Abstract
Implanted biomaterials often fail because they elicit a foreign body response (FBR) and concomitant fibrotic encapsulation. To design clinically relevant interference approaches, it is crucial to first examine the FBR mechanisms. Here, we report the development and validation of infrared-excited nonlinear microscopy to resolve the three-dimensional (3D) organization and fate of 3D-electrospun scaffolds implanted deep into the skin of mice and the following step-wise FBR process. We observed that immigrating myeloid cells (predominantly macrophages of the M1 type) engaged and became immobilized along the scaffold/tissue interface, before forming multinucleated giant cells. Both macrophages and giant cells locally produced vascular endothelial growth factor (VEGF), which initiated and maintained an immature neovessel network, followed by the formation of a dense collagen capsule two- to four-weeks post-implantation. Elimination of the macrophage/giant-cell compartment, by clodronate and/or neutralization of VEGF by VEGF Trap, significantly diminished giant-cell accumulation, neovascularization and fibrosis. Our findings identify macrophages and giant cells as incendiaries of the fibrotic encapsulation of engrafted biomaterials via VEGF release and neovascularization, and therefore as targets for therapy.
Similar content being viewed by others
Tissue exposure to biomaterials triggers a foreign body response (FBR), a step-wise process consisting of inflammation, wound healing and, if not resolved, end-stage tissue fibrosis and scarring1–5. The FBR follows vascular damage and is initiated by absorption of plasma proteins onto the foreign body surface, including fibrinogen and complement2. This local damage response causes the recruitment and activation of monocytes/macrophages, their fusion to form multinucleated giant cells and the generation of functional vessels followed by fibrotic encapsulation of the object4,6. Not unlike in chronic wounds7–9, monocytes/macrophages represent the primary orchestrator of the FBR. Depending on local stimuli, macrophages can be polarized into pro-inflammatory (M1) or anti-inflammatory (M2) macrophages, as well as intermediate types7,8,10. Based on their function profiles, including phagocytosis, adhesion and cytokine release11, the polarization to M1 and M2 macrophages defines the type and outcome of immune defence and tissue regeneration. M1 macrophages are highly phagocytic and produce inflammatory cytokines, which control an early innate damage response, while M2 polarization supports late-stage resolution of inflammation and tissue repair11–14. Both M1 and M2 type macrophages are implicated in organizing the inflammation and fibrous scar formation in response to implant materials9,15–17. However, their mechanistic roles in either enhancing or counteracting fibrotic outcome remain poorly understood18.
Clinically, the FBR reaches relevance when instigated by medical devices and/or implants, including sensors, pacemaker, prostheses (for example, hip and knee prostheses, breast implants, heart valves), devices for release of bioactive compounds and, more recently, scaffolds used in tissue engineering and regenerative medicine3,7. As detrimental processes, inflammation and fibrosis compromise implant integration and long-term functionality, impairing transplant bioactivity due to the fibrous barrier or biomaterial degradation2,7. Therefore, intervention strategies to control the inflammatory phase of the FBR and to limit fibrosis are critical to improve implant integration and long-term function.
Different concepts have been explored to attenuate the FBR and fibrosis experimentally, including improving the biomimetic properties of the biomaterial, for example, by geometrical and/or biochemical surface modifications19,20. Likewise, molecular interference has been explored, including: local immunosuppression by corticosteroid deposition3; dampening leukocyte and fibroblast activation by anti-transforming growth factor-antibody or halofunginone2,21; or stimulating vessel development to improve perfusion and performance of the bioactive implants by pro-angiogenic vascular endothelial growth factor (VEGF)2,3,22,23. These approaches have resulted in incomplete and often transient efficacy, with ongoing chronic inflammation, fibrosis and even graft failure as outcomes2,3. As a potentially stringent approach, the depletion of phagocytes by liposomal clodronate bisphosphonate or genetic ablation reduces the onset of inflammation during an intraperitoneal FBR19,24, implicating monocytes/macrophages as early effectors and potential targets of an ill-fated FBR.
