Abstract
In this study, we demonstrated that plasma collected from women who subsequently developed preeclampsia caused increased heme oxygenase-1 (HO-1) production and decreased levels of nitric oxide (NO) markers in endothelial cells (HUVECs). Conversely, no changes in HO-1 or NO markers were found when HUVECs were treated with plasma from women who remained healthy throughout pregnancy. These alterations in HO-1 and NO markers were prevented by cotreatment with the polyphenol resveratrol, which also improved GSH levels. In addition, we evaluated changes induced by plasma incubation in the expression of genes and their related pathways associated with antioxidant defenses, such as Nrf2, ARE activity, and GSR. Collectively, our findings suggest that even before the appearance of clinical symptoms of preeclampsia, plasma from affected women is able to induce modifications in endothelial cells with respect to HO-1 production and NO markers. We believe that this in vitro strategy may offer an attractive alternative to the exploitation of candidate markers or screening molecules, such as resveratrol, for the prevention and management of preeclampsia.
Similar content being viewed by others
Introduction
Preeclampsia is a pregnancy-related hypertensive disorder characterized by hypertension after 20 weeks of gestation accompanied by proteinuria, thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, or cerebral/visual symptoms [1]. It is a leading cause of maternal and fetal morbidity and mortality worldwide, and the only treatment is delivery of the placenta [2,3,4]. A common feature in preeclampsia etiology is an oxidative stress imbalance, which contributes to decreased nitric oxide (NO) bioavailability and endothelial dysfunction associated with this syndrome [5,6,7,8,9,10]. This characteristic justifies the interest in using antioxidants such as vitamins and phytochemicals in the prevention or treatment of preeclampsia [7, 11]. However, clinical trials conducted to verify whether supplementation with antioxidants, such as vitamin C and E, could reduce the risk of preeclampsia development have yielded no convincing evidence [12, 13]. Conversely, polyphenol resveratrol has been studied in preeclampsia [14,15,16,17,18], showing promising results. One study showed that resveratrol is a safe and effective adjuvant of oral nifedipine for attenuating hypertensive symptoms among preeclamptic patients [14], while other investigations showed that resveratrol can improve trophoblast and endothelial dysfunction in human cells [15,16,17,18], suggesting it may have potential in the treatment and prevention of preeclampsia.
Resveratrol is found in a variety of fruits, and its most common source is the red grape. In addition to being an antioxidant, resveratrol modulates several targets through physical interaction or indirect effects, such as activation of endothelial nitric oxide synthase (eNOS) and the nuclear factor-erythroid-derived 2-related factor-2 (Nrf2) [19]. Nrf2 binds to the antioxidant response element (ARE) promoter sequence, regulating the expression of antioxidant proteins, including heme oxygenase-1 (HO-1) and glutathione reductase (GSR), which are able to counter oxidative stress and balance the redox state in cells [20].
Incubation of endothelial cells with plasma/serum from preeclamptic women is a well-established in vitro model of the two stages of preeclampsia that supports a better understanding of the molecular mechanisms of endothelial dysfunction in this syndrome [6, 8, 21,22,23]. Interestingly, plasma from pregnant women collected early before the development of preeclampsia has been shown to modulate gene expression in endothelial cells [24] and impair the endothelial function of myometrial vessels [25].
In this regard, the present study aimed to investigate the effect of resveratrol incubated with plasma collected from women before the development of preeclampsia on modulating key antioxidant defenses and vasodilator factors, such as Nrf2, HO-1, GSR, glutathione (GSH), and NO in endothelial cells. We hypothesized that resveratrol could reduce oxidative stress by improving these pathways in endothelial cells even before the manifestation of clinical symptoms of preeclampsia.
Materials and methods
Study population
This study is part of a broader observational study performed in two Brazilian cities (Brazilian Ribeirao Preto and São Luis Birth Cohort Studies - BRISA) [26,27,28]. The study was approved by the Institutional Review Board at Ribeirao Preto Medical School, Brazil (reference 4116/2008, approval date 11 November 2008), following the principles of the Declaration of Helsinki, and all subjects gave written informed consent. A total of 1417 pregnant women at 20–25 weeks of gestation were evaluated at Hospital das Clinicas of Ribeirao Preto–University of Sao Paulo. Of these women, 17 did not return, and 460 gave birth in other units. Of the 940 pregnant women remaining, 30 developed preeclampsia (cases), of which 14 were classified as severe cases. For this in vitro study, six samples of women who developed severe preeclampsia and six samples of those who remained healthy throughout pregnancy (controls) were randomly selected. Diagnosis and severity criteria of preeclampsia were defined by the American College of Obstetricians and Gynecologists [1]. Maternal venous blood samples were collected in tubes containing EDTA. The tubes were rapidly centrifuged (1000 × g for 3 min) at room temperature, and plasma samples were stored at −80 °C.
