Abstract
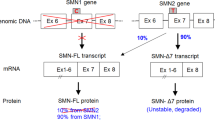
Spinal muscular atrophy (SMA) is a neuromuscular disease caused by loss of the SMN1 gene and low SMN protein levels. Although lower motor neurons are a primary target, there is evidence that peripheral organ defects contribute to SMA. Current SMA gene therapy and clinical trials use a single intravenous bolus of the blood-brain-barrier penetrant scAAV9-cba-SMN by either systemic or central nervous system (CNS) delivery, resulting in impressive amelioration of the clinical phenotype but not a complete cure. The impact of scAAV9-cba-SMN treatment regimens on the CNS as well as on specific peripheral organs is yet to be described in a comparative manner. Therefore, we injected SMA mice with scAAV9-cba-SMN either intravenously (IV) for peripheral SMN restoration or intracerebroventricularly (ICV) for CNS-focused SMN restoration. In our system, ICV injections increased SMN in peripheral organs and the CNS while IV administration increased SMN in peripheral tissues only, largely omitting the CNS. Both treatments rescued several peripheral phenotypes while only ICV injections were neuroprotective. Surprisingly, both delivery routes resulted in a robust rescue effect on survival, weight, and motor function, which in IV-treated mice relied on peripheral SMN restoration but not on targeting the motor neurons. This demonstrates the independent contribution of peripheral organs to SMA pathology and suggests that treatments should not be restricted to motor neurons.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Additional data are available from the corresponding author upon request.
References
Kolb SJ, Kissel JT. Spinal muscular atrophy. Neurol Clin. 2015;33:831–46.
Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–65.
Feldkötter M, Schwarzer V, Wirth R, Wienker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time LightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002;70:358–68.
Monani UR, Lorson CL, Parsons DW, Prior TW, Androphy EJ, Burghes AH, et al. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum Mol Genet. 1999;8:1177–83.
Lorson CL, Androphy EJ. An exonic enhancer is required for inclusion of an essential exon in the SMA-determining gene SMN. Hum Mol Genet. 2000;9:259–65.
Sumner CJ, Paushkin S, Ko C-P. Spinal muscular atrophy: disease mechanisms and therapy. Academic Press; 2016.508p.
Boyer JG, Murray LM, Scott K, De Repentigny Y, Renaud J-M, Kothary R. Early onset muscle weakness and disruption of muscle proteins in mouse models of spinal muscular atrophy. Skelet Muscle. 2013;3:24.
Boyer JG, Deguise M-O, Murray LM, Yazdani A, De Repentigny Y, Boudreau-Larivière C, et al. Myogenic program dysregulation is contributory to disease pathogenesis in spinal muscular atrophy. Hum Mol Genet. 2014;23:4249–59.
Bricceno KV, Martinez T, Leikina E, Duguez S, Partridge TA, Chernomordik LV, et al. Survival motor neuron protein deficiency impairs myotube formation by altering myogenic gene expression and focal adhesion dynamics. Hum Mol Genet. 2014;23:4745–57.
Rudnik-Schöneborn S, Heller R, Berg C, Betzler C, Grimm T, Eggermann T, et al. Congenital heart disease is a feature of severe infantile spinal muscular atrophy. J Med Genet. 2008;45:635–8.
Shababi M, Habibi J, Yang HT, Vale SM, Sewell WA, Lorson CL. Cardiac defects contribute to the pathology of spinal muscular atrophy models. Hum Mol Genet. 2010;19:4059–71.
Deguise M, Baranello G, Mastella C, Beauvais A, Michaud J, Leone A, et al. Abnormal fatty acid metabolism is a core component of spinal muscular atrophy. Ann Clin Transl Neurol. 2019;6:1519–32.
Zolkipli Z, Sherlock M, Biggar WD, Taylor G, Hutchison JS, Peliowski A, et al. Abnormal fatty acid metabolism in spinal muscular atrophy may predispose to perioperative risks. Eur J Paediatr Neurol EJPN Off J Eur Paediatr Neurol Soc. 2012;16:549–53.
Bowerman M, Swoboda KJ, Michalski J-P, Wang G-S, Reeks C, Beauvais A, et al. Glucose metabolism and pancreatic defects in spinal muscular atrophy. Ann Neurol. 2012;72:256–68.
Davis RH, Miller EA, Zhang RZ, Swoboda KJ. Responses to fasting and glucose loading in a cohort of well children with spinal muscular atrophy type II. J Pediatr. 2015;167:1362–.e1.
Deguise M-O, De Repentigny Y, McFall E, Auclair N, Sad S, Kothary R. Immune dysregulation may contribute to disease pathogenesis in spinal muscular atrophy mice. Hum Mol Genet. 2017;26:801–19.