Nonlinear imaging of biomaterials
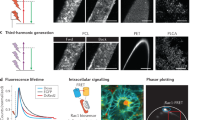
To explore the cause–consequence relationships of the FBR, we developed an intravital window model and applied nonlinear multiphoton microscopy to monitor the fate of engrafted material in mice and in response to molecular intervention. As a currently clinically investigated scaffold design and material platform technology, we applied calcium phosphate-coated medical grade poly(ɛ-caprolactone) (mPCL-CaP) implants, which were fabricated by melt-electrospinning with a fully interconnected honeycomb pore network (average pore size 100 μm, 90% porosity, Fig. 1a)25–28. In previous studies, we observed that mPCL-CaP scaffolds combined with bone morphogenetic protein-7 (BMP-7) form bone after ectopic implantation in mice, whereas BMP-7 free scaffolds induce a physiological FBR29,30. Hence, we hypothesized that these scaffolds would be a suitable porous implant to explore the mechanisms of the FBR in four dimensions. To detect both mPCL-CaP fibres and deriving biological responses by multiphoton microscopy, infrared excitation and higher harmonic generation were used for deep tissue penetration, for improved detection of red fluorophores and minimized phototoxicity (Supplementary Fig. 1)31,32.
a, Macroscopic overview of the melt-electrospun PCL scaffold by bright-field microscopy. Scale bar, 300 μm. b, 3D reconstruction of the mPCL-CaP scaffold by multiphoton-excited label-free SHG and THG detection (forward direction). Left, overview image; the inset on the right panel shows calcium deposits (arrowheads). Scale bars, 0.5 mm (left) and 200 μm (right). c, High-resolution SHG/THG projection of a single fibre in the horizontal (x y) and orthogonal (x z) directions, and signal intensity analysis in the x-direction. Lines denote calcium coating. Detailed representations in the bottom three panels show a PCL fibre as single and merged channels (SHG and THG). d, Co-localization analysis of SHG and THG signal intensity (R2 = 0.06, P > 0.001). e, Forward and backward direction detection of a PCL fibre shown as single channels (SHG and THG) and merged. Scale bar, 10 μm. f, Comparison of forward versus backward detection of orthogonal projections of (e). SHG and THG signal intensities decay in a single fibre in the z-direction.
Scaffold fibres elicited strong second (SHG) and third harmonic generation (THG) signals, using excitation/emission wavelengths of 1,090/545 and 1,180/393 nm, respectively (Fig. 1b). SHG/THG z-scanning resolved both individual fibre geometry in three dimensions (Fig. 1b) and an additional weak THG signal from the calcium phosphate coating (Fig. 1b, arrowheads). Remarkably, SHG-/THG-positive substructures were present throughout the fibre core, with strong individual signal profiles and insignificant co-localization (R2 = 0.06, P < 0.001, Fig. 1c,d). Thus, SHG/THG signals resolve different structural PCL subdomains after three-dimensional (3D) printing, such as the crystalline phase of the melt electrospun fibres33.
To extend the applicability of nonlinear SHG/THG microscopy for other implant types used in clinical applications, we analysed a series of biomaterials next to mPCL-CaP, including polyester, polysulfone (PSU), and biodegradable ones, such as polyglycolic lactic acid (PGLA) and gelatin. All tested materials elicited a strong THG signal, which predominantly originated from the scaffold interface independently from their microarchitecture (Supplementary Fig. 2). Likewise, a SHG signal was emitted by most materials, except PSU (Supplementary Fig. 2). The results establish the THG signal, in particular, as a universal interface-detection strategy for robust analysis across implant types.
As a prerequisite for intravital imaging in animals, we tested whether the SHG/THG signals induced by scaffold fibres were sufficient for reliable detection in the backward direction, which is mandatory for thick tissue microscopy32 (Fig. 1e,f). Signal intensity was fourfold decreased when detected in the backward, as compared with the forward, direction, and an additional 50% of signal decayed per 25 μm penetration into the fibre for each detection mode (Fig. 1e,f). Despite this scatter-induced signal loss, 40-μm-thick fibres were completely detected in either configuration (Fig. 1e). This establishes the combined SHG/THG signal detection as a tool for the non-destructive analysis of scaffold fibre geometry and of substructures of semicrystalline mPCL polymers.