Cell culture, plasma incubation, and trans-resveratrol intervention
Human umbilical vein endothelial cells (HUVEC) (CRL 2873, American Type Culture Collection (ATCC), VA, USA) were cultured in DMEM (Gibco, CA, USA) supplemented with 10% (v/v) fetal calf serum (FCS) (Gibco), 50 μg/ml penicillin, 50 μg/ml streptomycin, and 0.5 μg/ml amphotericin B (Gibco) at 37 °C in an incubator with a 5% CO2 atmosphere. After reaching 80–90% confluence, HUVECs were incubated in medium containing 10% (v/v) plasma from case and control patients and 1 μM of trans-resveratrol (Cayman Chemical®, MI, USA), the most predominant isoform responsible for many health benefits [29, 30], or the vehicle DMSO (Sigma-Aldrich®, Poole, UK) for 24 h in an incubator. The concentration of 1 μM was chosen based on literature research from studies using resveratrol in HUVECs [31,32,33]. Cells were used until the eighth passage.
Cell viability
After 24 h of plasma incubation from cases and controls in the presence or absence of trans-resveratrol in HUVECs, cell viability was assessed using a 3-(4,5-dimethylthiazol2-yl)−2,5-diphenyltetrazolium (MTT) assay as described previously [34]. MTT is reduced to blue formazan crystals by metabolically active cells. MTT solution (0.5 mg/ml PBS) (Sigma-Aldrich) was added to a 96-well plate, and the plate was incubated in the incubator for 3 h. Then, the MTT solution was removed, and DMSO (Sigma-Aldrich®) was added for 10 min. The optical density was measured at 570 nm on a multifunctional plate reader (Synergy 4, BioTek, VT, USA). DMSO alone was used as a blank. Each sample was performed in triplicate. Viability was compared to the control (untreated cells with vehicle DMSO, 100% viability).
Messenger RNA expression by qPCR
Total RNA from HUVECs incubated with plasma from cases and controls in the presence or absence of trans-resveratrol was isolated using a miRNeasy Micro Kit (Qiagen, Leusden, Netherlands) according to the manufacturer’s protocol. Isolated RNA from the samples was quantified using a NanoDrop Spectrophotometer (Thermo Scientific, MA, USA) and was consistently found to be pure. RNA was transcribed into cDNA using a High-Capacity cDNA Reverse Transcription Kit (Life Technologies, ON, Canada) according to the manufacturer’s protocol. qPCR was performed using Luna Universal qPCR Master Mix (New England BioLabs®, MA, USA). Each reaction contained 5 μL Luna Universal qPCR Master Mix, 100 nM of each primer (forward and reverse), 5 μL of cDNA (6.5 ng/μL), and a variable amount of nuclease-free water to yield a final volume of 10 μL. The following genes were analyzed: NFE2L2, HMOX1, GSR, and HPRT1. KiCqStart™ SYBR® Green Primers of the mentioned genes were purchased from Sigma-Aldrich. The HPRT1 gene was chosen as an endogenous control because it was the gene most stable in our samples according to the previous study [20]. Thermal cycling was performed under the following conditions: 1 min at 95 °C, 40 two-step cycles of 15 s at 95 °C and 60 s at 60 °C, and a final step for the dissociation curve. Relative expression was calculated using the comparative 2 (-Delta C(T)) method [35]. All PCRs were performed in duplicate for each sample.
Measurement of nuclear Nrf2
HUVECs were seeded into 25 cm2 bottles at a concentration of 0.7×106 cells. After reaching confluence and receiving the plasma and resveratrol intervention, the nuclear extract was separated and collected using a Nuclear Extraction Kit (Cayman Chemical®), according to the manufacturer’s protocol. Nrf2 quantification in the nuclear extract was assessed by ELISA using a Nrf2 Transcription Factor Assay Kit (Cayman Chemical®). First, samples and controls were added to a 96-well plate for overnight incubation. The next day, the plate was washed five times with wash buffer and incubated with a primary antibody for 1 h. Washes were repeated, and the second antibody was incubated for 1 h. After incubation, the washes were repeated, and a developing solution was added for 45 min under gentle agitation and protected from light. Then, stop solution was added, and the plate was read at 450 nm in a spectrophotometer (Synergy 4, BioTek®).
