We report a 26.7% incidence of ocular motility restriction after AADI implantation. However, none of our patients had a deviation (or change in preoperative deviation) in primary position and none reported diplopia. The AADI is more flexible than the Baerveldt implant and ocular motility disturbances between the two may not be directly comparable [1, 2]. Only two publications on the AADI detail on diplopia after surgery. One reported the incidence of motility disturbances to be 2% and the other reported a zero percent incidence of diplopia without specifying the details of motility restriction [5, 8]. Table 3 sums up the available literature on AADI. Considering the high incidence of ocular motility disturbances (which is not always accompanied by diplopia) we recommend routine evaluation of ocular motility disturbances after AADI implantation.
Twenty eyes in our series had an inferonasal placement of the implant. Inferior implantation is technically more difficult. Wound dehiscence and anterior exposure of the patch graft are considered to be more frequent with inferior implants due to a shorter fornix and poor wound healing. Some studies have reported higher post-operative incidence of transient diplopia and re-surgeries with inferior placement of GDD. The quadrant of implantation is however dictated by the preoperative state of the eye. Inferior placement of the GDD is preferred in patients with superior conjunctival scarring or when future posterior segment surgery is a possibility [17]. We report a higher incidence of ocular motility restriction with inferonasal placement of the AADI. Patient No. 5 with tube exposure (due to sideways rotation of the scleral patch graft) and patient No. 12 (endophthalmitis) had inferonasal placement of the AADI. The numbers in our study are however too small to permit an intergroup comparison.
To sum up, AADI implantation is a viable option for refractory glaucoma. Many patients continue to need anti-glaucoma medications for IOP control. Directly observing the tube tip position under the operating microscope, allowing sufficient tube length and repeated injections of viscoelastic under the iris can ensure proper tube positioning (and verification of the same in the post-operative period) and avoid damage to the lens zonules. Allowing overlap of the scleral/corneal patch graft and scleral flap has the potential to prevent peritubular leak. Melting of the donor scleral flap and conjunctiva may be an early sign of endophthalmitis. Use of 9–0 nylon sutures is recommended to anchor the scleral flap to prevent it from shifting position and exposing the tube.
The limitations of our study include a retrospective study design, small sample size and lack of information on endothelial cell counts. Specular microscopy before and after placement of the tube could have provided more information on endothelial cell loss after posterior chamber/pars plana implantation. Further studies on larger number of patients with diverse aetiologies are needed to elaborate on the success rates and motility restrictions.
Summary
What was known before
-
AADI implantation effectively reduces intraocular pressure
-
Current literature details on placement in the anterior chamber/pars plana
What this study adds
-
Sulcus/pars plana implantation of AADI results in a good short-term reduction of IOP many patients continue to require anti-glaucoma medications
-
The incidence of ocular motility restriction is 26.7%
-
Allowing overlap of the patch graft may help prevent peritubular leak
-
Melting of scleral flap in the post-operative period may be a early sign of endophthalmitis
-
Temporary suspension of anti-glaucoma medications on the 40th day may prevent hypotony related complications
References
Puthuran GV, Palmberg P, Wijesinghe HK, Krishnadas SR, Robin A. Intermediate-term outcomes of an affordable aqueous drainage implant in adults with refractory glaucoma. Ophthalmol Glaucoma. 2019;2:258–66. https://doi.org/10.1016/j.ogla.2019.03.009.
Puthuran GV, Palmberg PF, Wijesinghe HK, Pallamparthy S, Krishnadas SR, Robin AL. Intermediate-term outcomes of Aurolab aqueous drainage implant in refractory paediatric glaucoma. Br J Ophthalmol. 2019. pii: bjophthalmol-2019-314399. https://doi.org/10.1136/bjophthalmol-2019-314399.
Pathak Ray V, Rao DP. Surgical outcomes of a new low-cost nonvalved glaucoma drainage device in refractory glaucoma: results at 1 year. J Glaucoma. 2018;27:433–9.
