Abstract
The potential for using genetic modification (GM) to enhance the nutritional composition of crops (for either direct human consumption or as animal feed) has been recognized since the dawn of the GM era, with such ‘output’ traits being considered as distinct, if not potentially superior, to ‘input’ traits such as herbicide tolerance and insect resistance. However, while input traits have successfully been used and now form the basis of GM agriculture, output trait GM crops are still lagging behind after 20 years. This is despite the demonstrable benefits that some nutritionally enhanced crops would bring and the proven value of GM technologies. This Review considers the present state of nutritional enhancement through GM, highlighting two high-profile examples of nutritional enhancement—Golden Rice and omega-3 fish oil crops—systematically evaluating the progress, problems and pitfalls associated with the development of these traits. This includes not just the underlying metabolic engineering, but also the requirements to demonstrate efficacy and field performance of the crops and consideration of regulatory, intellectual property and consumer acceptance issues.
Similar content being viewed by others
Introduction
Research in plant biology entered a disruptive phase in the early 1980s with the advent of straightforward methods for the stable transformation of plant cells, allowing for the introduction of foreign DNA into the host genome1,2,3. There followed an explosion of interest in and uptake of this new technology, as the game-changing potential for plant sciences and agriculture became apparent. Among the first examples of successful plant genetic engineering, also known as genetically modified (or GM) crops, were those for tolerance to herbicides such as glyphosate4, with this innovation forming the cornerstone of the nascent agricultural biotechnology industry. Similarly, the demonstration that transgenic expression of Cry proteins from Bacillus thuringiensis could inhibit insect herbivory showed the power to transform crop protection5. These two traits, individually and in combination, now represent >99% of the ~180 Mha land growing GM crops across the planet6 as well as some of the fastest examples of technological innovation and uptake in the agricultural sector.
Given the potential of plant genetic engineering and the freedom it can bring from dependence on traditional breeding to introduce variation, it is surprising that similar advances are not so apparent for the GM plants in which nutritional composition has been improved. Such traits, sometimes called output traits (since they result in an alteration to the harvested product), also generally deliver a benefit to the consumer as opposed to delivering to the specific needs of the farmer (the case with the input traits of herbicide tolerance and insect resistance). Why do we not yet see any nutritionally enhanced GM crops being commercially grown in the field? Perhaps this is because nutritional quality, unlike herbicide tolerance, is a more difficult trait to quantify and demonstrate its efficacy, as well as being more genetically complex than these single-gene input traits. Nutritional enhancement can be achieved in different ways but for the purpose of this Review, we have defined it as the addition or elevation of a nutrient in a foodstuff through GM, as distinct from other forms of plant breeding or direct supplementation7.
On the basis of the success of the input traits, we know that the underpinning technology works well and at multiple scales. Equally, pre-existing supporting agricultural infrastructures are easily adapted (with low additional costs) to handling a GM crop. Yet the problems of malnutrition and poor diet remain for many populations, with more problems coming to the fore. The Green Revolution helped to lift many millions of people out of starvation but now of greater concern are metabolic pathologies such as type-2 diabetes, obesity and cardiovascular disease, arising from the over-consumption of calorie-rich but nutritionally poor foodstuffs7. This Review considers not only the progress towards developing nutritionally enhanced GM crops, but also looks at some of the other issues that have contributed to delaying these innovations. Specifically, we consider two well-known examples, Golden Rice and omega-3 fish oil crops, in terms of benefit against opposition, posing the question—if not now, when? Will it ever be feasible to deliver nutritional enhancement through GM crops? On the wider issue of other emerging examples of GM nutritional enhancement, the reader is pointed towards the recent review by Martin and Li7.