Intravital longitudinal imaging of the FBR
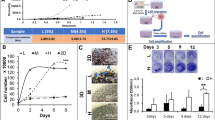
To monitor both extracellular matrix and cellular kinetics during the FBR in vivo, an mPCL-CaP scaffold of 5 × 5 × 0.2 mm was implanted in parallel to the deep dermis/subcutis interface in GFP-C57BL/6 mice expressing GFP in all cells and in nude (nu/nu) mice, and analysed through an optical imaging window over time (Fig. 2a and Supplementary Fig. 3a). The scaffolds integrated without macroscopic tissue trauma, inflammation or bleeding (Fig. 2b), similarly to the integration of scaffolds in window-free mice25. Fourteen days after implantation, a histologically significant FBR response emerged, characterized by a cell-rich infiltrate and multinucleated giant cells decorating the surface of fibres, high density of neovessels and emerging fibrosis between and surrounding the scaffold fibres (Fig. 2c). The composition of the inflammatory infiltrate, which indeed may vary with material type and geometry19,25,34,35, was further delineated by flow cytometry; macrophages/giant cells represented the predominant cell type of the immune infiltrate (47.2%), followed by T-cells (20%), granulocytes (9.1%) and B-cells (4%) (Supplementary Fig. 4a). Giant cells and macrophages accumulated with a ratio of about 1:6 and retained monocytic differentiation marker CD68 (Fig. 2d) and M1 polarization (IRF-5, interferon regulatory factor 5), but lacked the M2 profile marker CD163 overtime (up to seven weeks; Fig. 2e and Supplementary Fig. 4b). Thus, a macrophage-dominated pro-inflammatory M1-type response was induced by mPCL-CaP implants during early and late stages of the FBR, with no evidence for M2 polarization over time, in contrast with what occurs with other material types9,15,16.
a, Schematic representation of the model, showing a dorsal skin-fold chamber (DSFC) on the back of a C57BL/6 GFP mouse. A mPCL-CaP scaffold was implanted inside the DSFC within the subcutaneous tissue (x–y and x–z projections of the implantation site). b, Longitudinal macroscopic overview of the FBR after scaffold implantation within the DSFC. Dotted lines denote the position of the scaffold. c, Histology of the FBR in response to the scaffold implantation within the DSFC at day 14. Green arrowhead, giant FBR cells; blue arrowhead, blood vessels; asterisk, scaffold fibre. Right panels show magnified images of boxes in the left panel. Scale bar, 100 μm. d, CD68 expression detected by immunohistochemistry on day 14 post-implantation and graphical representation of all mononucleated (m) and giant cells (g) as percentage of total CD68-positive cells. The number of mononucleated cells that stably associated with the fibres was negligible (four fields per mouse; three mice). Image area, 500 × 700 μm (×5 μm). e, IRF5 and CD163 expression detected by immunohistochemistry at day 14 post-implantation. Scale bar, 50 μm. f,g, Longitudinal intravital imaging of FBR at days 4, 7 and 14, performed for different areas of the same lesions, chosen randomly. f, Merged multiparameter images representing: THG (red); GFP-positive cells (cyan); 70 kDa dextran (magenta); and SHG (green). g, Single-channel representations (top panels) and image-based quantifications of scaffold fibres (THG), GFP-positive infiltrate cells (white arrowheads), dextran-positive blood vessels (magenta arrowhead) and SHG, detecting PCL fibres (white asterisks) and fibrillar collagen (days 7 and 14; green asterisks). Scale bar, 50 μm. Bottom panel of (g) quantifies the signal-positive area (% of total image), obtained from four to eight independent fields per implant from four mice, for each channel (*P < 0.05; **P < 0.01; ***P < 0.001; one-way analysis of variance followed by Tukey’s HSD post hoc test).
When monitored longitudinally by intravital microscopy, the tissue surrounding the THG-positive mPCL-CaP scaffold became gradually infiltrated by GFP-positive cells (Fig. 2f,g), followed by sprouting of perfused neovessels and deposition of, initially loose and later bundled, fibrillar collagen (SHG) throughout the initially SHG-free wound bed (Fig. 2f,g and Supplementary Fig. 3b,c). The SHG signal intensity in the scaffold fibres was diminished in parallel to progressing collagen deposition, whereas the THG signal intensity remained largely unperturbed (Fig. 2g). This established the THG signal as the most robust detection mode in vivo, to monitor the positioning and integrity of the scaffold over time. Although the FBR kinetics in the GFP-C57BL/6 and nude mice were similar, neovascularization was enhanced in the nu/nu mice, consistent with angiogenic strength being dependent on the genetic background of mouse strains36,37. Despite severe inflammatory tissue remodelling, mPCL-CaP fibres were neither resorbed nor infiltrated by cells (Supplementary Fig. 3d,e), confirming the documented stability of this material in vivo28,38,39.