Senthil S, Gollakota SR, Ali MH, Turaga K, Badakere S, Krishnamurthy R, et al. Comparison of the new low-cost nonvalved glaucoma drainage device with ahmed glaucoma valve in refractory pediatric glaucoma in Indian eyes. Ophthalmol Glaucoma. 2018;1:167–74.
Maheshwari D, Dabke S, Rajagopal S, Kadar MA, Ramakrishnan R. Clinical outcome of a nonvalved Aurolab aqueous drainage implant in posterior segment versus anterior chamber. Indian J Ophthalmol. 2019;67:1303–8.
Kaushik S, Kataria P, Raj S, Pandav SS, Ram J. Safety and efficacy of a low-cost glaucoma drainage device for refractory childhood glaucoma. Br J Ophthalmol. 2017;101:1623–7.
Rathi SG, Seth NG, Kaur S, Thattaruthody F, Kaushik S, Raj S, et al. A prospective randomized controlled study of Aurolab aqueous drainage implant versus Ahmed glaucoma valve in refractory glaucoma: a pilotstudy. Indian J Ophthalmol. 2018;66:1580–5.
Philip R, Chandran P, Aboobacker N, DhavalikarM, Raman GV. Intermediate-term outcome of Aurolab aqueous drainage implant. Indian J Ophthalmol. 2019;67:233–8.
Babu N, Baliga G, Wijesinghe HK, Puthuran GV. Intermediate-term outcomes of pars plana tube insertion of Aurolab aqueous drainage implant for refractory glaucoma. Br J Ophthalmol. 2019. pii: bjophthalmol-2019-314639. https://doi.org/10.1136/bjophthalmol-2019-314639.
Tello C, Espana EM, Mora R, Dorairaj S, Liebmann JM, Ritch R. Baerveldt glaucoma implant insertion in the posterior chamber sulcus. Br J Ophthalmol. 2007;91:739–42.
Lee EK, Yun YJ, Lee JE, Yim JH, Kim CS. Changes in corneal endothelial cells after Ahmed glaucoma valve implantation: 2-year follow-up. Am J Ophthalmol. 2009;148:361–7.
Iwasaki K, Arimura S, Takihara Y, Takamura Y, Inatani M. Prospective cohort study of corneal endothelial cell loss after Baerveldt glaucoma implantation. PLoS ONE. 2018;13:e0201342.https://doi.org/10.1371/journal.pone.0201342.
Mendrinos E, Dosso A, Sommerhalder J, Shaarawy T. Coupling of HRT II and AS-OCT to evaluate corneal endothelial cell loss and in vivo visualization of the Ahmed glaucoma valve implant. Eye. 2009;23:1836–44.
Seo JW, Lee JY, Nam DH, Lee DY. Comparison of the changes in corneal endothelial cells after pars plana and anterior chamber ahmed valve implant. J Ophthalmol. 2015;2015:486832. https://doi.org/10.1155/2015/486832.
Christakis PG, Kalenak JW, Zurakowski D, Tsai JC, Kammer JA, Harasymowycz PJ, et al. The Ahmed Versus Baerveldt study: one-year treatment outcomes. Ophthalmology. 2011;118:2180–9.
Tsoukanas D, Xanthopoulou P, Charonis AC, Theodossiadis P, Kopsinis G, Filippopoulos T. Heterologous, fresh, human donor sclera as patch graft material in glaucoma drainage device surgery. J Glaucoma. 2016;25:558–64.
Sidoti PA. Inferonasal placement of aqueous shunts. J Glaucoma. 2004;13:520–3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajamani, M., Ramamurthy, C., Ramamurthy, S. et al. Outcome of a low-cost glaucoma drainage device with posterior chamber/pars plana insertion of the tube. Eye 35, 901–912 (2021). https://doi.org/10.1038/s41433-020-0994-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-0994-x