Golden Rice
It is reported that the concept of Golden Rice or more precisely, rice endosperm rendered yellow through the engineered accumulation of beta-carotene (pro-vitamin A), was first suggested in 1984 (ref. 8), placing it right at the dawn of plant genetic engineering. However, unlike herbicide tolerance, the development of this trait took well over a decade to demonstrate even the first steps towards making a rice grain that could be polished and still contain beta-carotene. In 1997, Burkhardt et al. showed that endosperm-specific expression of the daffodil (Narcissus pseudonarcissus) phytoene synthase gene directed the accumulation of phytoene (the precursor of beta-carotene)9 and in 2000, Ye et al. reported the first iteration of Golden Rice10 in which beta-carotene was made in the grain’s endosperm. With the benefit of hindsight, what was hailed as a landmark achievement then might now be viewed as a ‘proof-of-principle’ study, but still this achievement represents the first step towards the development of output traits and nutritionally enhanced crops. In 2000, the starting gun was fired on the race to make available a crop that could potentially radically improve the lives of millions, lifting them out of vitamin A deficiency and the associated pathologies such as childhood blindness10. Although some technical issues remained to be overcome (such as the modest accumulation of beta-carotene in the polished grain), in the view of the inventors it was issues such as intellectual property rights, non-governmental organization opposition and the regulatory burden that is imposed on any GM crop11,12,13 that stalled progress of Golden Rice becoming a viable crop. Attempting to pilot the development of such a GM trait outside agriculture biotechnology companies represented a further challenge, both in terms of costs and experience12,13. For all that, progress was made on many fronts, including introducing the Golden Rice trait into indica varieties of rice suitable for cultivation in the most needful geographical regions and also the development of a second iteration (Golden Rice 2 (GR2)) in which the endosperm levels of beta-carotene were increased at least tenfold through the replacement of the daffodil phytoene synthase gene with a similar activity from maize14. Importantly, the development of GR2 was carried out in collaboration with Syngenta, allowing the project to benefit from this company’s experience in intellectual property rights and regulatory approval14. By 2005, optimism was restored with expectations for the crop to be approved and grown on a large scale11,12,13.
Yet by the end of the same decade, the programme seemed to have stalled. Syngenta left the project and the lack of progress was being questioned8,13. Would the trait (by now backcrossed into the indica IR64 variety) perform in the field and be safe to eat? Sadly, environmental releases (GM field trials) in the Philippines, essential to validate the yield of the transgenic line and also to provide data for regulatory approval, were destroyed by anti-GM activists in 2013 (refs. 12,13). At the same time, concerns emerged that the lead event for GR2 (GR2-R1) was not performing as well in the field as non-GM lines. Molecular characterization of this lead event GR2-R1 indicated that the transgene cassette was integrated into the first exon of the rice gene for OsAux1, disrupting the transport of auxins15. It is possible that a lack of experience in carrying out pre-regulatory GM field studies contributed to this delay, although it has been reported by one of those involved in the project that these molecular problems were known but not understood before environmental release13.
In 2017, the Philippines Rice Research Institute (PhilRice) and the International Rice Research Institute (IRRI) submitted applications for a biosafety permit for the direct use in food, feed or for processing, of GR2-E1 (a back-up event produced by Syngenta14) to the Philippines’ Department of Agriculture-Bureau of Plant Industry, the US Food and Drug Administration, Food Standards Australia New Zealand and to Health Canada, marking an important step towards approval and release. Nearly 20 years after the original proof-of-principle, there is now a realistic prospect that GR2 will be approved for wide-scale growth and consumption—a simple intervention to lift millions out of a debilitating if not fatal deficiency.
Omega-3 fish oils
Interest in engineering plants to accumulate omega-3 long-chain polyunsaturated fatty acids (LC-PUFAs) began in the late 1990s16. This is because the omega-3 LC-PUFAs eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA) are known to play a crucial role in human health and development but are not present in any higher plant17. Increasing demand for EPA and DHA-containing oils (predominantly sourced from the oceans as fish oils) has raised questions about sustainability and the associated environmental footprint. Since most marine-sourced fish oils are used in aquaculture (fish farming), rather than for direct human nutrition, expansion in that sector places further demand on fish stocks17. Viewed from an economic perspective, the commodity price of fish oil is twice that of vegetable oil, making it an attractive trait to introduce into plants.