Antioxidant response element activation analyzed by a luciferase reporter assay
Antioxidant response element (ARE) activation was verified using an ARE Reporter Kit (BPS Bioscience, CA, USA). In brief, HUVECs were seeded at a concentration of 1×104 cells per well into a white 96-well plate. After reaching confluence, cells were transfected using Lipofectamine® 2000 (Thermo Scientific) with the ARE reporter or negative control reporter for 12 h. Then, the cells were incubated with plasma samples from the control and case groups and in the presence or absence of resveratrol. After 24 h of incubation, luminescence was measured in a multifunctional plate reader (Synergy 4, BioTek®) using the Dual-Glo® Luciferase Assay System (Promega, WI, USA). Trans-resveratrol was added at a concentration of 1 μM for 24 h (fold=1) as a positive control.
Measurement of HO-1 concentrations
HO-1 concentrations in cell supernatants were measured using the enzyme-linked immunosorbent assay kit Human Total HO-1/HMOX1 ELISA (R&D Systems, MN, USA) according to the manufacturer’s protocol. In brief, 50 μL of capture antibody was added to a half area of a 96-well plate and incubated overnight. Then, the plate was washed three times with washing buffer, and 50 μL of blocking buffer was added for 1–2 h. Washes were repeated, and 50 μL of standards and samples were added for 2 h. After incubation, the washes were repeated, and 50 μL of the detection antibody was added and incubated for 2 h. After the washes, 50 μL of streptavidin-HRP was added and incubated for 20 min. Then, final washes were performed, and 50 μL of substrate solution was added and incubated for more than 20 min; 25 μL of stop solution was added, and the plate was read at 450 nm in a spectrophotometer (Synergy 4, BioTek®). A standard curve was generated by the incubation of HO-1 solutions (156.25–100,00 pg/mL) with the previous reagents. The HO-1 concentration is expressed in pg/mL.
Measurement of nitrite by a Griess assay
Nitrite levels were assessed in the cell supernatants using Griess reagents [36]. In brief, 50 μL of each sample was added to a 96-well plate with 50 μL of 1% sulfanilamide solution in 5% phosphoric acid for 10 min protected from light. Then, 50 μL of 0.1% N- (1-naphthyl)-ethylenediamine dihydrochloride solution was added and incubated for 10 min. The plate was read at 540 nm in a spectrophotometer (Synergy 4, BioTek®). A standard curve was generated by incubation of nitrite solutions (0.39–50 μM) with the previous reagents. The nitrite concentration is expressed in μM. Each sample was performed in duplicate.
Measurement of glutathione levels
Total GSH levels were measured in cell lysates using a Glutathione Colorimetric Detection Kit (Invitrogen, MD, USA) according to the manufacturer’s protocol. For this assay, plasma samples from each group were pooled and incubated in HUVECs in a 12-well plate. In brief, 50 μL of each sample was added to a half-area 96-well plate with 25 μL of Colorimetric Detection Reagent and 25 μL of Reaction Mixture. Then, the plate was incubated at room temperature for 20 min. The plate was read at 405 nm in a spectrophotometer (Synergy 4, BioTek®). A standard curve was generated by the incubation of oxidized glutathione standard solutions (0.78–25 μM) with the previous reagents. GSH levels are expressed in μM. Each sample was performed in duplicate.
Measurement of intracellular ROS levels
The levels of intracellular ROS levels were evaluated by measuring the fluorescence of 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) (Cayman Chemical®). DCFH-DA is deacetylated by cellular esterases to a nonfluorescent compound, which is later oxidized by ROS into the highly fluorescent compound 2,7-dichlorofluorescein (DCF). In brief, after 24 h of plasma and trans-resveratrol incubation, HUVECs in a 96-well plate were incubated with 25 μM of DCFH-DA in PBS for 45 min in an incubator. Fluorescence was read on a multifunctional plate reader (Synergy 4, BioTek®) using excitation and emission wavelengths of 502 and 523 nm, respectively. Tert-butyl-hydroperoxide solution (Sigma-Aldrich®) at 250 μM for 2 h was used as a positive control. Each sample was performed in duplicate.
Statistical analysis
For comparison between the case and control groups, t-tests were performed, and for comparison within each group between the presence and the absence of trans-resveratrol, we applied a t-test. These statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, CA, USA). For all tests, a p value ≤0.05 (two-tailed) was considered significant.
Results
The study workflow is shown in Fig. 1. The clinical characteristics of the women enrolled in the study are shown in Table 1. At the time of blood collection, all the clinical characteristics were similar between the groups. In the case group, the gestational age (GA) at delivery was significantly lower than that for controls.
First, we verified the cell viability of HUVECs incubated with plasma from the control group (those who remained healthy throughout pregnancy) and the case group (pregnant women who developed preeclampsia), in the absence (−R) and presence (+R) of 1 μM trans-resveratrol. No significant differences in cell viability among groups were found, independently of the addition of resveratrol (all p > 0.05).