Khairallah M-T, Astroski J, Custer SK, Androphy EJ, Franklin CL, Lorson CL. SMN deficiency negatively impacts red pulp macrophages and spleen development in mouse models of spinal muscular atrophy. Hum Mol Genet. 2017;26:932–41.
Thomson AK, Somers E, Powis RA, Shorrock HK, Murphy K, Swoboda KJ, et al. Survival of motor neurone protein is required for normal postnatal development of the spleen. J Anat. 2017;230:337–46.
Gombash SE, Cowley CJ, Fitzgerald JA, Iyer CC, Fried D, McGovern VL, et al. SMN deficiency disrupts gastrointestinal and enteric nervous system function in mice. Hum Mol Genet. 2015;24:5665.
Wang CH, Finkel RS, Bertini ES, Schroth M, Simonds A, Wong B, et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22:1027–49.
Dominguez E, Marais T, Chatauret N, Benkhelifa-Ziyyat S, Duque S, Ravassard P, et al. Intravenous scAAV9 delivery of a codon-optimized SMN1 sequence rescues SMA mice. Hum Mol Genet. 2011;20:681–93.
Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28:271–4.
Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N Engl J Med. 2017;377:1713–22.
Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65.
Novartis Gene Therapies. Phase I, Open-Label, Dose Comparison Study of AVXS-101 for Sitting But Non-ambulatory Patients With Spinal Muscular Atrophy [Internet]. clinicaltrials.gov; 2021 Jun [cited 2021 Jul 22]. Report No.: NCT03381729. Available from: https://clinicaltrials.gov/ct2/show/NCT03381729.
Bowerman M, Murray LM, Beauvais A, Pinheiro B, Kothary R. A critical smn threshold in mice dictates onset of an intermediate spinal muscular atrophy phenotype associated with a distinct neuromuscular junction pathology. Neuromuscul Disord NMD. 2012;22:263–76.
Eshraghi M, McFall E, Gibeault S, Kothary R. Effect of genetic background on the phenotype of the Smn2B/- mouse model of spinal muscular atrophy. Hum Mol Genet. 2016;25:4494–506.
Deguise M-O, Pileggi C, De Repentigny Y, Beauvais A, Tierney A, Chehade L, et al. SMN depleted mice offer a robust and rapid onset model of nonalcoholic fatty liver disease. Cell Mol Gastroenterol Hepatol. 2021;12:354–.e3.
Deguise M-O, De Repentigny Y, Tierney A, Beauvais A, Michaud J, Chehade L, et al. Motor transmission defects with sex differences in a new mouse model of mild spinal muscular atrophy. EBioMedicine. 2020;55:102750.
Murray L, Gillingwater TH, Kothary R. Dissection of the transversus abdominis muscle for whole-mount neuromuscular junction analysis. JoVE J Vis Exp. 2014;83:e51162.
Wurster CD, Steinacker P, Günther R, Koch JC, Lingor P, Uzelac Z, et al. Neurofilament light chain in serum of adolescent and adult SMA patients under treatment with nusinersen. J Neurol. 2020;267:36–44.
Murray LM, Comley LH, Thomson D, Parkinson N, Talbot K, Gillingwater TH. Selective vulnerability of motor neurons and dissociation of pre- and post-synaptic pathology at the neuromuscular junction in mouse models of spinal muscular atrophy. Hum Mol Genet. 2008;17:949–62.
Park G-H, Maeno-Hikichi Y, Awano T, Landmesser LT, Monani UR. Reduced survival of motor neuron (SMN) protein in motor neuronal progenitors functions cell autonomously to cause spinal muscular atrophy in model mice expressing the human centromeric (SMN2) gene. J Neurosci. 2010;30:12005–19.
Hua Y, Sahashi K, Rigo F, Hung G, Horev G, Bennett CF, et al. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature. 2011;478:123–6.
Hua Y, Liu YH, Sahashi K, Rigo F, Bennett CF, Krainer AR. Motor neuron cell-nonautonomous rescue of spinal muscular atrophy phenotypes in mild and severe transgenic mouse models. Genes Dev. 2015;29:288–97.
Besse A, Astord S, Marais T, Roda M, Giroux B, Lejeune F-X, et al. AAV9-mediated expression of SMN restricted to neurons does not rescue the spinal muscular atrophy phenotype in mice. Mol Ther. 2020;28:1887–901.
Meyer K, Ferraiuolo L, Schmelzer L, Braun L, McGovern V, Likhite S, et al. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose–response study in mice and nonhuman primates. Mol Ther. 2015;23:477–87.
Audic F, de la Banda MGG, Bernoux D, Ramirez-Garcia P, Durigneux J, Barnerias C, et al. Effects of nusinersen after one year of treatment in 123 children with SMA type 1 or 2: a French real-life observational study. Orphanet J Rare Dis. 2020;15:148.