Unlike Golden Rice and beta-carotene, the biosynthesis of EPA and DHA involves many enzymes and is non-native to higher plants. The organisms responsible for the primary biosynthesis of these omega-3 LC-PUFAs are marine microorganisms such as microalgae, forming the base of the aquatic foodwebs in which these fatty acids accumulate at every trophic level17. Conceptually, the transfer of algal genes is straightforward but 20 years ago, the molecular identity of these biosynthetic activities was unknown16. A phase of gene discovery for the desaturases and elongases required to make EPA and DHA resulted in a toolbox of sequences with which to attempt the heterologous synthesis of these omega-3 fatty acids. Initial attempts in both yeast and plants to make EPA resulted in disappointingly low yields and revealed some metabolic bottlenecks that resulted from the inefficient recognition of non-native substrates by endogenous lipid metabolism18. These first results set the course for further intensive studies of this pathway in transgenic plants and the adoption of iterative rationales to overcome often poorly understood biochemical obstacles. These advances, from several different research teams, have delivered plant seed oils that contain levels of EPA and DHA the same or greater than that found in bona fide fish oils19,20. More recently, field trials of GM camelina and canola accumulating EPA and DHA have been carried out by several groups in the UK, USA and Australia20,21. Animal feeding studies in which the oil from GM camelina has been used to feed salmon, sea bream and mice have been published, confirming the efficacy of this plant-derived source of EPA and DHA to serve as a direct drop-in replacement for marine oils22,23. A substantial body of data demonstrates the feasibility of using these plants to generate a terrestrial, de novo source of omega-3 fish oils. This production platform (for example, transgenic camelina or canola) can service the needs of not just the aquafeed sector but also direct human nutrition, terrestrial animal nutrition and pharmaceutical applications, since it generates a chemical ‘feedstock’ (triacylglycerols containing EPA and/or DHA) identical in properties to the triacylglycerols that comprise fish oils.
Diversity of traits and drivers
Perhaps the most obvious differentiating factor between the Golden Rice trait and that for omega-3 fish oils, is that while the former was conceived as conforming to public good/humanitarian use, the latter has a notable economic component in addition to delivering nutritional and sustainability benefits. Also, as noted above, there is increased fiscal value associated with a plant oil that has been enhanced by the presence of the omega-3s EPA and DHA. Conversely, in the case of Golden Rice, not only is there a less obvious commercial value associated with enhanced amounts of beta-carotene, there are strong moral imperatives (established by the Humanitarian Golden Rice project) to prevent ‘profiteering’ from vitamin-A deficiency and human misery12. Unfortunately, this can be viewed as putting the two output traits (Golden Rice and omega-3) on distinct paths, since the drivers and the end-user pull are different. Specifically, the desirability and value of the omega-3 trait is recognized by the end-users of such an oil (predominantly private companies involved in the aquafeed sector), allowing for the establishment of value chains and business models based around economics. In the case of Golden Rice, although the absence of monetization is fundamental to the ethical use of the trait, the concomitant absence of a commercially motivated plan for its use can be viewed as a disadvantage. This became pertinent with the exit of Syngenta from the Golden Rice project in 2005 (refs. 12,13). It is interesting to note that multiple groups from publicly funded and private industry have focused on the omega-3 trait, whereas studies on Golden Rice have been associated with fewer teams, predominantly from the public sector. The plant omega-3 trait can only be achieved using a GM solution, whereas the accumulation of pro-vitamin A formed part of the contemporaneous conventional breeding HarvestPlus (www.harvestplus.org) crop biofortification programme (most notably orange cassava). Golden Rice faced a problem of consumer acceptance (in terms of technology and selection of regional varieties) that the omega-3 commodity trait does not. However, regulatory approval for both traits (omega-3, Golden Rice) is being taken forward by organizations other than those who established the first wave of GM input traits.
Barriers and lessons
The problems that beset the Golden Rice project occurred for the most part not during the research phase, but in the development stage11,24. As identified by the Golden Rice inventors, intellectual property represented a major roadblock for establishing freedom-to-operate—that is, ensuring that you do not infringe other parties’ patents—and subsequent entry into the regulatory approval process12. However, this situation is not unique to Golden Rice and probably more complicated for the omega-3 trait where the metabolic engineering is considerably more complicated (more genes mean more patents). Also, the field has many active parties, all generating their own intellectual property. Several generic problems confront any entity wishing to determine their freedom-to-operate status. First, the patent landscape for plant biotechnological processes is complicated and congested. Second, progress through the patenting process is (understandably, given the volumes) slow, with many complex filings still not at the granted stage after several years. This means that it is often hard to determine the relevance and breadth of a patent because the final scope of the claims has not yet been decreed. Moreover, the acceptance by examiners of some of the broad claims often included in patent applications has considerably reduced over the years, meaning that grants nowadays are more restricted in scope. Unfortunately, this does not apply retrospectively to earlier patents, meaning that some older patents have a reach that would not be granted now.