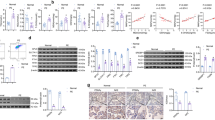
Next, we verified the gene expression of NFE2L2, HMOX1, and GSR in HUVECs (Fig. 1). For NFE2L2 (Fig. 2a) and HMOX1 expression (Fig. 2b), there was no significant difference between the groups, and resveratrol did not alter the expression. Regarding GSR expression (Fig. 2c), there was no difference between the groups, and resveratrol significantly decreased the expression only in the control group. Moreover, HMOX1 and GSR expression was higher in the case group with resveratrol than in controls receiving the same treatment.
Relative expression of the genes NFE2L2 (a), HMOX1 (b), and GSR (c) in HUVECs. HUVECs were incubated with 10% (v/v) plasma samples from pregnant women who subsequently developed preeclampsia (case) and who remained healthy during gestation (control) (n = 6 per group) for 24 h in the absence (−R) and presence of trans-resveratrol (+R) at 1 μM. Resveratrol significantly decreased GSR expression in the control group. HMOX1 and GSR expression was higher in the case group with resveratrol than in the controls with the same treatment. DMSO was used as a vehicle for trans-resveratrol. Values were normalized using HPRT1 as an endogenous control. Values are expressed as the mean ± S.E.M. #p ≤ 0.05 vs. respective group –R; †p ≤ 0.05 vs. control group+R
To examine the Nrf2 pathway, Nrf2 expression in the nucleus (Fig. 3a) and ARE activity (Fig. 3b) were assessed. Plasma from the case group did not alter Nrf2 expression or ARE activity compared with that in the controls; however, resveratrol increased the Nrf2 activity by ~15% and the ARE activity by ~72% only in the control group.
Nrf2 expression in the nucleus (a) and ARE activity (b). HUVECs were incubated with 10% (v/v) plasma samples from pregnant women who subsequently developed preeclampsia (cases) and who remained healthy during gestation (controls) (n = 6 per group) for 24 h in the absence (−R) or presence of trans-resveratrol (+R) at 1 μM. Resveratrol increased Nrf2 expression and ARE activity only in the control group. DMSO was used as a vehicle for trans-resveratrol. Values are expressed as the mean ± S.E.M. #p ≤ 0.05 vs. respective group –R
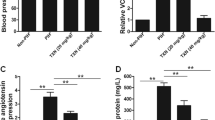
The HO-1 concentration in the cell supernatant (Fig. 4a) was increased ~2.4-fold in cultures incubated with plasma from the case group compared with that in the control. Furthermore, in the case group, resveratrol decreased the HO-1 concentration by ~15%, but this concentration did not change in the control group. In the case group treated with resveratrol, we observed a higher HO-1 concentration than that in the controls with the same treatment.
HO-1 levels (a) and nitrite concentration (b) in cell supernatants, total GSH in cell lysates (c) and intracellular ROS levels (d). HUVECs were incubated with 10% (v/v) plasma samples from pregnant women who subsequently developed preeclampsia (cases) and who remained healthy during gestation (controls) (n = 6 per group) for 24 h in the absence (−R) or presence of trans-resveratrol (+R) at 1 μM. For ROS levels, the mean and standard error for the positive control was 4.45 ± 0.23 square root of fluorescence intensity ×103. Plasmas from the case group increased the HO-1 concentration and decreased the nitrite levels compared with those in the controls. Resveratrol decreased the HO-1 concentration and increased the nitrite and glutathione levels in the case group. The HO-1 concentration was higher in the case group with resveratrol than in the controls with the same treatment. DMSO was used as a vehicle for trans-resveratrol. Values are expressed as the mean±S.E.M. *p ≤ 0.05 vs. control –R; #p ≤ 0.05 vs. respective group –R; †p ≤ 0.05 vs. control group+R
The nitrite level (Fig. 4b) was significantly decreased by ~25% in the case group compared with that in the control. However, only in the case group was resveratrol able to increase the nitrite levels.
We also investigated the total GSH concentration in cell lysates (Fig. 4c). There was no significant difference between the control and case groups in the absence of resveratrol. Resveratrol increased the GSH concentration in the case group but not in the control group.
Intracellular ROS levels (Fig. 4d) were not significantly different between groups, and the addition of resveratrol did not alter ROS levels. The positive control was 4.45 ± 0.23 square root of fluorescence intensity ×103.
Discussion
In this study, we showed for the first time that (1) plasma collected from women who subsequently develop preeclampsia (case group) was able to increase HO-1 production and decrease NO markers in HUVECs, while (2) no changes in HO-1 or NO markers were found when HUVECs were treated with plasma from women who remained healthy throughout pregnancy (control). (3) These alterations in HO-1 and NO markers were prevented by cotreatment with polyphenol resveratrol, which was followed by an improvement in GSH levels. In addition, we found that resveratrol increased NRF2 expression and ARE activity in cells incubated with plasma from controls but not from cases. Moreover, in the case group, resveratrol increased the gene/protein expression of HO-1 and GSR expression compared with that in the control group with the same treatment. Collectively, these findings suggest that even before the appearance of clinical symptoms of preeclampsia, plasma from the women is able to induce modifications in endothelial cells through these mechanisms and that resveratrol is able to prevent this induction.
HO-1 catalyzes heme degradation into carbon monoxide, free iron, and biliverdin and is further degraded to bilirubin, which has antioxidative properties. Recently, the role of HO-1 has been studied in preeclampsia [37,38,39]. HO-1 inhibition stimulated soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng) production in endothelial cells; both of these antiangiogenic markers are implicated in preeclampsia pathophysiology [39]. We showed that plasma from the case group stimulated HO-1 production by HUVECs, and because we used an immortalized healthy cell line, this finding may reflect a protective mechanism of these cells against circulating factors, including oxidative stress inducers, even before development of the syndrome. Indeed, studies have already shown that oxidative stress markers, such as malondialdehyde (MDA) and oxidized low-density lipoprotein (oxLDL), are increased in serum collected in the first trimester from women who develop preeclampsia [40,41,42]. In our study, we did not observe a difference in ROS production by cells incubated with plasma from the case group compared with that in the control group, which also suggests that a protective mechanism might occur, probably by HO-1 induction and not by glutathione, as we observed. As mentioned before, we used an immortalized healthy cell line of HUVECs, which is a limitation of the study since it is known that even before preeclampsia development, the maternal endothelium is already impaired [43, 44].
It is well known that in preeclampsia, NO bioavailability is deficient and contributes to development of the hypertension characteristic of this syndrome [45,46,47,48]. More interestingly, in serum/plasma from women in the first trimester of pregnancy, before the manifestation of the clinical symptoms of preeclampsia, the NO concentration was decreased compared with that in healthy controls during the first trimester of pregnancy [45, 49]. Our in vitro results also demonstrated that the NO concentration was decreased in endothelial cell supernatants incubated with plasma from the case group compared with the control group. The mechanisms involved in this decrease could be as follows: (1) the presence of ROS in plasma from these women, which could scavenge NO; [7] (2) an antiangiogenic factor, such as sFlt-1 and sEng, which is elevated in serum from women before preeclampsia development [50, 51] and could inhibit NO formation [48] and (3) asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthase that is also elevated before preeclampsia development [52].
As a potent antioxidant with several targets, resveratrol has been extensively studied as a possible therapeutic tool and for the prevention of cancer [53], diabetes [54], obesity [55], cardiovascular diseases [56], and disorders in pregnancy [57,58,59]. For instance, perinatal resveratrol supplementation is able to prevent the development of hypertension and improve vascular function in adult spontaneously hypertensive rat offspring [60]. Roberts et al. [61]. demonstrated that resveratrol use during pregnancy in nonhuman primates improves some maternal factors (maternal weight loss, improved glucose tolerance, increased uterine artery blood flow volume, and decreased placental inflammation, and liver triglyceride deposition); however, resveratrol enlarged the fetal pancreatic mass by 42%. This finding raises concerns for resveratrol use by pregnant women; nonetheless, it is difficult to know if this effect would occur in humans, as noted by Hannan et al. [16].
In preeclampsia, although the results might be promising, few studies have investigated the role of resveratrol [14,15,16,17,18], and to the best of our knowledge, no study has focused on prevention. Our findings showed that the addition of resveratrol incubated with plasma from the case group decreased the HO-1 concentration and increased the NO and GSH concentrations. In fact, it was demonstrated in endothelial cells with an oxidative environment that resveratrol is able to increase NO bioavailability [19] and stimulate antioxidant defenses [62, 63], such as the GSH content. Regarding the decrease in the HO-1 concentration caused by resveratrol, we suggest that since resveratrol can modulate targets directly and activate other protective mechanisms, HO-1 induction was probably not triggered. We also found that resveratrol did not induce Nrf2 activation to the nucleus or ARE activity in the case group, which supports this hypothesis. In the control group, resveratrol was able to activate Nrf2 and ARE; however, these activations did not alter the HO-1 concentration. In the current study, we also observed that resveratrol incubated with plasma from the case group increased the gene and protein expression of HO-1 and GSR compared with that in controls with the same treatment. ROS levels were not altered by resveratrol in either group, which was not expected as it is well established that resveratrol scavenges ROS [64]. As a limitation of our study, this lack of a difference may be due to the low concentration of resveratrol that was used and/or the fact that the time of incubation was not enough to evidence this difference.