To demonstrate in vivo applicability of THG imaging for an independent material, PSU implants were efficiently monitored, including early recruitment of GFP-positive cells, robust neovascularization and collagen deposition (Supplementary Fig. 5), similar to the response to mPCL-CaP.
Kinetic and mapping of infiltrating cells
Unlike their composition, the dynamics of FBR infiltrate cells remain unknown. We mapped infiltrate kinetics and the accumulation of foreign body giant cells along the scaffold surface and performed time-lapse recordings and single-cell tracking of infiltrating GFP-positive cells. Relative to the implant position, we identified two kinetic subpopulations that were recruited over time, including interstitially moving and fibre-attached resident cells (Fig. 3a–c and Supplementary Videos 1–3). A population of mononuclear interstitial cells retained mostly kinetic behaviours and moved along and between collagen fibrils, without directly contacting scaffold fibres (Fig. 3a,d; speed up to 3 μm min−1). These were interstitial leukocytes, also detected by histology (Fig. 2c). Conversely, scaffold-attached cells were typically multinucleated, as detected by intravital injection of Hoechst dye (Fig. 3b, arrowhead), with high positional stability despite vigorous cytoplasmic dynamics (Fig. 3d and Supplementary Video 4). These were stably anchored resident giant cells, as predicted7; they nonetheless retained slow dynamic behaviours, locally exploring the material surface by ruffling lamellipodia4 (Fig. 3b,d; average speed at day 14: 0.07 μm min−1).
a,b, Dynamics of GFP-positive infiltrate cells at day 7, monitored by time-lapse intravital microscopy and analysed by single-cell tracking. Sequential frames obtained at different time points from Supplementary Video 2, which was used to track cells and classify them as interstitial (a) or scaffold-associated (b). White arrowheads denote single-cell positioning over time. GFP-positive cells, green; SHG and Hoechst, red and grayscale (single channel). Scale bar, 20 μm. Lower panels denote the speed as heatmaps from ten representative cells. c, Mean ± s.d. of interstitial or mPLC-CaP fibre-associated infiltrate cells per imaging field over time. Area, 360 × 360 μm (×40 μm). *P < 0.05; ***P < 0.01; n.s., non significant; analysed by unpaired two-tailed Student’s t-test. d, Frequency distributions of the average speed for interstitial and scaffold-associated cells over time. Three mice per time point and two fields per mouse were analysed. Data show one representative image sequence per time point from three independent experiments.
Neoangiogenesis and fibrotic capsule development during FBR
Whether neovessels contribute to enhance or resolve the FBR remains unclear2,3,22,23. Both immunodeficient and immunocompetent mice carrying mPCL-CaP material developed a dense neovascular network (Fig. 2f,g and Supplementary Fig. 3b,c), with an irregular-shaped morphology, an enhanced number of branch points and a decreased inter-branch length, compared with the linear organization of normal dermal blood vessels (Fig. 4a,b). These features are similar to those of tumour neovessels40,41, where dysfunctional angiogenesis supports cancer lesion growth, progression and metabolic deregulation42. Neovessels preferentially oriented in parallel or perpendicular to the scaffold fibres, defined by an angle distribution favouring 0 ° and 90 ° (Fig. 4c). This indicated that neovessels sense and undergo guidance by the pre-defined architecture of the scaffold, but thereafter fail to normalize over time, similar to what occurs with dysfunctional vessels in tumour-associated desmoplastic tissue42.
a, Regular skin and scaffold-elicited vasculature. Scale bar, 100 μm. b, Quantification of neovessel organization, including: number of branches, interbranching distance and total vessel length. Mean + s.d., three fields per mouse from three mice. **P < 0.01; ***P < 0.001 (unpaired two-tailed Student t-test). c, Frequency distribution of vessel-to-scaffold angle. Dashed line, boundary of PCL-CaP fibre. Data represent three fields per mouse from three mice. d, Collagen staining by Masson’s Trichrome on day 14. The red boxes indicate the location of magnified image(s) in panels to the right. Dotted line (left panel), region of implant. Asterisks, scaffold fibre. Scale bar, 1 mm (left panel). e, Collagen-wrapped mPLC-CaP fibres at indicated imaging depths (SHG, green; THG, red). Scale bar, 100 μm. f, Frequency distribution of the angle of collagen fibrils relative to the scaffold fibre orientation (three fields per mouse, three mice).