With hindsight, it is clear that the transitioning of a project from a research phase onto a development pathway requires a great deal of planning, most of which should have occurred at a very early phase in the project12. However, unless the programme of work was initiated with the specific goal of progressing all the way through to regulatory approval and commercialization (as might be the case in industry), then lack of consideration at the start can result in problems later. For example, the use of an antibiotic resistance marker for the selection of transgenic plants is acceptable for laboratory-based research but might prove problematic when seeking approval for commercial release. Similarly, the use of genetic elements (promoters, genes, vectors and so on) that are covered by third-party patents is usually without problem when used for basic research (under the so-called research or safe-harbour exemptions enshrined in patent law) but would require licensing if their use extended beyond that. This presupposes that the aim of all fundamental research is the generation of outputs that are suitable for translation. Even with the recent increased emphasis on research impact and delivery of tangible benefits from basic research, most projects do not have an applied goal. However, an awareness of the intellectual property landscape associated with your field of endeavour is a good thing, as is a basic understanding of the process by which a transgenic event might be evaluated for regulatory approval and commercialization. In the case of Golden Rice, initial research was carried out in an academic environment in which intellectual property and regulatory approval were (understandably) given less consideration than the primary goal of making beta-carotene in the rice endosperm. For the omega-3 trait, the combination of a more market-driven trait and the presence of several companies working in this field resulted in greater sensitivity to these issues, although the above-mentioned issue of protracted time for a patent to get to grant means that freedom-to-operate is a continually shifting landscape. Attempts have been made to make aspects of intellectual property management easier, for example through Public Intellectual Property Resource for Agriculture (PIPRA) establishment of ‘patent pools’ for plant transformation25.
Influencing the narrative
Beyond the problems of restrictive intellectual property, expensive regulatory approval and the economics of business development, additional factors need to be considered, especially those of public engagement and societal consent. With hindsight, it is widely felt that in the early years of the agriculture biotechnology industry, this new technology (in the form of herbicide-tolerant crops) was rolled out irrespective of the consumer’s views or sentiments and in a manner that was unreceptive to criticism or examination. Nowadays, the situation is different and the end-users are introduced to new technologies at a much earlier stage in development with a view to obtaining social license for their use. In the case of Golden Rice, the first introduction the public had to this trait was through the front cover of Time magazine in 2000, proclaiming “This rice could save a million kids a year” (see Fig. 4 in ref. 13). Although this represented wide-reaching coverage, it also coincided with growing antagonism towards GM crops and plant genetic engineering, at least in Europe, where several well-organized non-governmental organizations focused efforts to critique and question the ethos of the Golden Rice project13. As recorded by the inventors of Golden Rice, this antipathy then radiated out to developing countries, including the Philippines where the IRRI-led field trials of GR2 were subsequently vandalized12.
This poses the question as to why would anyone want to destroy a research project, especially one that could deliver great potential health benefits. It could be argued that people were unfamiliar or fearful of this new technology, but could that explain such a reaction? More likely, a few activists ideologically opposed to GM technology were able to project a loud and persuasive voice in support of their views, in the absence of facts to the contrary. However, the temptation to fight fire with fire must be avoided—scientists are not spin-doctors or public relations experts but instead have a duty to report advances in a factual manner, not resorting to hype. This can seem bland and unexciting to the media looking to cover a story, especially in the face of hyperbolic claims from opponents.