In conclusion, plasma from women who subsequently develop preeclampsia appears to contain factors that lead to alterations in HO-1 and NO markers in endothelial cells. Moreover, we showed that resveratrol was able to increase NO and GSH levels when incubated with plasma from these women. We believe that the understanding of these early events using our in vitro strategy may offer an attractive alternative to exploiting candidate markers for the prevention and management of preeclampsia, as we have demonstrated for resveratrol in the present study.
References
American College of Obstetricians and Gynecologists, Task Force on Hypertension in pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–31.
Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–7.
Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403.
Dekker GA. Management of preeclampsia. Pregnancy Hypertens. 2014;4:246–7.
McCarthy C, Kenny LC. Therapeutically targeting mitochondrial redox signalling alleviates endothelial dysfunction in preeclampsia. Sci Rep. 2016;6:32683.
Scalera F, Fischer T, Schlembach D, Beinder E. Serum from healthy pregnant women reduces oxidative stress in human umbilical vein endothelial cells. Clin Sci. 2002;103:53–7.
Williamson RD, McCarthy C, McCarthy FP, Kenny LC. Oxidative stress in pre-eclampsia; have we been looking in the wrong place? Pregnancy Hypertens. 2017;8:1–5.
Sankaralingam S, Xu H, Davidge ST. Arginase contributes to endothelial cell oxidative stress in response to plasma from women with preeclampsia. Cardiovasc Res. 2010;85:194–203.
English FA, McCarthy FP, McSweeney CL, Quon AL, Morton JS, Sawamura T, et al. Inhibition of lectin-like oxidized low-density lipoprotein-1 receptor protects against plasma-mediated vascular dysfunction associated with pre-eclampsia. Am J Hypertens. 2013;26:279–86.
Chen J, Gao Q, Jiang L, Feng X, Zhu X, Fan X, et al. The NOX2-derived reactive oxygen species damaged endothelial nitric oxide system via suppressed BKCa/SKCa in preeclampsia. Hypertens Res. 2017;40:457–64.
Shi DD, Guo JJ, Zhou L, Wang N. Epigallocatechin gallate enhances treatment efficacy of oral nifedipine against pregnancy-induced severe pre-eclampsia: A double-blind, randomized and placebo-controlled clinical study. J Clin Pharm Ther. 2018;43:21–5.
Rumbold A, Ota E, Hori H, Miyazaki C, Crowther CA. Vitamin E supplementation in pregnancy. Cochrane Database Syst Rev. 2015: CD004069.
Rumbold A, Ota E, Nagata C, Shahrook S, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database Syst Rev. 2015: CD004072.
Ding J, Kang Y, Fan Y, Chen Q. Efficacy of resveratrol to supplement oral nifedipine treatment in pregnancy-induced preeclampsia. Endocr Connect. 2017;6:595–600.
Gurusinghe S, Cox AG, Rahman R, Chan ST, Muljadi R, Singh H, et al. Resveratrol mitigates trophoblast and endothelial dysfunction partly via activation of nuclear factor erythroid 2-related factor-2. Placenta. 2017;60:74–85.
Hannan NJ, Brownfoot FC, Cannon P, Deo M, Beard S, Nguyen TV, et al. Resveratrol inhibits release of soluble fms-like tyrosine kinase (sFlt-1) and soluble endoglin and improves vascular dysfunction - implications as a preeclampsia treatment. Sci Rep. 2017;7:1819.
Al-Ani B. Resveratrol inhibits proteinase-activated receptor-2-induced release of soluble vascular endothelial growth factor receptor-1 from human endothelial cells. EXCLI J. 2013;12:598–604.
Cudmore MJ, Ramma W, Cai M, Fujisawa T, Ahmad S, Al-Ani B, et al. Resveratrol inhibits the release of soluble fms-like tyrosine kinase (sFlt-1) from human placenta. Am J Obstet Gynecol. 2012;206:e10–5.
Xia N, Forstermann U, Li H. Resveratrol and endothelial nitric oxide. Molecules. 2014;19:16102–21.
Kweider N, Huppertz B, Kadyrov M, Rath W, Pufe T, Wruck CJ. A possible protective role of Nrf2 in preeclampsia. Ann Anat. 2014;196:268–77.
Caldeira-Dias M, Luizon MR, Deffune E, Tanus-Santos JE, Freire PP, Carvalho RF, et al. Preeclamptic plasma stimulates the expression of miRNAs, leading to a decrease in endothelin-1 production in endothelial cells. Pregnancy Hypertens. 2018;12:75–81.
Sandrim VC, Dias MC, Bovolato AL, Tanus-Santos JE, Deffune E, Cavalli RC. Plasma from pre-eclamptic patients induces the expression of the anti-angiogenic miR-195-5p in endothelial cells. J Cell Mol Med. 2016;20:1198–200.
Calicchio R, Buffat C, Mathieu JR, Ben Salem N, Mehats C, Jacques S, et al. Preeclamptic plasma induces transcription modifications involving the AP-1 transcriptional regulator JDP2 in endothelial cells. Am J Pathol. 2013;183:1993–2006.
Mackenzie RM, Sandrim VC, Carty DM, McClure JD, Freeman DJ, Dominiczak AF, et al. Endothelial FOS expression and pre-eclampsia. BJOG. 2012;119:1564–71.
Myers J, Mires G, Macleod M, Baker P. In preeclampsia, the circulating factors capable of altering in vitro endothelial function precede clinical disease. Hypertension. 2005;45:258–63.
da Silva AA, Simoes VM, Barbieri MA, Cardoso VC, Alves CM, Thomaz EB, et al. A protocol to identify non-classical risk factors for preterm births: the Brazilian Ribeirao Preto and Sao Luis prenatal cohort (BRISA). Reprod Health. 2014;11:79.
Pereira TB, Thomaz EB, Nascimento FR, Santos AP, Batista RL, Bettiol H, et al. Regulatory cytokine expression and preterm birth: case-control study nested in a cohort. PLoS ONE. 2016;11:e0158380.
Rocha-Penha L, Bettiol H, Barbieri MA, Cardoso VC, Cavalli RC, Sandrim VC. Myeloperoxidase is not a good biomarker for preeclampsia prediction. Sci Rep. 2017;7:10257.
Omar JM, Yang H, Li S, Marquardt RR, Jones PJ. Development of an improved reverse-phase high-performance liquid chromatography method for the simultaneous analyses of trans-/cis-resveratrol, quercetin, and emodin in commercial resveratrol supplements. J Agric Food Chem. 2014;62:5812–7.
Anisimova NY, Kiselevsky MV, Sosnov AV, Sadovnikov SV, Stankov IN, Gakh AA. Trans-, cis-, and dihydro-resveratrol: a comparative study. Chem Cent J. 2011;5:88.
Yang J, Wang N, Li J, Zhang J, Feng P. Effects of resveratrol on NO secretion stimulated by insulin and its dependence on SIRT1 in high glucose cultured endothelial cells. Endocrine. 2010;37:365–72.
Takizawa Y, Kosuge Y, Awaji H, Tamura E, Takai A, Yanai T, et al. Up-regulation of endothelial nitric oxide synthase (eNOS), silent mating type information regulation 2 homologue 1 (SIRT1) and autophagy-related genes by repeated treatments with resveratrol in human umbilical vein endothelial cells. Br J Nutr. 2013;110:2150–5.
Boncler M, Rozalski M, Krajewska U, Podsedek A, Watala C. Comparison of PrestoBlue and MTT assays of cellular viability in the assessment of anti-proliferative effects of plant extracts on human endothelial cells. J Pharmacol Toxicol Methods. 2014;69:9–16.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Dias-Junior CA, Neto-Neves EM, Montenegro MF, Tanus-Santos JE. Hemodynamic effects of inducible nitric oxide synthase inhibition combined with sildenafil during acute pulmonary embolism. Nitric Oxide. 2010;23:284–8.
Tong S, Kaitu’u-Lino TJ, Onda K, Beard S, Hastie R, Binder NK, et al. Heme oxygenase-1 is not decreased in preeclamptic placenta and does not negatively regulate placental soluble fms-Like tyrosine kinase-1 or soluble endoglin secretion. Hypertension. 2015;66:1073–81.
George EM, Granger JP. Heme oxygenase in pregnancy and preeclampsia. Curr Opin Nephrol Hypertens. 2013;22:156–62.
Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–97.
Genc H, Uzun H, Benian A, Simsek G, Gelisgen R, Madazli R, et al. Evaluation of oxidative stress markers in first trimester for assessment of preeclampsia risk. Arch Gynecol Obstet. 2011;284:1367–73.
D’Souza V, Rani A, Patil V, Pisal H, Randhir K, Mehendale S, et al. Increased oxidative stress from early pregnancy in women who develop preeclampsia. Clin Exp Hypertens. 2016;38:225–32.
Asiltas B, Surmen-Gur E, Uncu G. Prediction of first-trimester preeclampsia: Relevance of the oxidative stress marker MDA in a combination model with PP-13, PAPP-A and beta-HCG. Pathophysiology. 2018;25:131–5.
Brandao AH, Felix LR, Patricio Edo C, Leite HV, Cabral AC. Difference of endothelial function during pregnancies as a method to predict preeclampsia. Arch Gynecol Obstet. 2014;290:471–7.
Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122:478–87.
Matsubara K, Matsubara Y, Hyodo S, Katayama T, Ito M. Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. J Obstet Gynaecol Res. 2010;36:239–47.
Matsubara K, Higaki T, Matsubara Y, Nawa A. Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. Int J Mol Sci. 2015;16:4600–14.
Khalil A, Hardman L, OB P. The role of arginine, homoarginine and nitric oxide in pregnancy. Amino Acids. 2015;47:1715–27.
Sandrim VC, Palei AC, Metzger IF, Gomes VA, Cavalli RC, Tanus-Santos JE. Nitric oxide formation is inversely related to serum levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluble endogline in preeclampsia. Hypertension. 2008;52:402–7.
Teran E, Escudero C, Vivero S, Molina G, Calle A. NO in early pregnancy and development of preeclampsia. Hypertension. 2006;47:e17.
Bian Z, Shixia C, Duan T. First-trimester maternal serum levels of sFLT1, PGF and ADMA predict preeclampsia. PLoS ONE. 2015;10:e0124684.
Baumann MU, Bersinger NA, Mohaupt MG, Raio L, Gerber S, Surbek DV. First-trimester serum levels of soluble endoglin and soluble fms-like tyrosine kinase-1 as first-trimester markers for late-onset preeclampsia. Am J Obstet Gynecol. 2008;199:266 e1–6.
Boger RH, Diemert A, Schwedhelm E, Luneburg N, Maas R, Hecher K. The role of nitric oxide synthase inhibition by asymmetric dimethylarginine in the pathophysiology of preeclampsia. Gynecol Obstet Invest. 2010;69:1–13.
Singh CK, Ndiaye MA, Ahmad N. Resveratrol and cancer: challenges for clinical translation. Biochim Biophys Acta. 2015;1852:1178–85.
Szkudelski T, Szkudelska K. Resveratrol and diabetes: from animal to human studies. Biochim Biophys Acta. 2015;1852:1145–54.
Aguirre L, Fernandez-Quintela A, Arias N, Portillo MP. Resveratrol: anti-obesity mechanisms of action. Molecules. 2014;19:18632–55.
Bonnefont-Rousselot D. Resveratrol and cardiovascular diseases. Nutrients. 2016;8:E250.
Singh CK, Kumar A, Lavoie HA, Dipette DJ, Singh US. Diabetic complications in pregnancy: is resveratrol a solution? Exp Biol Med. 2013;238:482–90.
Furuya H, Taguchi A, Kawana K, Yamashita A, Inoue E, Yoshida M, et al. Resveratrol protects against pathological preterm birth by suppression of macrophage-mediated inflammation. Reprod Sci. 2015;22:1561–8.
Bariani MV, Correa F, Leishman E, Rubio APD, Arias A, Stern A, et al. Resveratrol protects from lipopolysaccharide-induced inflammation in the uterus and prevents experimental preterm birth. Mol Hum Reprod. 2017;23:571–81.
Bhatt SR, Lokhandwala MF, Banday AA. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur J Pharmacol. 2011;667:258–64.
Roberts VH, Pound LD, Thorn SR, Gillingham MB, Thornburg KL, Friedman JE, et al. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J. 2014;28:2466–77.
Sayin O, Arslan N, Guner G. The protective effects of resveratrol on human coronary artery endothelial cell damage induced by hydrogen peroxide in vitro. Acta Clin Croat. 2012;51:227–35.
Liu L, Gu L, Ma Q, Zhu D, Huang X. Resveratrol attenuates hydrogen peroxide-induced apoptosis in human umbilical vein endothelial cells. Eur Rev Med Pharm. 2013;17:88–94.
Truong VL, Jun M, Jeong WS. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors. 2018;44:36–49.
Acknowledgements
We thank Naiara Cinegaglia for assistance with the cell culture experiments. This work was supported by the National Council for Scientific and Technological Development (CNPq-Brazil) [Grant Number #2014-5/305587], by the São Paulo Research Foundation (FAPESP-Brazil) [Grant Numbers #2008/53593-0, #2015/20461-8], and in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Caldeira-Dias, M., Montenegro, M.F., Bettiol, H. et al. Resveratrol improves endothelial cell markers impaired by plasma incubation from women who subsequently develop preeclampsia. Hypertens Res 42, 1166–1174 (2019). https://doi.org/10.1038/s41440-019-0243-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0243-5