Abstract
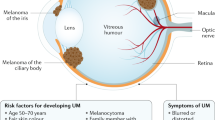
Metastasis to the eye can involve the choroid (90%), ciliary body (2%), iris (8%), and retina, optic disc, vitreous, and/or lens capsule (<1–4%). The mean number of uveal metastasis per eye (1.7), mean tumour base (11.6 mm) and thickness (3.2 mm), tumour colour (86% yellow), and presence of subretinal fluid (72%), are all clinical features suggestive of the diagnosis. Imaging with ultrasonography demonstrates an echodense mass (80%) and optical coherence tomography shows a “lumpy bumpy” choroidal surface (64%), both important diagnostic features. Uveal metastases typically emanate from primary cancer of the breast (37%), lung (27%), kidney (4%), gastrointestinal tract (4%), cutaneous melanoma (2%), lung carcinoid (2%), prostate (2%), thyroid (1%), pancreas (1%), and other sites (3%). Occasionally, fine needle aspiration biopsy is employed if the primary site is not known. In 16% of cases, the primary site remains unknown. Rarely, metastases affect the retina, vitreous, and lens capsule, most often originating from cutaneous melanoma and in patients previously treated with checkpoint inhibitor therapy. Kaplan–Meier analysis in a series of 1111 patients with uveal metastasis revealed 32% survival at 3 years and 24% at 5 years. Patients with uveal metastasis from carcinoid tumour showed most favourable survival at 5-years (92%), whereas pancreatic and kidney cancer demonstrated least favourable survival (0%). The 5-year survival was better for females (versus (vs.) males) (31% vs. 21%) and older adults (vs. children) (40% vs. 0%). In this review, we examine several large-cohort publications on the topic of ocular metastasis.
摘要
肿瘤可以转移至眼的脉络膜(90%), 睫状体(2%), 虹膜(8%)和视网膜, 视盘, 玻璃体和/或晶状体囊(<1–4%)等部位。每只眼脉络膜转移的平均数量为1.7, 平均肿瘤基底部的宽度为11.6 mm, 厚度为3.2 mm, 肿瘤颜色(86%黄色), 视网膜下积液(72%)这些临床特征都是诊断依据。超声成像提示有回声肿块(80%), 光学相干断层扫描(OCT)提示脉络膜表面有“块状隆起” (64%), 为重要的诊断特征。葡萄膜的转移性肿瘤通常来源于乳腺(37%), 肺(27%), 肾(4%), 胃肠道(4%), 皮肤黑色素瘤(2%), 肺类癌(2%), 前列腺(2%), 甲状腺(1%), 胰腺(1%)和其他部位(3%)。少数原发部位未明的眼部转移肿瘤可采用细针穿刺进行活检。在16%的病例中, 转移肿瘤的原发部位仍然未知。在极少数情况下, 转移肿瘤会侵犯视网膜, 玻璃体, 晶状体囊, 这些肿瘤多数来源于皮肤黑色素瘤和既往使用免疫检查点抑制剂治疗过的病人。对1111名有葡萄膜转移肿瘤的病人使用Kaplan-Meier生存分析, 结果提示3年生存率为32%, 5年生存率为24%。其中, 类癌葡萄膜转移的病人5年生存率最高(92%),而前列腺和肾脏来源的葡萄膜肿瘤生存率最低(0%)。女性(vs.男性)(31% vs.21%)和老年人(vs.儿童)(40% vs.0%)的5年生存率更好。在这篇综述中, 我们验证了一些以眼部转移肿瘤为主题的大型队列研究的成果。
Similar content being viewed by others
Introduction
In the 2012 Cambridge symposium, Victoria M.L. Cohen, FRCOphth, and the Chief of Ocular Oncology at Moorfields Eye Hospital, London UK, discussed the current status of ocular metastases, including clinical features, investigations, and treatments [1]. At that time, there was substantial knowledge regarding tumour origin, diagnostic procedures, and treatment of ocular metastasis, but little was known on the outcomes of ocular metastasis in general and per specific tumour or differences in outcomes based on patient age and sex, as well as timing of metastatic tumours before or after the primary cancer.