It is important to be open, honest and transparent, presenting the facts as they are and not trying to avoid difficult issues. In our experience of carrying out omega-3 trait GM field trials in the UK20, effective communication to a wide and varied audience is essential, and effectively conveying the same facts about the research while tailoring the message to the recipient’s interests ensures engagement and the initiation of dialogue. Engagement in a two-way conversation, aiming to listen to the views, concerns and opinions of others and demonstrating a preparedness to respond to such concerns, in some instances even including the way that the research is conducted, is important. For example, in 2014, at Rothamsted Research a public dialogue focusing on the work of the organization with industry was conducted. The outcomes26 of this public dialogue exercise were used to inform the development of the organization’s knowledge exchange and commercialization strategy, which in turn guided the approach to translation of the omega-3 trait. Additionally, when the first permission was granted to Rothamsted to conduct field trials of GM camelina in 2014, there was no requirement in terms of biosafety to cover the transgenic crop with a net. However, in discussions with neighbouring honey producers and commercial organic farmers there were concerns raised about the pollen flow. In response to this, the crop was covered by a net during flowering to reassure the stakeholders that they had been heard. None of this guarantees that the public will be receptive to a new technology, nor should it be the sole motivation for such dialogue activities but it can help better frame innovation in a wider context. In addition, the responsible research and innovation framework that has been developed for use by Research Councils UK (now UK Research and Innovation (UKRI)) and the communities they support27 has been extensively used in the recent stages of engagement for the omega-3 project at Rothamsted Research. Adoption of the responsible research and innovation principles was manifested through institutional communications and engagement strategy28. One could argue that in the omega-3 example at Rothamsted, the research, the researchers and potentially the future consumer of the possible direct or indirect product have all benefited from the responsible research and innovation process. In general, the move from experimental stages to commercialization and the associated opportunities for informed consumer choice, dictate, now more than ever, that social scientists have an important role to play alongside the research and development teams. In the specific examples of nutritionally enhanced crops developed to address health and malnutrition challenges considered here, probably the need is even more pressing and relevant. It is worth noting that the omega-3 GM field trials have been taking place since 2014 in the UK without incidents of vandalism or protest, and feedback from stakeholders has informed the design of the experiment in the field at different times. Being able to conduct these trials without disruption has enabled refinement and improvement of the trait and brings it closer to commercial application—the route that can benefit the consumer and the environment.
Discussion
In this Review, we have not only tried to record the progress made by the two best-known examples of GM crops with enhanced nutritional properties, but also to take a critical look at some of the factors that might have contributed to the slow progress in the application of fundamental research in this area. The primary issues can be listed as follows:
-
intellectual property (freedom-to-operate, licensing and volume)
-
economic value (viable business proposition versus social value)
-
regulatory approval (cost and time)
-
consumer and societal acceptance
From the perspective of private industry, who also represent the developers of virtually all deregulated (in other words, approved) and commercialized GM crops (predominantly input traits), the most important factor will be value and financial return. What of the situation for nutritional enhancement output traits? As discussed above, the academic developers of Golden Rice made it explicit that it should be not-for-profit but equally, the lack of an economic pull (in the form of monetizable consumer demand) could be an impediment to faster progress. As evidence for this, although GR2 has recently gained regulatory approval process for food and feed use in some (non-target) countries such as Canada, the USA and Australia, an omega-3 canola event making DHA developed by Nuseed was already approved for similar use by Food Standards Australia New Zealand in 2017 and commercial cultivation in the USA in 2018 (ref. 21). However, there is hope that 2019 will see approval for cultivation of GR2E in Bangladesh, as well as regulatory field trials in the Philippines.
There is a pressing need for a different pathway by which health-beneficial traits in GM plants are delivered to consumers, such that it is not reliant on market forces and economics. This may need to be supported by the public purse rather than by private industry, but this would be offset by cost-savings to national health services as a result of a healthier population. In such a scenario, public funding would continue beyond the research phase, helping to navigate a project through regulatory approval and onto a level ready for consumer uptake and end-use. Such a scenario would only be applicable to examples where the technology demonstrably had the capacity to deliver meaningful improvements to diets and would still be dependent on societal acceptance. Thus, a situation where the consumer would receive a tangible benefit (improved diet and health) and also be an actively involved stakeholder shaping the development of the technology/trait (as well as being a shareholder by contributing to its funding as a tax payer and funder of the public purse) might be met with less resistance than a privately owned input trait. That good nutrition should be central to healthcare is a concept gaining traction7,29.
In the future, additional nutritional enhancement traits will probably progress and there are some promising examples—black tomatoes with extra anthocyanins, cereals fortified with vital micronutrients and maize stacked with several different extra vitamins7,30,31,32. Equally exciting is the emergence of a new generation of transgenic plants with nutritional enhancements, such as the aSTARice (which builds on Golden Rice). aSTARice can synthesize a tailored range of carotenoids and ketocarotenoids (such as astaxanthin and canthaxanthin) with health benefits, primarily in the form of antioxidants33, and the eye-catching expression of betalain pigments that also act as potent antioxidants34. These are just a few examples—many are still at the research phase but will hopefully be viable to make the transition to product. As Marc Van Montagu, one of the pioneers of plant biotechnology, notes: “Genetically engineered plants and plant biotechnology have the potential to revolutionize agriculture in a sustainable manner; …and profoundly improve the health, quality of life and livelihood of mankind”35. To build on the present advances and successes, in addition to a vibrant research base, we need a culture of translation and impact, where even fundamental results are framed with a view to how they might be of use and benefit.