In parallel to neoangiogenesis, collagen deposition occurred in an incremental fashion (Fig. 2f,g), histologically forming a fibrillar capsule surrounding and penetrating the scaffold architecture by day 14 and thereafter (Fig. 4d). In a 3D reconstruction, two subsets of SHG-positive collagen fibres and bundles were detected, including fibres wrapping around (60–90 ° orientation) or aligning in parallel (0–15 °) to scaffold fibres (Fig. 4e,f). This indicated fibrous scarring as a mechanically precise process, leading to functionally distinct subsets of collagen bundles. In aggregate, giant-cell development is accompanied by a desmoplasia-like process consisting of leukocyte influx, primordial vascularization, and progressive collagen deposition and encapsulation of the porous implant.
Therapeutic targeting of macrophages and neoangiogenesis
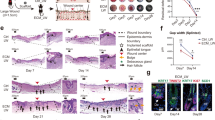
To test whether infiltrating macrophages and multinucleated giant cells are the cause or consequence of neovascularization and late-stage fibrosis, we depleted the macrophage lineage of scaffold-bearing mice with clodronate liposomes before graft implantation. After internalization, clodronate induces apoptosis of phagocytes but no other cells43. Clodronate treatment stopped the recruitment of infiltrate cells and giant-cell development, as previously reported19,24, and further blocked scaffold-associated neovascularization and fibrosis (Fig. 5a,b and Supplementary Fig. 6a). To directly test whether neovessel development and late-stage scarring were linked and dependent on the presence of macrophages and giant cells, lesions containing the scaffold were stained for VEGF-A, the predominant proangiogenic cytokine in regenerating and cancerous tissue44 and an important cytokine produced by M1 macrophages8,9. VEGF-A was prominently expressed in mononucleated stromal cells and giant cells (Fig. 5c, left panel); after clodronate treatment both VEGF-positive cell subsets were absent, without compensatory up-regulation of VEGF-A in other tissue regions (Fig. 5c, right panel), and there was an absence of neoangiogenesis (Fig. 5a). This identified myeloid and giant cells as the main source for VEGF-A and as drivers of neovessel formation.
a, Histology (H & E staining) of FBR to PCL scaffolds day 14 post-implantation in control mice and in mice after clodronate liposome (Cl), VEGF Trap (VT) and VEGF Trap + clodronate liposome (Cl + VT) treatment. Green arrowhead, giant FBR cell; blue arrowhead, blood vessels. Insets, magnification of giant cell. Scale bar, 100 μm. Analysis was performed for multi-image representations from entire scaffolds from three mice per group. Image area, 500 × 600 μm (×5 μm). b, Quantification of giant-cell and vessel numbers represented in panel a. c, VEGF-A expression in control- and clodronate liposome-treated mice, detected by immunohistochemistry and counted as percentage of VEGF-A positive cells of all mononucleated (m) and giant cells (g) (four fields per mouse; three mice). Image area, 500 × 700 μm (×5 μm). Scale bar and inset-image size, 100 μm (overviews). d, Longitudinal intravital multiphoton microscopy and analysis of the FBR in untreated and treated mice (example images represent 3D reconstructions from day 14). The graphs on the right show signal area (mean + s.d.) at different time points. Three fields per mouse; three mice; two experiments. Scale bars, 50 μm. **P < 0.01; ***P < 0.001; one-way analysis of variance followed by Tukey’s HSD post hoc test.
We next aimed to clarify whether neovascularization is an independent mechanism that enhances or counteracts the severity of the FBR and scarring. To minimize neovessel establishment directly, without targeting giant cells, we administered VEGF Trap to prevent VEGF availability45, using a dosing scheme effective for anti-angiogenic therapy in patients46,47. VEGF Trap stopped vessel formation and significantly diminished the numbers of giant cells and collagen deposition (Fig. 5a,b and Supplementary Fig. 6a). VEGF Trap monotherapy was slightly less efficient than clodronate treatment, but combining both agents resulted in near-complete blocking of giant cells and fibrosis development; although, this was not an improvement on the already profound effects observed by clodronate treatment alone (Fig. 5a,b).
When addressed longitudinally by intravital microscopy, clodronate-mediated cell depletion, effective by day 7, preceded the blocking of neovascularization and fibrous encapsulation of scaffold fibres by day 14 (Fig. 5d and Supplementary Fig. 6b). This failure to mount both neovessels and fibrosis after macrophage/giant-cell targeting indicated that myeloid cells are non-redundant drivers of FBR-associated tissue remodelling. VEGF Trap inhibited tissue infiltration by GFP-positive cells after, but not before, day 7 (Fig. 5d), suggesting a two-step process with early-onset inflammation mediated by VEGF-independent pre-existing vessels, followed by secondary exacerbation via VEGF-dependent neovessels.
Cell accumulation near the implant was strongly diminished by clodronate and/or VEGF Trap (Supplementary Fig. 7a,b), but the positional stability of residual scaffold-associated cells and the mobility distribution of interstitial cells remained unperturbed, compared with untreated lesions (Supplementary Fig. 7c–e). These results indicated that both clodronate and VEGF targeting act by limiting cell entry into the tissue rather than mobility within the lesion.
To investigate the long-term efficacy of macrophage and neovessel depletion on the FBR and fibrotic encapsulation beyond the two-week observation period achievable using the window system, window-free mice bearing scaffolds were treated with clodronate liposomes and/or VEGF Trap and assessed by ex vivo 3D SHG/THG microscopy and histology on day 28 (Fig. 6a–c and Supplementary Fig. 6a, lower panels). Suppression of the FBR, including diminished inflammation, vascularization and fibrosis, was sustained by anti-angiogenesis therapy and, with stronger effects, by clodronate and combined clodronate and VEGF Trap treatment (Fig. 6b,c).
a, Long-term treatment schedule for analyses shown in b–c. b, Histological analysis (H & E staining; left) and quantification (graphs) of end-stage FBR in untreated and treated (clodronate liposome (Cl), VEGF Trap (VT) and VEGF Trap + clodronate liposome (Cl + VT)) mice at day 28. Green arrowhead, giant cell; blue arrowhead, blood vessels. Analysis was performed for multi-image representations from entire scaffolds from three to five mice per group. Scale bar, 100 μm. c, Ex vivo multiphoton microscopy and analysis of scaffold 28 d post-implantation after different treatments and quantification of SHG surface area. Scale bar, 50 μm. d, Therapy withdrawal schedule for analyses shown in e–h. Mice were treated for 21 days and analysed 35 or 49 days after scaffold implantation. e, Histological analysis (H & E staining) of end-stage FBR in untreated and treated mice at day 49. Green arrowhead, giant cell; blue arrowhead, blood vessels. f, Ex vivo multiphoton microscopy and analysis of scaffold at day 49. Scale bar, 50 μm g,h. Quantification of SHG surface area, vessel and giant-cell number at day 35 (g) and day 49 (h) after therapy withdrawal on day 21. Analysis was performed for multi-image representations from entire scaffolds from three to five mice per group. Image area, 500 × 700 μm (×5 μm). *P < 0.05; **P < 0.01; ***P < 0.001; one-way analysis of variance followed by Tukey’s HSD post hoc test.
Lastly, we tested whether the therapeutic effects persisted after discontinuation of clodronate and/or VEGF Trap administration, by monitoring FBR giant cells, vessels and collagen deposition 14 and 28 days after the treatment was suspended (Fig. 6d). For all therapy settings, the FBR severity remained significantly diminished until at least four weeks post therapy withdrawal, including stable collagen levels and vessel numbers, and a decreased but partly relapsing density of giant cells (Fig. 6e–h). This indicated that dampening the initial inflammatory phase is crucial to reach a sustained anti-angiogenic and anti-fibrotic response.
Discussion
In summary, by implementing nonlinear intravital microscopy to monitor 3D porous mPCL-CaP scaffolds and the related cell and tissue dynamics, we identified the connection between giant cells and VEGF-induced neovessels as the central pathogenic axis driving the FBR and late-stage fibrosis. In a reciprocal process, implant-associated myeloid cells, including giant cells, locally release VEGF-A, which is required to induce neovessels that, in turn, maintain additional cell recruitment and enhance myeloid inflammation and local encapsulation of the graft site by collagen fibres (Fig. 7). The results suggest that an immature neovessel network aggravates the FBR, similar to the way that tumour-associated neovessels support inflammation, metabolic deregulation and desmoplastic remodelling of the tumour stroma42. Because macrophage recruitment, giant-cell development and emerging neovascularization cooperate in series to entertain the fibrosis response, therapeutic interference with either process is suited to limit tissue remodelling and scar formation24.
Implantation of a mPCL-CaP scaffold into vascularized tissue triggers a FBR. Early influx of monocytes from normal skin vessels is followed by differentiation to macrophages and multinucleated giant cells decorating the foreign material. By releasing VEGF, macrophages/giant cells induce the formation of a primitive neovasculature that resembles primordial vessels of tumour and desmoplastic tissues and supports further cell recruitment, inflammation, and accumulation of fibrillar collagen that borders both graft material and site of inflammation. Continuous arrows represent migratory events or release; dashed arrows represent induction of a phenomenon; 1 and 2 highlight steps of interference with macrophage recruitment and VEGF functions, respectively.
A rich set of cytokines is released during a FBR, including IL-1b, IL-4, IL-6, IL-8, IL-10, IL-13, MCP-1, TGF-β and VEGF2,7,9,48,49. However, their pathogenic impact on whether they aggravate or limit late stages (in particular) of the FBR is poorly understood2. Besides confirming macrophages and giant cells as central drivers of FBR, we identified a major role for VEGF-releasing M1 polarized cells as important exacerbators of the FBR to mPCL-CaP, with persistent and probably dysfunctional neovascularization and fibrosis as outcomes. Targeting macrophages and giant cells, either by non-specific depletion using clodronate liposome treatment, or by a targeted molecular therapy to remove specific subsets (for example, M1- or M2-skewed cells50), represents a promising strategy to modulate both early inflammation (days), as shown recently19, and late-stage tissue remodelling (weeks) to minimize a chronic FBR and detrimental scarring.
Our results further establish anti-VEGF therapy as an intervention principle48 to efficiently reduce both neovascularization and subsequent macrophage influx. Thus, anti-VEGF therapy might be specified for grafts that do not require sustained vascularization for full functionality, but suffer from inflammation and fibrosis, including implanted prosthesis, valves, pacemakers, catheters and defibrillators3,7.
Depleting myeloid cells and neovascularization using targeted (combination) therapy, may produce a largely avascular and non-inflamed integration site, which may be critical towards achieving a scar-free long-term integration. Given their proven safety profile in clinical application, where they are generally tolerated well with no long-term adverse effects on the immune system51–53, individual or combined administration of bisphosphonates and anti-angiogenesis regimens represent promising systemic, or local, topical strategies to improve long-term integration and functionality of material-based grafts.
Methods
Scaffold design and fabrication
mPCL (Purac Biochem) was 3D printed by means of melt electrospinning writing (MEW)54. Briefly, mPCL was melted at 94 °C in a glass syringe and extruded through a 23G blunt-end needle at 10 μl h−1 and at 20 mm distance from a rotating metallic collector, which was mounted on an x–y stage under an electrical field of 11–12 kV. Scaffolds were surface-treated with NaOH etching and/or CaP coating to reduce the hydrophobicity of PCL. For NaOH etching, scaffolds were soaked in 70% ethanol for 15 min, washed with distilled water, subjected to a 3M NaOH solution (1 h, 37 °C) and then washed with distilled water until pH 7.0 was reached. CaP coating was performed as described in ref. 54. After surface treatment, scaffolds were sterilized by immersion in 70% ethanol and air dried under exposure to ultraviolet light.
Dorsal skinfold chamber model and mPCL-CaP scaffold implantation
Animal studies were approved by the Institutional Animal Care and Use Committee of The University of Texas, M.D. Anderson Cancer Center and performed according to the institutional guidelines for animal care and handling. Dorsal skin-fold chambers were transplanted onto 10- to 12-week-old C57BL/6-Tg (UBC-GFP) 30Scha/J (Jackson Lab) or athymic nu/nu mice (experimental radiation oncology, MD Anderson), as described55. During surgery, a 5 × 5 × 0.2 mm scaffold was surgically implanted into the subcutaneous tissue of either imaging window bearing or window-free mice. This was monitored for up to 14 and 49 days, respectively. For surgery, mice were anaesthetized using Ketamine/Xylazine and the wound was closed by 4.0 polypropylene sutures.
Intravital microscopy, scaffold imaging and image analysis
For intravital microscopy, mice were anaesthetized with isofluorane and stably mounted onto a temperature-controlled platform (37 °C). The FBR elicited by an implanted mPCL-CaP scaffold was monitored using a custom intravital multiphoton microscope (LaVision BioTech)31 with three Ti:Sapphire lasers (Chameleon-XR, Coherent) and two Optical Parametric Oscillators (APE/Coherent), resulting in a tunable excitation range from 800 to 1,300 nm (Supplementary Fig. 1). Multi-spectral detection was performed using up to five backward or two forward photomultipliers (PMTs) and up to three excitation wavelengths in two consecutive scans, to separate the following excitation and emission channels: GFP (920 nm; 525/50 nm); Hoechst 33342 (920 nm; 450/60 nm), Rhodamine (1090 nm; 595/40 nm), SHG (1090 nm; 525/50 nm), THG (1180 nm; 387/15 nm) and AlexaFluor750 (1180 nm; 810/90 nm).
For intravital detection, long-working distance ×16 NA 0.8 water or ×25 NA 1.05 multi-immersion oil/water objectives (Olympus) were used. Sequential 3D stacks were obtained of 5–10 μm step-size, reaching up to 200 μm penetration depth. Images were acquired in a random fashion within the subcutaneous tissue up to the dermis. Perfused blood vessels were visualized by intravenous injection of Rhodamine- or AlexaFluor750-conjugated dextran (70 kDa; Invitrogen; 1 mg per mouse). mPCL-CaP scaffolds, in vitro, were analysed using SHG and THG imaging.
Digital image processing, segmentation and quantitative analysis
Images were reconstructed, stitched and analysed using FIJI (W. Rasband, NIH). Individual 3D scan fields representing z-projections of 50 to 300 μm were stitched to large-field montages for both overview and detailed analysis. Quantitative analysis of THG, SHG and fluorescent channels was performed on 3D stacks of 360 × 360 μm, with 10 μm step intervals in the z direction. For standardized enumeration of the local density of GFP-positive cells or vascular density, single channel z-stacks were masked, thresholded (default or Li algorithm), converted to binary images and the signal-positive area was obtained. For each sample, the relative fluorescence density was obtained from ten slices per z-stack, averaged and represented as the percentage of the total area. Four to eight independent fields were averaged per implant from at least four mice per condition. The experiment was repeated three times.
For topography-controlled analysis of fibrillar collagen density in association with scaffold fibres, the SHG intensity of 40 × 40 μm regions were quantified as an average from two adjacent slices preceding the appearance of the PCL fibre, as identified by the THG signal (three to five PCL fibres per sample; three mice per group). Each region was masked, thresholded (Li algorithm) and quantified for the signal-positive area fraction. Single cell velocities from time-lapse sequences were obtained by computer-assisted cell tracking (Autozell). For giant-cell counts, any cell displaying ≥2 nuclei was counted as multinucleated. The mean number of nuclei per giant cell was 5.95 ± 2, quantified from 60 random giant-cells; only a minority of giant cells retained only two nuclei.
Therapeutic intervention
Mice bearing mPCL-CaP scaffold in the subcutaneous tissue received VEGF Trap (500 ng per week, once per week) starting four days after scaffold implantation. VEGF Trap is a recombinant fusion protein with VEGF-A neutralizing activity combining the binding portions of VEGF receptors 1 and 2, with the Fc region of the human IgG146. Clodronate liposomes (200 μl per mouse) were administered every two to three days, starting three days before scaffold implantation to deplete macrophages by the day of implantation. For combination therapy, both regimens were combined. For intravital microscopy, three independent fields were averaged per implant, with three mice per group. The experiment was repeated twice.
Histological analysis
Mice were euthanized 14, 28, 35 or 49 days after implantation of the scaffold. Scaffold-bearing skin was excised, fixed (4% buffered formaldehyde) and embedded in paraffin for hematoxylin and eosin (H & E) or Masson’s Trichrome staining (five sections per sample, 5 μm thick, three to four samples per treatment). The experiment was repeated twice.
Flow cytometry
Infiltrate cells associated with the FBR in vivo were isolated and phenotyped by flow cytometry (LSRII FACS, Becton-Dickinson; Diva). To reach sufficient cell numbers, 20 scaffolds were recovered 14 days post implantation, mechanically disaggregated to generate a single-cell suspension and pooled. Phenotyping was performed using the following rat monoclonal antibodies (BD Pharmingen): PE-Cy 7 anti-CD45; PerCP-Cy 5.5 anti-CD11b; