Abstract
Objective
To evaluate the efficacy of immunomodulatory therapy (IMT) in paediatric anterior uveitis.
Methods
Chart review of all patients ≤ 18 years treated for anterior uveitis using a stepladder approach during a 10-year period. The type and duration of IMT were noted. The data were analysed depending on chronicity, aetiology, and type of IMT using appropriate statistical tests. The outcome measures included ocular complications, the need for surgical intervention, and visual outcomes.
Results
One hundred and thirty-four patients (191 eyes) were analyzed. The median age at diagnosis was 7 years (interquartile range (IQR): 7.5 years). The median follow-up was 4 years (IQR: 6 years). The most common causes of anterior uveitis were Juvenile idiopathic arthritis (64 patients, 47.8%) and undifferentiated (33 patients, 24.6%). All patients were started on topical steroids and cycloplegics. 94 (70%) patients required IMT. 92 (68.6%) were started on Methotrexate as the first agent, of which 21 (22%) were switched to a different agent owing to side effects. Biologic agent was added in 55 (41%) patients. 21 (16%) required switch to a second biologic agent, 5 (3.7%) to third, and 1 (0.8%) to fourth biologic agent. At the last exam, 11 (8%) had persistent inflammation. 55 (41%) had ocular complications, and 113 (84%) had a best corrected visual acuity ≥ 20/40.
Conclusion
Early introduction of IMT and switch to different agents may be required to control anterior uveitis and reduce the complications in children. IMT is safe and effective in treating paediatric anterior uveitis.
Similar content being viewed by others
Introduction
Non-infectious uveitis accounts for 75–88% of childhood uveitis in the United States with infections contributing to the remaining 10–15% of cases [1,2,3,4]. Anterior uveitis is the most common location in children affecting 40–60% of cases [1, 2, 4]. The prevalence of uveitis in the paediatric population is less than in adults; however, it is believed to be severe and can lead to significant visual complications, if left uncontrolled. Studies have reported an overall visual impairment of 17–22% in at least one affected eye, with up to one-third of all children experiencing permanent visual deterioration [5,6,7,8,9]. Ocular complications such as cataract, glaucoma, band keratopathy, macular oedema, and amblyopia have been reported in up to 76% of all paediatric uveitis cases [5].
The insidious onset, delay in diagnosis, difficulties during eye examination, lack of consistent monitoring of anterior uveitis, and side effects of topical and systemic therapy are all challenges in successfully treating and monitoring paediatric anterior uveitis. Currently, the preferred management strategy for treating anterior uveitis is the “step-ladder approach” [10,11,12,13]—the first step includes the beginning of treatment using topical corticosteroids with cycloplegics, the second step is the addition of steroid-sparing immunomodulatory therapy (IMT) with the most preferred agent being Methotrexate (MTX), and the third step is the addition of biologic agents. Tumour necrosis factor (TNF) α inhibitors such as Adalimumab and Infliximab are the most commonly used biologic agents in paediatric uveitis [10,11,12,13,14]. Adalimumab is a fully-humanized monoclonal antibody that is given subcutaneously every 2 weeks, while Infliximab is a chimeric (human-murine) monoclonal antibody that is given as a monthly intravenous infusion and serves as a viable alternative for those who have failed Adalimumab treatment [14]. If the uveitis persists after both agents have been tried for the optimal dose and frequency, other biologic agents such as Tociliuzumab, Abatacept, or Golimumab are used as the next step of management [13].
The main objective of this study is to evaluate the safety and efficacy of the current treatment modalities, specifically, systemic IMT in a cohort of paediatric anterior uveitis patients treated at a tertiary care centre.
Methods
The study was approved by the Institutional Review Board of Boston Children’s Hospital. It was conducted in compliance with the United States Health Insurance Portability and Accountability Act (HIPAA) and conformed with the principles of the Declaration of Helsinki.
We conducted a retrospective chart review of all patients ≤ 18 years at the time of initial presentation who were treated in our department for anterior uveitis during the 10-year period between June 2010 and June 2020. The patients were identified using ICD-9 and ICD-10 billing codes.
Anterior uveitis was diagnosed clinically by the presence of cells and/or flare in the anterior chamber using the slit-lamp examination and was graded using Standardization of uveitis nomenclature (SUN) criteria [15]. Patients with spill-over cells in the vitreous were included for analysis. Patients with intermediate uveitis, posterior uveitis, traumatic uveitis, vasculitis, or with in-complete data or lack of regular follow-up were excluded from the analysis.
Data collection
The demographic data that was collected included: age at diagnosis, gender, duration of follow-up, ocular symptoms at presentation, aetiology of uveitis, systemic associations, grade of inflammation, and chronicity of uveitis. Uveitis was classified as acute, chronic, or recurrent. Chronic uveitis was defined as persistent inflammation >3 months while on continuous treatment. Recurrent uveitis was defined as uveitis that recurred within 3 months of discontinuing the treatment. All patients underwent complete systemic examination by the paediatric rheumatologist after the diagnosis of the first episode of uveitis to rule out associated systemic diseases. The uveitis workup was sent in collaboration with the rheumatologist depending on the presenting symptoms and signs. In some cases, uveitis was noted on regular screening examination that was part of the protocol owing to the patient’s systemic disease [11].
The type and duration of topical and systemic treatment were noted. All ocular medications (dose and frequency of administration) and prior or current systemic treatment (dosage and frequency) were included. Persistent uveitis was defined as lasting >3 months or worsening uveitis and/or failure to wean ocular corticosteroids to ≤3 drops of prednisolone acetate 1% (PA) daily and/or failure to control the systemic disease.
Outcome measures
The outcome measures that were analyzed were ocular complications, the need for surgical intervention, and visual outcomes. The presence of ocular complications was noted at the time of presentation and at the last follow-up visit. The documented complications included band keratopathy (BK), ocular hypertension (OHT), glaucoma, posterior synechiae, hypotony, cystoid macular oedema (CME) and amblyopia. Glaucoma was defined as intraocular pressure (IOP) >21 mmHg with glaucomatous optic nerve head and visual field changes. Ocular hypertension was defined as IOP >21 mm Hg on more than 2 visits with no anatomical changes in optic nerve head or visual field changes. The need for surgical intervention at the last follow-up visit was recorded. Best-corrected visual acuity (BCVA) was documented at the initial and last visit using an age-appropriate method including LEA symbols, HOTV, or Snellen Chart. Visual impairment was defined as BCVA <20/40 with worsened VA from baseline. The primary cause of visual impairment was noted.
Active anterior segment inflammation corresponded to cell grade 0.5+ or higher as described by SUN criteria. Controlled uveitis corresponded to <0.5+ cells on ≤2 drops of PA per day. Steroid remission was calculated from the last date of known steroid use to the last visit date.
Statistical analysis
The quantitative data were analyzed using mean, median, standard deviation (SD), IQR, minimum and maximum quartiles. Categorical data was configured by using frequency (count) and relative frequency (percentage) per each variable. Comparisons between quantitative variables were made using the parametric paired samples t-test. For comparing categorical data, Chi-square (χ2) test and Fisher’s exact test were performed. P values < 0.05 were considered statistically significant.
Results
A total of 134 patients (191 eyes) with paediatric anterior uveitis were included for analysis. Forty-three patients were excluded of which 18 patients had intermediate/posterior uveitis, 13 were on IMT for their systemic association without evidence of uveitis, 6 patients lacked regular follow-up at our institution, and an additional 6 patients were older than 18 years at the initial presentation.
The baseline clinical and demographic characteristics of these patients are summarized in Table 1. Of the 134 patients, 88 patients (65.6%) were females. Overall, 77 patients (57.4%) had unilateral uveitis with left eye involvement found in 44 patients (32.8%). The median age at presentation was 7.0 years (IQR: 7.5, mean: 7.0 ± 4.6 years, range: 1–16 years). The median follow-up was 4 years (IQR: 6 years, mean: 4.6 ± 4.2 years, range: 1–9.2 years). 8 patients (4 unilateral and 4 bilateral cases) were classified with granulomatous uveitis. 85 patients (64%) had chronic uveitis, 34 patients (25%) had acute uveitis, and 15 patients (11%) had recurrent uveitis.
Aetiology
A total of 101 patients (75.3%) had an underlying systemic association (Table 2). Juvenile idiopathic arthritis (JIA) was the most common cause of anterior uveitis in 64 (47.8%), followed by undifferentiated uveitis in 33 (24.6%) patients. Other systemic associations included TINU (5 patients, 3.7%), Kawaski disease (4 patients, 3%), Lyme disease (3 patients, 2.2%), leukaemia (2 patients, 1.5%), Crohn’s disease (1 patient, 0.8%), type 1 diabetes (1 patient, 0.8%), and Blau syndrome (1 patient, 0.8%). The mean age of presentation was lower (5.0 ± 2.7 years) in patients with JIA. 80% (51/64) patients with JIA were females. In contrast, the prevalence of undifferentiated uveitis was similar in males and females. Table 2 enumerates the other systemic associations noted with anterior uveitis in our population.
In patients with JIA, 78% (50/64) had no symptoms at the time of uveitis diagnosis. In contrast, 91% (30/33) patients with undifferentiated uveitis and 89% (33/37) patients with other causes were symptomatic at the time of uveitis diagnosis. The most common symptoms included red eye, photophobia, decreased vision, and eye pain.
Medical therapy
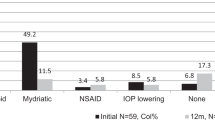
The first line of treatment for all patients was topical steroids in the form of PA 1% and cycloplegic agents. The frequency of topical steroids was determined by the degree of anterior chamber inflammation. Steroids were tapered every 2–3 weeks after the inflammation was controlled (<grade 0.5). In 30% patients (40/134), the uveitis was controlled on topical treatment alone. The mean duration for which these patients needed topical treatment was about 2 months (range 1–5 months). The time since cessation of steroid (remission) was approximately 25 ± 29 months (median: 15 months; range: 1–137 months) in these patients.
In 27% (37/134) of patients with continued inflammation, oral steroids were used to control the inflammation and served as a bridge to IMT. The children were started on single course of oral prednisolone 1 mg/kg for first 10 days followed by a taper over next 4–8 weeks. No patient was given a repeat course of oral steroids.
70% of patients (94/134) needed IMT. 68.6% (92/134) received MTX as the first systemic immunomodulatory agent. The mean duration between the first exam with documented uveitis until the start of MTX was 4.81 months ± 6.9 months. The average duration of MTX treatment was 4.59 ± 3.56 years, and MTX was prescribed to 97% (62/64) of patients with JIA-associated uveitis (Table 3). The mean duration of MTX treatment in JIA-associated uveitis was 6.2 ± 4.15 years (median: 6 years, range: 1–16 years). In comparison, 39% of undifferentiated uveitis patients received MTX for a mean duration of 3.5 ± 3.12 years (median: 2.5 years, range: 1–9 years). Forty patients (45%) reported side effects while on MTX. Most common side effects included nausea in 19 patients (22%), gastrointestinal upset/discomfort 12 (14%) and vomiting in 10 patients (11%). Out of the 88 patients on MTX, 21 patients (15.7%) had to discontinue due to side effects (Supplementary Table 1). These patients were switched to other agents such as mycophenolate Mofetil/Cellcept (10 patients), Leflunomide (10 patients) and Azathioprine/Imuran (1 patient).
Over time, 41% of patients (55/134) received a biologic agent. 20% (27/134) required ≥ 2 biologics during the treatment course. The patients were on optimal dose and frequency of biologic agent with persistent uveitis before they were switched to another agent. 15.7% (21/134) of patients transitioned to a 2nd biologic agent, 3.7% (5/134) required a 3rd biologic agent, and 0.8% (1/134) required a 4th biologic agent (Fig. 1). The average time from initial presentation to start of biologic agent was 6.56 ± 7.96 months. No child received a biologic agent as the primary medication. The biologic agents that were prescribed included Adalimumab (45/134, 33.5%), Infliximab (29/134, 22%), Tocilizumab (2/134, 1.5%) and Abatacept (2/134, 1.5%) (Table 3). The patients who were not compliant with medications or follow-up visits were started on Infliximab as the first biologic agent. All other patients were offered either Adalimumab or Infliximab as the first biologic agent. Supplementary Table 1 enumerates the number of patients in each treatment group, the number of patients with side effects in each group, and the number of patients who required substitution or discontinuation of treatment owing to side effects or persistence of uveitis. The need for the addition of biologic agents was higher in patients with JIA-associated uveitis (39/64, 61%) than in those with undifferentiated uveitis (6/33,18.2%). 38% of patients (21/55) reported systemic side effects on biologic agents. Infliximab had the highest number of recorded side effects in 10.5% (14 patients), followed by 4% (5 patients) on Adalimumab, and in 1 patient each on Tocilizumab and Abatacept. The most common side effects of Infliximab were infusion reactions (causing tachycardia, dyspnoea, facial flush, and chest pain). 3 patients on Adalimumab developed intolerance to injections over time. Overall, 20% of patients on biologic agents developed mild infections involving the gastrointestinal tract, respiratory tract, or skin (all of which responded well to antibiotics). 17 patients had to switch biologic agents owing to systemic side effects (Table 4).
At the last follow-up, 92% of patients (123/134) had controlled inflammation. 8% of patients (11/134) had persistent uveitis at a mean follow-up of 55 months. 8 patients had JIA, 2 patients had undifferentiated uveitis and 1 patient had tubulointerstitial nephritis and uveitis (TINU). Five of these patients were on MTX only, 5 were on combination of MTX + biologic agent, and 1 patient was on topical steroids only.
Complications
A total of 44% of patients (59/134) presented with complications at the first exam. The most common complication at initial presentation was posterior synechiae in 29% of patients (46 eyes of 39 patients), followed by band keratopathy in 11% of patients (15/134). Three patients had complicated cataracts at initial presentation (Supplementary Figure). The patients who presented with complications at the first exam had a higher rate of complications (75%, 44/59, p < 0.001) at the last exam, a higher need for surgical intervention (36%, 21/59, p < 0.004), and poor visual outcomes (p = 0.4).
By the final visit, complications were found in 41% (55/134) of the cohort. The most common complications were band keratopathy in 21% (28/134), posterior synechiae in 20% (26/134), cataract in 19% (25/134), glaucoma in 15% (20/134), deprivation amblyopia in 10% (13/134), and ocular hypertension in 10% (13/134). JIA patients were more likely to develop complications (45%) during the treatment course compared with undifferentiated patients (42%), though the difference was not statistically significant (p = 0.82) (Table 4). A total of 12% of patients (16/134) had cataract surgery. Seven percent of patients (9/134) had posterior subcapsular cataract at the last visit, of whom only two patients had visually significant cataract.
Need for surgical intervention
Surgical interventions were needed in 16% of patients (22/134) and most patients required multiple procedures (76%) (Table 4). The most common surgeries included cataract extraction in 12% of patients (24 eyes of 16 patients), glaucoma surgery (12 eyes of 12 patients), YAG capsulotomy (4 eyes of 4 patients), EDTA chelation (4 eyes of 4 patients), and vitrectomy (1 eye of 1 patient). The patients with JIA-associated uveitis required more surgical procedures compared with patients with undifferentiated uveitis as noted in Table 4.
Visual outcomes
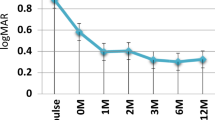
At the final follow-up visit, 84% of patients (113/134) had a BCVA ≥ 20/40, 8% (11/134) had <20/40–20/100, and 7.5% (10/134) had < 20/100 (Table 4). Overall, 56.7% of patients had improved final BCVA compared with their initial presentation (p < 0.001). Four patients (5 eyes) were not included in mean BCVA due to non-numerical visual acuities (fix and follow). In patients with BCVA < 20/100, 5 patients had band keratopathy and deprivation amblyopia, 2 had cataract (not operated), 2 had corneal opacities, and 1 had iris atrophy with derivation amblyopia.
Discussion
In our study, one-fourth of patients with anterior uveitis responded to topical steroids alone. The remaining three-fourths of patients required IMT (one-fourth responded to MTX alone and the remaining half needed the addition of biologic agents). Thirty percent of patients (39/134) had to be switched to a different agent owing to systemic side effects. Despite the difficulties in determining which IMT worked best with the least amount of side effects, we were able to control the uveitis in 88% of patients. 84% had a BCVA ≥ 20/40 at the final follow-up visit.
Immunomodulatory therapy has revolutionized the management of uveitis and provides steroid-sparing treatment that is critical in the paediatric population [10,11,12,13,14]. It is very important to minimize the use of systemic as well as topical steroids owing to numerous systemic and ocular side effects in a growing child. The introduction of IMT creates anxiety for the patient and their families due to concerns about safety and efficacy in children. The aim of this study was to evaluate the effectiveness of IMT in a paediatric population at a tertiary care centre and to report clinical outcomes in children with anterior uveitis.
Our study highlights the fact that there were two main issues in the management of paediatric uveitis using IMT. First, there may be persistent uveitis which can be managed using the stepladder approach (first increasing the dose and frequency of the current agents or by adding a new IMT agent). Second, there may be systemic side effects from IMT. These side effects were not life-threatening but required switching to a different IMT agent.
Our study supports the current evidence of higher prevalence of anterior uveitis in females [3, 4, 6, 7, 11, 16,17,18]. We found that 48% of our patients with paediatric anterior uveitis had JIA, similar to that reported in other studies [10, 14]. 78% of patients with JIA were asymptomatic at presentation. Several studies reported asymptomatic uveitis in 20% of patients with oligoarticular type and less frequently in those with negative rheumatoid factor [19, 20]. This highlights the importance of screening exams in the early diagnosis of uveitis before the development of complications.
The rate of ocular complications (41%) in our cohort was lower compared with the previously published data. Curragh et al. [12] reported complications in 65% of patients (94 UK patients mean follow-up: 5 years); de Boer and colleagues [5] reported complications in 76% of patients93 Dutch patients, mean follow-up: 3 years); and Ferrera et al. [16] reported complications in 76.8% of patients with anterior uveitis (177 US patients, mean follow-up 5 years). The reduced incidence of complications found in our study may be attributable to regular screening regimens in our patients leading to early diagnosis, early institution of IMT, and addition or switch to a different biologic agent when inflammation was persistent. Our study found that the most common complications were band keratopathy (21%) and posterior synechiae (20%). The incidence of cataract formation (19%) was low in our population compared with other studies, which may be due to steroid-sparing treatment [18]. Ferrera and colleagues [16] reported that cataract was the most common complication in their cohort developing in 43.84% of affected eyes. Another study by Cann et al. [10] also found that cataract was the most frequent complication to occur at a rate of 0.05/EY. Supplementary Table 2 compares the rates of complications of JIA-associated uveitis in our study to previous studies.
The need for surgical intervention was also lower (16%) in our study. The prevalence of surgeries in various studies ranges from 7.8% to 45.9% [7, 16, 21]. This is closely related to the low rate of complications noted in our cohort.
The rates of visual impairment vary in different studies and range from VA worse than 20/50 in 18%-36% to VA worse than 20/200 in 4–24% [4, 8, 18, 19, 21, 22]. In our study, 84% had BCVA > 20/40, 8% had BCVA 20/40–20/100, and 7.5% had BCVA < 20/100. Cann and colleagues [10] reported the incidence rate of visual impairment with vision >0.3 logMAR to be 0.05/EY (>0.3logMAR) and with vision ≥ 1.0 log-MAR to be 0.02/EY. Thorne et al. [23], who focused on topical corticosteroids solely, recorded that the incidence rates of visual impairment of 6/15 or worse (≥0.4 logMAR) and 6/60 or worse (≥1.0 logMAR) were 0.10/EY and 0.08/EY) respectively. Gregory et al. [21] found a statistically significant reduction in the risk of vision loss when patients were prescribed immunosuppressive drugs in their treatment regimen.
The SITE Cohort study depicted the importance of timely diagnosis and efficacy of systemic IMT and showed a lower incidence of visual impairment (visual acuity <20/200) when using immunosuppressive drugs over a median follow-up of 2.62 years [21]. Our study showed that early introduction of IMT, stepladder approach, and switch to different agents when uveitis is persistent lead to a lower rate of complications, less need for surgical interventions, and improvement in visual outcomes. Prolonged course and complications can cause significant morbidity in children which can eventually affect the psychosocial well-being of the child as well as the family [21].
As with all retrospective studies, our results must be interpreted with caution. The limitations of our study include its retrospective design and potential biases. Referral bias is likely, given that all these patients were referred to our tertiary care clinic after some level of initial treatment.
There was a selection bias in the way IMT was prescribed to certain patients. Some patients were started on monthly inpatient transfusions when reliability and compliance were factored into the decision about which therapeutic to prescribe. We lacked an adequate sample size for each specific IMT used. We did not evaluate treatment adherence in our cohort. But our centre has combined paediatric rheumatology and paediatric uveitis clinic such that the patient can be seen in the same visit which helps in timely diagnosis and consistent follow-up.
In summary, IMT is safe and effective in the management of paediatric anterior uveitis, helps improve visual outcomes with fewer complications, and causes less need for surgical interventions. Early introduction of IMT in a timely manner using a step-ladder approach is the key to achieving steroid-free remission and helps reduce long-term complications of steroids and uncontrolled uveitis. The multi-disciplinary approach that works collaboratively with a rheumatology clinic ensues timely diagnosis and consistent follow-up, especially for those with systemic associations. Despite the advances in IMT, paediatric uveitis remains a challenging condition associated with visually significant complications.
Summary
What was known before
-
Anterior uveitis is associated with high morbidity in children owing to higher rate of complications.
-
Steroid sparing immunomodulatory therapy using step ladder approach is recommended to treat paediatric uveitis.
What this study adds
-
Immunomodulatory therapy in children may require switching through different agents to find the agent that is safe and effective.
-
Early introduction of steroid sparing therapy reduces the complications and improves the visual outcomes.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kump LI, Cervantes-Castañeda RA, Androudi SN, Foster CS. Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology. 2005;112:1287–92.
Rosenberg KD, Feuer WJ, Davis JL. Ocular complications of pediatric uveitis. Ophthalmology. 2004;111:2299–306.
Thorne JE, Suhler E, Skup M, Tari S, Macaulay D, Chao J, et al. Prevalence of noninfectious uveitis in the united states: A claims-based analysis. JAMA Ophthalmol. 2016;134:1237–45.
Smith JA, Mackensen F, Sen HN, Leigh JF, Watkins AS, Pyatetsky D, et al. Epidemiology and course of disease in childhood uveitis. Ophthalmology. 2009;116:1544–51. 1551.e1541
de Boer J, Wulffraat N, Rothova A. Visual loss in uveitis of childhood. Br J Ophthalmol. 2003;87:879–84.
Cunningham ET Jr. Uveitis in children. Ocul Immunol Inflamm. 2000;8:251–61.
Nagpal A, Leigh JF, Acharya NR. Epidemiology of uveitis in children. Int Ophthalmol Clin. 2008;48:1–7.
Kadayifçilar S, Eldem B, Tumer B. Uveitis in childhood. J Pediatr Ophthalmol Strabismus. 2003;40:335–40.
Tugal-Tutkun I. Pediatric uveitis. J Ophthalmic Vis Res. 2011;6:259–69.
Cann M, Ramanan AV, Crawford A, Dick AD, Clarke SLN, Rashed F, et al. Outcomes of non-infectious paediatric uveitis in the era of biologic therapy. Pediatr Rheumatol Online J. 2018;16:51.
Wentworth BA, Freitas-Neto CA, Foster CS. Management of pediatric uveitis. F1000Prime Rep. 2014;6:41.
Curragh DS, O’Neill M, McAvoy CE, Rooney M, McLoone E. Pediatric uveitis in a well-defined population: Improved outcomes with immunosuppressive therapy. Ocul Immunol Inflamm. 2018;26:978–85.
Angeles-Han ST, Ringold S, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. 2019 American College of Rheumatology/arthritis foundation guideline for the screening, monitoring, and treatment of juvenile idiopathic arthritis-associated uveitis. Arthritis Care Res. 2019;71:703–16.
Miraldi Utz V, Bulas S, Lopper S, Fenchel M, Sa T, Mehta M, et al. Effectiveness of long-term infliximab use and impact of treatment adherence on disease control in refractory, non-infectious pediatric uveitis. Pediatr Rheumatol Online J. 2019;17:79.
Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140:509–16.
Ferrara M, Eggenschwiler L, Stephenson A, Montieth A, Nakhoul N, Araùjo-Miranda R, et al. The challenge of pediatric uveitis: Tertiary referral center experience in the united states. Ocul Immunol Inflamm. 2019;27:410–7.
Couto C, Frick MM, LaMattina K, Schlaen A, Khoury M, Lopez MM, et al. Chronic anterior uveitis in children. Ocul Immunol Inflamm. 2016;24:392–6.
Clarke LA, Guex-Crosier Y, Hofer M. Epidemiology of uveitis in children over a 10-year period. Clin Exp Rheumatol. 2013;31:633–7.
Zulian F, Martini G, Falcini F, Gerloni V, Zannin ME, Pinello L, et al. Early predictors of severe course of uveitis in oligoarticular juvenile idiopathic arthritis. J Rheumatol. 2002;29:2446–53.
Kotaniemi K, Kautiainen H, Karma A, Aho K. Occurrence of uveitis in recently diagnosed juvenile chronic arthritis: a prospective study. Ophthalmology. 2001;108:2071–5.
Gregory AC 2nd, Kempen JH, Daniel E, Kaçmaz RO, Foster CS, Jabs DA, et al. Risk factors for loss of visual acuity among patients with uveitis associated with juvenile idiopathic arthritis: the systemic immunosuppressive therapy for eye diseases study. Ophthalmology. 2013;120:186–92.
Woreta F, Thorne JE, Jabs DA, Kedhar SR, Dunn JP. Risk factors for ocular complications and poor visual acuity at presentation among patients with uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol. 2007;143:647–55.
Thorne JE, Woreta F, Kedhar SR, Dunn JP, Jabs DA. Juvenile idiopathic arthritis-associated uveitis: Incidence of ocular complications and visual acuity loss. Am J Ophthalmol. 2007;143:840–6.
Funding
This study is funded by Children’s Hospital Ophthalmology Foundation.
Author information
Authors and Affiliations
Contributions
All the authors were responsible for designing the review protocol, writing the protocol and manuscript, conducting the search, extracting and analyzing data, interpreting results, updating reference lists and creating’Summary of findings’ tables.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Huynh, E., Elhusseiny, A.M. & Nihalani, B.R. Paediatric anterior uveitis management in the USA: a single-centre, 10-year retrospective chart review exploring the efficacy and safety of systemic immunomodulatory therapy. Eye 37, 1325–1330 (2023). https://doi.org/10.1038/s41433-022-02121-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02121-3
This article is cited by
-
Encouraging visual outcomes in children with idiopathic and JIA associated uveitis: a population-based study
Pediatric Rheumatology (2023)