Lavie M, Diamant N, Cahal M, Sadot E, Be’er M, Fattal-Valevski A, et al. Nusinersen for spinal muscular atrophy type 1: Real-world respiratory experience. Pediatr Pulmonol. 2021;56:291–8.
Valori CF, Ning K, Wyles M, Mead RJ, Grierson AJ, Shaw PJ, et al. Systemic delivery of scAAV9 expressing SMN prolongs survival in a model of spinal muscular atrophy. Sci Transl Med. 2010;2:35ra42.
Gavrilina TO, McGovern VL, Workman E, Crawford TO, Gogliotti RG, DiDonato CJ, et al. Neuronal SMN expression corrects spinal muscular atrophy in severe SMA mice while muscle-specific SMN expression has no phenotypic effect. Hum Mol Genet. 2008;17:1063–75.
Martinez TL, Kong L, Wang X, Osborne MA, Crowder ME, Van Meerbeke JP, et al. Survival motor neuron protein in motor neurons determines synaptic integrity in spinal muscular atrophy. J Neurosci Off. J Soc Neurosci. 2012;32:8703–15.
Gogliotti RG, Quinlan KA, Barlow CB, Heier CR, Heckman CJ, DiDonato CJ. Motor neuron rescue in spinal muscular atrophy mice demonstrates that sensory-motor defects are a consequence, not a cause, of motor neuron dysfunction. J Neurosci. 2012;32:3818–29.
Paez-Colasante X, Seaberg B, Martinez TL, Kong L, Sumner CJ, Rimer M. Improvement of neuromuscular synaptic phenotypes without enhanced survival and motor function in severe spinal muscular atrophy mice selectively rescued in motor neurons. PLoS ONE. 2013;8:e75866.
Didonna A, Opal P. The role of neurofilament aggregation in neurodegeneration: lessons from rare inherited neurological disorders. Mol Neurodegener. 2019;14:19.
de Waegh SM, Lee VM, Brady ST. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell. 1992;68:451–63.
Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci. 2009;10:519–29.
Hess PL, Al‐Khalidi HR, Friedman DJ, Mulder H, Kucharska‐Newton A, Rosamond WR, et al. The metabolic syndrome and risk of sudden cardiac death: the atherosclerosis risk in communities study. J Am Heart Cardiovasc. 2017;6:e006103.
Van Alstyne M, Tattoli I, Delestrée N, Recinos Y, Workman E, Shihabuddin LS, et al. Gain of toxic function by long-term AAV9-mediated SMN overexpression in the sensorimotor circuit. Nat Neurosci. 2021;24:930–40.
Acknowledgements
The authors thank Dr. Ronald Booth and Adrienne Rowan from the Eastern Ontario Regional Laboratory Association at The Ottawa Hospital for assistance with some of the NfL assays. RK was supported by Muscular Dystrophy Association (USA) (grant number 575466); Muscular Dystrophy Canada; and Canadian Institutes of Health Research (CIHR) (grant number PJT-156379). AR was supported by a CNMD STAR Award from the University of Ottawa Brain and Mind Institute. MOD was supported by Frederick Banting and Charles Best CIHR Doctoral Research Award.
Author information
Authors and Affiliations
Contributions
A.R. and R.K. designed research. A.R., M.O.D., A.B., R.Y., S.T., and D.R.T. performed experiments. B.L.S. and V.T.C. provided material support. A.R., M.O.D., and N.H. analyzed the data. A.R., N.H., and R.K. wrote the manuscript with input from all authors. R.K. designed the study.
Corresponding author
Ethics declarations
Competing interests
R.K. received honoraria and travel accommodations from Roche as an invited speaker at their global and national board meetings in 2019. R.K. and the Ottawa Hospital Research Institute have a licensing agreement with Biogen for the Smn2B/− mouse model. MOD received honoraria and travel accommodations from Biogen for speaking engagements at the SMA Summit 2018 held in Montreal, Canada and SMA Academy 2019 held in Toronto, Canada. These COIs are outside the scope of this study. All other authors have no competing interests to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Reilly, A., Deguise, MO., Beauvais, A. et al. Central and peripheral delivered AAV9-SMN are both efficient but target different pathomechanisms in a mouse model of spinal muscular atrophy. Gene Ther 29, 544–554 (2022). https://doi.org/10.1038/s41434-022-00338-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-022-00338-1
This article is cited by
-
Neurofilaments as biomarkers in neurological disorders — towards clinical application
Nature Reviews Neurology (2024)
-
Optimization of base editors for the functional correction of SMN2 as a treatment for spinal muscular atrophy
Nature Biomedical Engineering (2023)