What we do know is that metastatic tumours to the eye are classically detected within the uvea, mostly within the post-equatorial choroid, and in patients with known systemic cancer [1,2,3,4,5,6,7]. Metastatic tumours reach the uvea due to the rich uveal blood supply. Tumour emboli can seed along the vascular uveal tract and produce growing and symptomatic tumours at various sites. Less often, metastasis to the eye can present in other sites of the uvea such as the iris or ciliary body. Quite rarely, metastatic foci reach other intraocular sites such as the retina, optic disc, vitreous, and lens capsule. Many patients with metastatic tumours to the eye are not seen by an ophthalmologist because they have disseminated metastatic foci with more serious systemic cancer concerns, so the ocular metastasis is not documented.
Historically, metastatic tumours to the eye were thought to be rare. A well-known ophthalmic textbook in 1966 stated that few surgeons had observed more than one case of ocular metastasis during their career [8]. Later, it was realised that ocular metastases were more common, and over the past 50 years there have been several fairly large pathology reports on the incidence of patients with metastatic tumours to the eye [9,10,11,12,13,14,15]. Albert et al. found that 2% of 213 patients with known systemic cancer with metastases demonstrated choroidal metastases on histopathology [10]. Bloch and Gartner reported that 8% of eyes in 230 patients with autopsy-proven carcinomas had histopathologically-confirmed uveal metastatic foci [12]. Nelson and coworkers, in an autopsy study, noted that 4% of patients who had died from carcinoma demonstrated ocular metastases, and they estimated that 22,000 patients who died of cancer in 1983 had ocular metastatic disease [15]. As noted herein, however, most reports on ocular metastases have emanated from pathology laboratories or from general cancer centres where patients have had known primary cancers and/or metastatic disease and the eyes were subsequently examined following death. These studies have basically focused on the primary tumour source and histopathologic features of the tumour (derived from pathology or autopsy) [8,9,10,11,12,13,14,15].
More recently, there have been a few comprehensive clinical reports from ocular oncology centres on the tumour features, treatments, and outcomes of patients with uveal metastatic disease [3, 6, 7, 16,17,18]. Most recently, large-cohort studies on uveal metastasis in 1111 patients from an ocular oncology centre was documented and the clinical features and outcomes based on primary tumour site [3], patient age [16], sex [17], and timing of onset of metastasis [18] was studied. Furthermore, there are now reports on fairly large series of specific cancers from a single organ that metastasised to the eye such as cancer of the breast, lung, skin melanoma, uveal melanoma, and others [19,20,21,22,23,24,25,26]. Other reports have focused on tumour imaging features for diagnosis, fine needle aspiration biopsy (FNAB) results, treatments for globe preservation, and ocular outcomes following management of ocular metastatic foci [27,28,29,30,31,32,33,34,35,36,37,38,39]. There are only few reports on systemic survival following diagnosis and treatment of ocular metastasis [3, 19].
Herein, we review the various sites of ocular metastasis by highlighting large-data reports on the sites with description of clinical features, imaging, treatments, and outcomes.
Metastatic tumours to the uvea (iris, ciliary body, choroid)
Uveal metastasis by primary site, age, sex, and timing
The uveal tract is the most common ophthalmic site for hematogenous dissemination of metastatic tumours from systemic cancer [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. The most comprehensive publication on uveal metastatic disease is an analysis of 2214 metastatic tumours in 1111 affected patients [3]. In that analysis, mean patient age was 60 years, Caucasian race (88%) and female sex (64%) were noted. The tumour was unilateral (82%) and the primary tumour site was known before uveal metastasis (67%). The primary tumour originated from cancer of the breast (37%), lung (26%), kidney (4%), gastrointestinal tract (4%), cutaneous melanoma (2%), lung carcinoid (2%), prostate (2%), thyroid (1%), pancreas (1%) and other sites (3%), with primary tumour site remaining unknown, despite systemic evaluation in 16% of cases. Overall prognosis was poor with 5-year survival at 24% and the poorest survival occurred in those with pancreatic metastasis (mean 4.2 months) whereas most favourable survival was with lung carcinoid metastasis (92% at 5 years).
Additional analysis of this large cohort of 1111 patients with uveal metastasis was performed regarding outcomes based on age, sex, and timing of the metastasis [16,17,18]. Regarding age, it was noted that uveal metastases rarely occur in children (0–20 years) (<1%), and young adults (21–40 years) (7%), but more often occur in middle-aged (41–60 years) (42%), older-aged (61–80 years) (46%), and senior-aged (81–100 years) (4%) adults [16]. The primary cancer to most commonly metastasise to the uvea was either breast or lung cancer in all age groups except children where the metastasis was from rare sarcomas (n = 3). The Kaplan–Meier survival estimates (at 1 and 5 years) for children (33%, 0%), young adults (48%, 23%), middle adults (60%, 29%), older adults (62%, 25%), and senior adults (76%, 40%) revealed senior adults with most favourable life prognosis.
Regarding patient sex, of 1111 consecutive patients with uveal metastasis, the majority were females (64%) [17]. Comparison (female versus (vs.) male) revealed differences in mean age at metastasis diagnosis (58 vs 63 years, p < 0.001), presence of bilateral disease (21% vs 11%, p < 0.001), and mean number of metastases per eye (1.8 vs 1.6 tumours per eye, p =0.04). Overall survival was more favourable for females (20 vs. 13 months, p = 0.03), likely related to the underlying primary cancer. Kaplan–Meier survival estimates (at 5 years) showed better survival in females (31% vs. 21%, p < 0.001). For specific primary cancers, the survival differed only for lung cancer (24% vs. 9%, p = 0.009).
Regarding timing, of 1111 consecutive patients with uveal metastasis, the primary cancer presented before (67%) or after (33%) detection of the uveal metastasis [18]. Those with known primary cancer had a mean delay between primary site and uveal metastasis of 5.2 years, but it differed per primary cancer site including gastrointestinal (2.1 years), lung (2.2 years), breast (6.5 years), and thyroid (13 years). The Kaplan–Meier survival estimates (at 5 years) revealed no difference whether the primary site was known (before vs. after vs. never found) (28% vs. 20%, vs. 33%).
Uveal metastasis by primary cancer
Other reports have specifically focused on a single primary cancer. In 2002, Demirci et al. evaluated 264 patients with choroidal metastasis from breast cancer alone and found Kaplan–Meier survival at 1 year at 65% and at 5 years at 24% [20]. Shah et al. reviewed 229 eyes of 194 patients with lung cancer metastasis to the eye, noting 1-year mortality at 54% [21]. DeBustros et al. presented 12 eyes with intraocular metastasis from cutaneous melanoma and indicated that palliative radiotherapy could assist in slowing the foci and vision loss [23]. Zografos et al. presented a single case and reviewed 28 similar cases in the literature of cutaneous melanoma metastatic to the retina and vitreous, finding patient survival at only 22 months [24]. Harbour et al. studied carcinoid tumour metastatic to the uvea, indicating that this rare disease represented only 2% of all metastasis and tends to arise in the bronchus and with a distinct orange colour [25]. Masoomian et al. reported on 13 patients with uveal melanoma that demonstrated metastatic disease to the contralateral eye [26]. In that cohort, death from uveal melanoma metastasis elsewhere occurred in 11 patients at a mean of 17 months.
Uveal metastasis imaging
There are several methods for imaging of uveal metastasis, particularly choroidal metastasis. By ultrasonography, most tumours appear dome-shaped or placoid, often with echodense appearance (Fig. 1). On fluorescein angiography, choroidal metastases demonstrate early hypofluorescence with late staining and multifocal retinal pigment epithelial pinpoint leaks. Unlike melanoma that can show “double circulation” on angiography from both retinal and dilated choroidal vessels, metastasis tends to not demonstrate the dilation of choroidal vessels. On indocyanine green angiography, choroidal metastasis show hypocyanescence with minimal late staining as opposed to melanoma that commonly demonstrates hypercyanescence..
Optical coherence tomography shows characteristic features in eyes with choroidal metastasis [27,28,29,30]. Al-Dahmash et al. reviewed 14 eyes with choroidal metastasis and found choriocapillaris compression (93%), a “lumpy bumpy” anterior contour (64%), and subretinal fluid (79%) [28] (Fig. 2). The “lumpy bumpy” contour is uncharacteristic for choroidal melanoma, nevus, and haemangioma, but can be seen with choroidal lymphoma and uveal effusion [27, 29, 30].
Fine needle aspiration biopsy
In cases where the diagnosis of uveal metastasis remains obscure, FNAB can be helpful [31,32,33]. Williams et al. studied posterior segment FNAB for 494 patients with an intraocular tumour and found that adequate tumour sample was achieved in 85% cases and the remainder (15%) had quantity not sufficient for diagnosis [33]. The most common final diagnosis was choroidal melanoma (44%), choroidal metastasis (39%), and choroidal lymphoma (5%). They concluded that FNAB is a reliable technique for cytopathologic confirmation of posterior segment intraocular tumours with clinically challenging diagnoses.