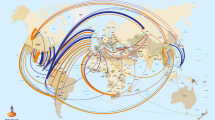
Before we conclude, it is important to note that the implementation of innovation, at least in agriculture, often takes time—just under 30 years on average36 and in many other cases, a lot longer. For example, the first experiments to generate hybrid vigour in maize were successfully carried out in 1877 by William Beal (MSU) but it was not until 1933 that the first commercial plantings of hybrid maize occurred in the US. That the fastest example (at 17 years) of an agricultural innovation being translated into practice is the GM Roundup-Ready herbicide-tolerance trait demonstrates, at least in part, the potential of plant biotechnology36 (Fig. 1).
The length of the line is proportional to the indicated time in years between initial conception and application. In the case of Golden Rice, the regulatory approval process is ongoing, hence the broken line. Timescales are described in the text. The omega-3 canola trait is described in ref. 21. Credits: Images adapted from Rothamstead Research and from the image collection of the International Rice Research Institute (IRRI).
References
Bevan, M. W., Flavell, R. B. & Chilton, M. -D. A chimaeric antibiotic resistance gene as a selectable marker for plant cell transformation. Nature 304, 184–187 (1983).
Fraley, R. T. et al. Expression of bacterial genes in plant cells. Proc. Natl Acad. Sci. USA 80, 4803–4807 (1983).
Herrera-Estrella, L., Depicker, A., Van Montagu, M. & Schell, J. Expression of chimaeric genes transferred into plant cells using a Ti-plasmid-derived vector. Nature 303, 209 (1983).
Shah, D. M. & et al. Engineering herbicide tolerance in transgenic plants. Science 233, 478–481 (1986).
Vaeck, M. et al. Transgenic plants protected from insect attack. Nature 328, 33–37 (1987).
Global Status of Commercialized Biotech/GM Crops: 2016 (ISAAA, 2016).
Martin, C. & Li, J. Medicine is not health care, food is health care: plant metabolic engineering, diet and human health. New Phytol. 216, 699–719 (2017).
Enserink, M. Tough lessons from golden rice. Science 320, 468–471 (2008).
Burkhardt, P. K. et al. Transgenic rice (Oryza sativa) endosperm expressing daffodil (Narcissus pseudonarcissus) phytoene synthase accumulates phytoene, a key intermediate of provitamin A biosynthesis. Plant J. 11, 1071–1078 (1997).
Ye, X. et al. Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287, 303–305 (2000).
Al-Babili, S. & Beyer, P. Golden Rice—five years on the road—five years to go?. Trends Plant Sci. 10, 565–573 (2005).
Potrykus, I. Lessons from the ‘Humanitarian Golden Rice’ project: regulation prevents development of public good genetically engineered crop products. New Biotechnol. 27, 466–472 (2010).
Dubock, A. The present status of Golden Rice. J. Huazhong Agric. Univ. 33, 69–84 (2014).
Paine, J. A. et al. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat. Biotechnol. 23, 482–487 (2005).
Bollinedi, H. et al. Molecular and Functional characterization of GR2-R1 event based backcross derived lines of Golden Rice in the genetic background of a mega rice variety Swarna. PLoS ONE 12, e0169600 (2017).
Broun, P., Gettner, S. & Somerville, C. Genetic engineering of plant lipids. Annu Rev. Nutr. 19, 197–216 (1999).
Napier, J. A., Usher, S., Haslam, R. P., Ruiz-Lopez, N. & Sayanova, O. Transgenic plants as a sustainable, terrestrial source of fish oils. Eur. J. Lipid Sci. Technol. 1179, 1317–1324 (2015).
Domergue, F., Abbadi, A. & Heinz, E. Relief for fish stocks: oceanic fatty acids in transgenic oilseeds. Trends Plant Sci. 10, 112–116 (2005).
Petrie, J. R. et al. Metabolic engineering plant seeds with fish oil-like levels of DHA. PLoS ONE 7, e49165 (2012).
Usher, S., Haslam, R. P., Ruiz-Lopez, N., Sayanova, O. & Napier, J. A. Field trial evaluation of the accumulation of omega-3 long chain polyunsaturated fatty acids in transgenic Camelina sativa: Making fish oil substitutes in plants. Metab. Eng. Commun. 9, 93–98 (2015).
Napier, J. A., Olsen, R. E. & Tocher, D. R. Update on GM canola crops as novel sources of omega-3 fish oils. Plant Biotechnol. J. 17, 703–705 (2019).
Betancor, M. B. et al. A nutritionally-enhanced oil from transgenic Camelina sativa effectively replaces fish oil as a source of eicosapentaenoic acid for fish. Sci. Rep. 5, 8104 (2015).
Tejera, N. et al. A Transgenic Camelina sativa seed oil effectively replaces fish oil as a dietary source of eicosapentaenoic acid in mice. J. Nutr. 146, 227–235 (2016).
Lee, H. & Krimsky, S. The rrested development of Golden Rice: the scientific and social challenges of a transgenic biofortified crop. J. Soc. Sci. Stud. 4, 51–64 (2016).
Chi‐Ham, C. L. et al. An intellectual property sharing initiative in agricultural biotechnology: development of broadly accessible technologies for plant transformation. Plant Biotechnol. J. 10, 501–510 (2012).
Turrall, S. Evaluation of a public dialogue on Rothamsted Research working with industry (Rothamsted Research, 2014).
Stilgoe, J., Owen, R. & Macnaghten, P. Developing a framework for responsible innovation. Res. Policy 42, 1568–1580 (2013).
Stilgoe, J. A tale of two trials. Responsible Innovation https://jackstilgoe.wordpress.com/2015/09/04/a-tale-of-two-trials/ (2015).
Martin, C. A role for plant science in underpinning the objective of global nutritional security? Ann. Bot. 24, 541–553 (2018).
Butelli, E. et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 26, 1301–1308 (2008).
Borrill, P., Connorton, J. M., Balk, J., Miller, A. J., Sanders, D. & Uauy, C. Biofortification of wheat grain with iron and zinc: integrating novel genomic resources and knowledge from model crops. Front. Plant Sci. 21, 53 (2014).
Naqvi, S. et al. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc. Natl Acad. Sci. USA 106, 7762–7767 (2009).
Zhu, Q. et al. From Golden Rice to aSTARice: Bioengineering astaxanthin biosynthesis in rice endosperm. Mol. Plant 11, 1440–1448 (2018).
Polturak, G. et al. Engineered gray mold resistance, antioxidant capacity, and pigmentation in betalain-producing crops and ornamentals. Proc. Natl Acad. Sci. USA 114, 9062–9067 (2017).
Van Montagu, M. It Is a long way to GM agriculture. Annu. Rev. Plant Biol. 62, 1–23 (2011).
Alston, J. M., Andersen, M. A., James, J. S. & Pardey, P. G. Persistence Pays Vol. 34 (Springer, 2009).
Acknowledgements
The authors thank BBSRC (UK) for financial support under Institute Strategic Programme Grants BBS/E/C/000I0420 and BBS/E/C/00005207.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Plants thanks Shan Lu, Mark Taylor and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Napier, J.A., Haslam, R.P., Tsalavouta, M. et al. The challenges of delivering genetically modified crops with nutritional enhancement traits. Nat. Plants 5, 563–567 (2019). https://doi.org/10.1038/s41477-019-0430-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-019-0430-z
This article is cited by
-
Agricultural technology as a driver of sustainable intensification: insights from the diffusion and focus of patents
Agronomy for Sustainable Development (2024)
-
CRISPR-Cas9 System Mediated Genome Editing Technology: An Ultimate Tool to Enhance Abiotic Stress in Crop Plants
Journal of Soil Science and Plant Nutrition (2024)
-
Plant Co-expression Annotation Resource: a web server for identifying targets for genetically modified crop breeding pipelines
BMC Bioinformatics (2021)
-
CRISPR/Cas9-mediated mutation of 5-oxoprolinase gene confers resistance to sulfonamide compounds in Arabidopsis
Plant Biotechnology Reports (2021)
-
Multiplying the efficiency and impact of biofortification through metabolic engineering
Nature Communications (2020)