Uveal metastasis management
Management of uveal metastasis involves methods of radiotherapy including external beam radiotherapy (EBRT), proton beam radiotherapy, and plaque radiotherapy as well as systemic chemotherapy, systemic targeted medications and photodynamic therapy [34,35,36,37,38,39]. Rudoler et al. reviewed 233 consecutive eyes with metastatic tumours treated with EBRT using 30–40 Gy target dose. There were anticipated radiation-related side effects including cataract, retinopathy, papillopathy, keratopathy, iris neovascularization, and glaucoma [34]. Rudoler et al. provided another report on visual outcomes following EBRT of choroidal metastasis and noted that 98% of treated eyes had globe salvage, 57% regained some vision, and 36% of legally blind eyes regained useful vision [35] (Fig. 3).
Shields et al. reported on plaque radiotherapy for solitary choroidal metastasis in 36 consecutive cases and found tumour control in 94% following a single session, including those that failed previous EBRT [36, 37]. Radiation side effects were noted in 8% cases. Photodynamic therapy using verteporfin dye for treatment of choroidal metastasis was studied in 58 consecutive cases and accomplished tumour control in 1 session (71%) or 2 sessions (7%) and failed to achieve tumour control in 22% cases who were later treated with radiotherapy. For those successfully treated, visual outcome was 20/20-20/40 in 66% of eyes [39].
Iris metastasis
There are a number of solid (melanocytic and non-melanocytic) and cystic tumours that can occur in the iris and iris metastasis represents only 2% of all cases [40,41,42,43]. Iris metastasis can manifest as a single or multiple yellow-white nodules within the iris stroma, occasionally with debris (pseudohypopyon) in the anterior chamber and hyphema that can lead to glaucoma [42, 43] (Fig. 4). In a comprehensive study of 160 iris metastases presenting in 104 patients, with median age at presentation of 60 years, the most common symptoms were pain (32%) and blurred vision (30%) [43].
Iris metastasis was more common in women (62%) and the primary cancer site in all patients included breast (33%), lung (27%), skin melanoma (12%), kidney (7%), and oesophagus (3%). The iris findings included corectopia (37%), secondary glaucoma (37%), iris neovascularization (27%), keratic precipitates (25%), hyphema (11%), ectropion uveae (10%), and pseudohypopyon (8%), often mistaken as uveitis [43]. Management included EBRT (41%), plaque radiotherapy (24%), systemic chemotherapy (22%), surgical excision (5%), and enucleation (4%). The mean time between detection of iris metastasis and death was 24 months [43]. Chen et al. and Goduni et al. documented iris metastasis treated with novel systemic medications [44, 45].
Metastatic tumours to the retina
Metastatic tumours to the eye are typically found in the choroid (88%), iris (9%), or ciliary body (2%), but rarely in the retina (<1%) [2, 6, 46,47,48,49]. In the largest published analysis of retinal metastases, only 8 cases were documented [46]. These tumours originated from cutaneous melanoma (n = 3), breast cancer (n = 2), oesophageal cancer (n = 1), and lung cancer (n = 1), all established before the retinal metastasis, and there was one case of cutaneous melanoma (n = 1) discovered following retinal metastasis (Fig. 5). The primary tumour origin for retinal metastasis was substantially different from uveal metastasis as cutaneous melanoma represented 50% of retinal metastasis and only 2% of uveal metastasis [3, 46].
In the series of 8 patients with retinal metastasis, all were Caucasian, the mean patient age was 62 years, and the median interval from primary cancer to retinal metastasis was 33 months. This long delay was related to misdiagnosis elsewhere before referral as infectious or inflammatory retinitis (n = 5), retinal haemangioma (n = 1), choroidal neovascular membrane (n = 1), or nerve fibre layer infarction (n = 1). The tumour was unilateral (n = 7), white or yellow colour (n = 6) and with mean basal dimension of 7.4 mm and mean thickness of 2.3 mm. Vitreous tumour seeds (n = 3), vitreous haemorrhage (n = 2), subretinal fluid (n = 4), and intraretinal exudation (n = 1) were present. Management included plaque radiotherapy (n = 1) for localised disease, enucleation (n = 3) for extensive disease, or observation (n = 4) for pre-terminal patients. Systemic cancer-related death (n = 5, 63%) occurred within 1 month of retinal metastasis as established by ocular oncology. Patient survival with retinal metastasis is far poorer than with uveal metastasis.
Metastatic tumours to the optic disc
Metastatic tumours to the optic disc represent only 4% of all metastases to the eye [50]. In a series of 30 cases, the most common primary cancer sites to metastasise to the optic disc include breast (43%), lung (27%), gastrointestinal tract (3%), kidney (3%), and prostate (3%) cancer [50]. In 20% of cases the primary site was not found. Optic disc metastasis is typically unilateral (97%) and often with adjacent choroidal component (74%). Clinical features include swollen disc, often with surrounding nerve fibre layer haemorrhages. Systemic survival is poor at mean 13 months.
Metastatic tumours to the vitreous
Metastases to the vitreous are notably rare [51,52,53,54]. In 1998, Gunduz et al. reported 4 eyes in 3 patients with metastatic cutaneous melanoma to the vitreous, appearing at mean interval of 25 months since skin diagnosis and with findings of sheets or strands of golden yellow or brown cells as well as neovascular glaucoma [51]. At that time, most cases of vitreous metastasis were single case reports. Since then, a higher frequency of vitreous metastasis from treated cutaneous melanoma has been observed, especially in those treated with checkpoint inhibitor medications. Francis et al. reported a multicentre retrospective cohort of 14 eyes of 11 patients with metastatic cutaneous melanoma to the vitreous, most of whom had received checkpoint inhibitors during their course [52]. The eyes showed amelanotic (50%) or melanotic (50%) vitreous cells and the intraocular pressure was elevated (36%) Additionally, vitreous metastasis from other primary cancers have been documented [53].
Metastatic tumours to the lens capsule
There are only a few published case reports on metastatic tumour to the lens capsule, most of which have originated from cutaneous melanoma [51, 55,56,57,58]. In 2011, Solomon et al. reported a 71 year-old female with 1 year history of skin melanoma who developed complete opacification of the posterior lens capsule from melanoma metastasis [56]. Given the intraocular dissemination with evidence of vitreous debris on ultrasonography and lack of systemic metastasis, enucleation was performed. Metastatic cutaneous melanoma to the lens capsule, pseudophakic lens, anterior chamber, trabecular meshwork, vitreous, and retina was confirmed and the patient remained without systemic metastasis at 7 months follow up. In 2018, Paul et al. managed a 71 year-old male with known metastatic cutaneous melanoma on checkpoint inhibitor therapy (pembrolizumab) who developed bilateral pseudo-uveitis, lens capsule nodules, and cataract that proved on capsulorhexis at the time of cataract surgery to represent metastases from cutaneous melanoma [57]. The patient died from abdominal metastasis 7 months later. In 2020, Quhill et al. recorded an 80 year-old male with cutaneous melanoma 3 years prior who demonstrated anterior uveitis, ‘mutton fat’ keratic precipitates, and vision loss, ultimately demonstrating tumour deposition on the posterior lens capsule, confirmed on vitrectomy as metastatic cutaneous melanoma to the lens capsule [58]. Further widespread systemic metastasis led to guarded prognosis.
Summary
What is known about this topic
-
Metastasis to the eye most often involve the choroid, and rarely affect the ciliary body, and iris.
-
In select cases, metastases can manifest in the retina, optic disc, vitreous, and/or lens capsule.
-
Most metastatic tumours appear yellow in color, located within the choroid and producing an overlying serous retinal detachment.
-
Standard treatment includes systemic chemotherapy, if there are remote metastases, or external/plaque radiotherapy, if the condition is localized to the eye.
What this study adds
-
Uveal metastases most often arise from primary cancer of the breast (37%), lung (27%), kidney (4%), gastrointestinal tract (4%), cutaneous melanoma (2%), lung carcinoid (2%), prostate (2%), thyroid (1%), pancreas (1%), and other sites (3%).
-
At the time of detection, based on recent literature review, the mean number of uveal metastasis per eye is 1.7, mean tumour base is 11.6 mm and thickness is 3.2 mm.
-
Imaging with ultrasonography typically reveals an echodense mass and optical coherence tomography depicts a classic “lumpy bumpy” tumour surface, both suggestive of the diagnosis and important in tumour detection.
-
If the primary site is not known, then fine needle aspiration biopsy can be used to aspirate cells for identification of tumour type.
-
In a series of 1111 patients with uveal metastasis, Kaplan–Meier analysis revealed only 24% survival at 5 years.
-
The most favorable 5-year survival was in those with uveal metastasis from carcinoid tumour (92%) and the least favorable survival were those with primary sites in the pancreas or kidney (0%).
-
Newer studies have documented a slowly changing landscape in the incidence of cancer and its management [59]. This could impact outcomes in the future.
References
Cohen VML. Ocular metastases. Eye. 2013;27:137–41.
Shields JA, Shields CL. Metastatic tumors to the uvea retina and optic disc. In: Shields JA, Shields CL, editors. Intraocular tumors. an atlas and textbook. 3rd ed. Philadelphia, PA: Lippincott Wolters Kluwers; 2016, pp. 213–45.
Shields CL, Welch RJ, Malik K, Acaba-Berrocal LA, Selzer EB, Newman JH, et al. Uveal metastasis: clinical features and survival outcome of 2214 tumors in 1111 patients based on primary tumor origin. Middle East Afr J Ophthalmol. 2018;25:81–90.
Konstantinidis L, Damato B. Intraocular metastases–a review. Asia Pac J Ophthalmol. 2017;6:208–14.
Arepalli S, Kaliki S, Shields CL. Choroidal metastases: origin, features, and therapy. Indian J Ophthalmol. 2015;63:122–7.
Shields CL, Shields JA, Gross NE, Schwartz G, Lally S. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104:1265–76.
Stephens RF, Shields JA. Diagnosis and management of cancer metastatic to the uvea. A study of 70 cases. Ophthalmology. 1979;86:1336–49.
Duke-Elder S. System of ophthalmology, vol 9: Diseases of the uveal tract. St Louis: CV Mosby; 1966, 917.
Hart WM. Metastatic carcinoma to the eye and orbit. Int Ophthalmol Clin. 1962;212:465–82.
Albert DM, Rubenstein RA, Scheie HG. Tumors metastasis to the eye. I. Incidence in 213 adult patients with generalized malignancy. Am J Ophthalmol. 1967;63:723–726.
Jensen OA. Metastatic tumors of the eye and orbit. A histopathological analysis of a Danish series. Acta Pathol Microbiol Scand. 1970;212:201–14. (suppl)
Bloch RS, Gartner S. The incidence of ocular metastatic carcinoma. Arch Ophthalmol. 1971;85:673–5.
Ferry AP, Font RL. Carcinoma metastatic to the eye and orbit. I. A clinicopathologic study of 227 cases. Arch Ophthalmol. 1974;92:276–86.
Eliassi-Rad B, Albert DM, Green WR. Frequency of ocular metastases in patients dying of cancer in eye bank populations. Br J Ophthalmol. 1996;80:125–8.
Nelson CC, Hertzberg BS, Klintworth GK. A histopathologic study of 716 unselected eyes in patients with cancer at the time of death. Am J Ophthalmol. 1983;95:788–93.
Shields CL, Acaba-Berrocal LA, Selzer EB, Mayro EL, Newman JH, Malik K, et al. Uveal metastasis based on patient age in 1,111 patients: Comparison of clinical features and outcomes per age category. Retina. 2020;40:204–13.
Welch RJ, Malik K, Considine SP, Acaba-Berrocal L, Selzer E, Newman J, et al. Uveal metastasis based on patient sex in 2214 tumors of 1111 patients. A comparison of female versus male clinical features and outcomes. Asia Pac J Ophthalmol. 2019;8:298–303.
Welch RJ, Malik K, Mayro EL, Newman J, Honig S, Ang SM, et al. Uveal metastasis in 1111 patients: Interval to metastasis and overall survival based on timing of primary cancer diagnosis. Saudi J Ophthalmol. 2019;33:229–37.
Freedman M, Folk JC. Metastatic tumors to the eye and orbit patient survival and clinical characteristics. Arch Ophthalmol. 1987;105:1215–1219.
Demirci H, Shields CL, Chao AN, Shields JA. Uveal metastasis from breast cancer in 264 patients. Am J Ophthalmol. 2003;136:264–71.
Shah SU, Mashayekhi A, Shields CL, Walia HS, Hubbard GB, Zhang J, et al. Uveal metastasis from lung cancer: clinical features, treatment, and outcome in 194 patients. Ophthalmology. 2014;121:252–7.
Shah SU, Shields CL, Bianciotto CG, Shields JA. Pancreatic cancer metastasis to choroid. Ophthalmology 2011;118:1483.
de Bustros S, Augsburger JJ, Shields JA, Shakin EP, Pryor CC. Intraocular metastases from cutaneous malignant melanoma. Arch Ophthalmol. 1985;103:937–40.
Zografos L, Mirimanoff RO, Angeletti CA, Frosini R, Beati D, Schalenbourg A, et al. Systemic melanoma metastatic to the retina and vitreous. Ophthalmologica. 2004;218:424–33.
Harbour JW, De Potter P, Shields CL, Shields JA. Uveal metastasis from carcinoid tumor. Clinical observations in nine cases. Ophthalmology. 1994;101:1084–90.
Masoomian B, Mashayekhi A, Shields JA, Shields CL. Uveal melanoma metastasis to the contralateral eye structures: a retrospective comparative analysis of 13 consecutive patients. Ophthalmol Retina. 2021;5:1036–42. https://doi.org/10.1016/j.oret.2020.12.025.
Shields CL, Pellegrini M, Ferenczy SR, Shields JA. Enhanced depth imaging optical coherence tomography (EDI-OCT) of intraocular tumors. From placid to seasick to rock and rolling topography. The 2013 Francesco Orzalesi Lecture. Retina. 2014;34:1495–512.
Al-Dahmash SA, Shields CL, Kaliki S, Johnson T, Shields JA. Enhanced depth imaging optical coherence tomography of choroidal metastasis in 14 eyes. Retina. 2014;34:1588–93.
Shields CL, Manalac J, Das C, Saktanasate J, Shields JA. Review of spectral domain enhanced depth imaging optical coherence tomography (EDI-OCT) of tumors of the choroid. Indian J Ophthalmol. 2015;63:117–21.
Shields CL, Manalac J, Das C, Saktanasate J, Shields JA. Review of spectral domain enhanced depth imaging optical coherence tomography (EDI-OCT) of tumors of the retina and retinal pigment epithelium in children and adults. Indian J Ophthalmol. 2015;63:128–32.
Shields JA, Shields CL, Ehya H, Eagle RC, Jr, DePotter P. Fine needle aspiration biopsy of suspected intraocular tumors. The 1992 Urwick Lecture. Ophthalmology. 1993;100:1677–84.
Shields CL, Manquez ME, Mashayekhi A, Danzig CJ, Ehya H, Shields JA. Fine needle aspiration biopsy of iris tumors in 100 consecutive cases. Tech complications Ophthalmol. 2006;113:2080–6.
Williams BK, Di Nicola M, Khoo CTL, Mashayekhi A, Ehya H, Shields JA, et al. Fine-needle aspiration biopsy of posterior segment intraocular tumors in 500 consecutive eyes. Submitted for publication.
Rudoler SB, Corn BW, Shields CL, DePotter P, Hyslop T, Shields JA, et al. External beam irradiation for choroid metastases: identification of factors predisposing to long-term sequelae. Int J Radiat Oncol Biol Phys. 1997;38:251–6.
Rudoler SB, Shields CL, Corn BW, DePotter P, Hyslop T, Curran WJ, et al. Functional vision is improved in the majority of patients treated with external-beam radiotherapy for choroid metastases: a multivariate analysis of 188 patients. J Clin Oncol. 1997;15:1244–51.
Shields CL, Shields JA, DePotter P, Quaranta M, Friere J, Brady LW, et al. Plaque radiotherapy for the management of uveal metastasis. Arch Ophthalmol. 1997;115:203–9.
Shields CL. Plaque radiotherapy for the management of uveal metastasis. Curr Opin Ophthalmol. 1998;9:31–7.
Kaliki S, Shields CL, Al-Dahmash SA, Mashayekhi A, Shields JA. Photodynamic therapy for choroidal metastasis in 8 cases. Ophthalmology. 2012;119:1218–22.
Shields CL, Khoo CTL, Mazloumi M, Mashayekhi A, Shields JA. Photodynamic therapy for choroidal metastasis. Tumor control and visual outcomes in 58 cases. The 2019 Burnier International Ocular Pathology Society Lecture. Ophthalmol Ret 2020;4:310–319.
Shields CL, Kancherla S, Patel J, Vijavargiya P, Suriano M, Kolbus E, et al. Clinical survey of 3680 iris tumors based on patient age at presentation. Ophthalmology. 2012;119:407–14.
Shields CL, Shields PW, Manalac J, Jumroendararasame C, Shields JA. Review of cystic and solid tumors of the iris. Oman J Ophthalmol. 2013;6:159–64.
Shields JA, Shields CL, Kiratli H, De Potter P. Metastatic tumors to the iris in 40 patients. Am J Ophthalmol. 1995;119:422–30.
Shields CL, Kaliki S, Crabtree GS, Peshtani A, Morton S, Anand RA, et al. Iris metastasis from systemic cancer in 104 patients: the 2014 Jerry A. Shields Lecture. Cornea. 2015;34:42–8.
Chen KJ, Chao AN, Wang CL. Iris metastasis regression following Osimertinib treatment. JAMA Ophthalmol. 2020;138:e202095. https://doi.org/10.1001/jamaophthalmol.2020.2095.
Goduni L, Ashkenazy N, Hansen E, Soyano-Muller A, Correa ZM, Harbour JW. Iris metastasis from breast cancer successfully treated with Abemaciclib and Letrozole. Retin Cases Brief Rep. 2022. https://doi.org/10.1097/ICB.0000000000001176.
Shields CL, McMahon JF, Atalay HT, Hasanreisoglu M, Shields JA. Retinal metastasis from systemic cancer in 8 cases. JAMA Ophthalmol. 2014;132:1303–8.
Whalen KE, Eagle RC Jr, Vrabec TR. A case of metastatic urothelial carcinoma of the retina and vitreous. Retin Cases Brief Rep. 2018;12:177–80.
Mano F, LoBue SA, Chang KC, Mano T. Multimodal imaging of retinal metastasis masquerading as an acute retinal necrosis. Int J Retin Vitreous. 2018;21:43. 4
Venkat A, Binkley EM, Srivastava S, Karthik N, Singh A. Immunotherapy-resistant vitreoretinal metastatic melanoma. Ocul Oncol Pathol. 2021;7:62–5.
Shields JA, Shields CL, Singh AD. Metastatic neoplasms in the optic disc. The 1999 Bjerrum Lecture. Part 2. Arch Ophthalmol. 2000;118:217–24.
Gündüz K, Shields JA, Shields CL, Eagle RC Jr. Cutaneous melanoma metastatic to the vitreous cavity. Ophthalmology. 1998;105:600–5.
Kellermann N, Maier M, Feucht N. Vitreous metastasis of malignant cutaneous melanoma during treatment with ipilimumab. Ophthalmologe. 2017;114:163–6.
Francis J, Berry D, Abramson DH, Barkert CA, Bergstrom C, Demirci H, et al. Intravitreous cutaneous metastatic melanonma in the era of checkpoint inhibition. Ophthalmology. 2020;127:240–8.
Gelman R, Lee SE, Rocha N, Kayserman LG, Vallar RV, Marr BP. Vitreous metastasis in a case of metastatic breast cancer. Ocul Oncol Pathol. 2020;6:323–7.
Bowman CB, Guber D, Brown CH 3rd, Curtin VT. Cutaneous malignant melanoma with diffuse intraocular metastases. Arch Ophthalmol. 1994;112:1213–16.
Solomon JD, Shields CL, Shields JA, Eagle RC Jr. Posterior capsule opacity as initial manifestation of metastatic cutaneous melanoma. Graefes Arch Clin Exp Ophthalmol. 2011;249:127–31.
Paul SK, Chou L, Shields CL. Cutaneous melanoma metastatic to anterior lens capsule. JAMA Ophthalmol. 2018;136:e180095.
Quhill H, Mudhar HS, Cornish KS, Rennie IG. Cutaneous malignant melanoma metastasis to the pseudophakic lens capsule with associated granulomatous intraocular inflammation. Ocul Oncol Pathol. 2020;6:339–43.
Glass AG, Lacey JV, Hoover RN. The rise in breast cancer incidence, 1960-2003 in Estrogen Receptor positive tumors. Proc Am Assoc Cancer Res. 2005;65:953–4.
Funding
Support provided in part by the Eye Tumor Research Foundation, Philadelphia, PA (CLS). The funders had no role in the design and conduct of the study, in the collection, analysis and interpretation of the data, and in the preparation, review or approval of the manuscript. CLS has had full access to all the data in the study and takes responsibility for the integrity of the data.
Author information
Authors and Affiliations
Contributions
CLS, NEK, MG, MS: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; Drafting the work or revising it critically for important intellectual content; Final approval of the version to be published; Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AL, AMVS, KA, SEL, JAS: Substantial contributions to the interpretation of data for the work; Drafting the work or revising it critically for important intellectual content; Final approval of the version to be published; Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shields, C.L., Kalafatis, N.E., Gad, M. et al. Metastatic tumours to the eye. Review of metastasis to the iris, ciliary body, choroid, retina, optic disc, vitreous, and/or lens capsule. Eye 37, 809–814 (2023). https://doi.org/10.1038/s41433-022-02015-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02015-4
This article is cited by
-
Klinisches Vorgehen bei soliden intraokulären Metastasen
Die Ophthalmologie (2024)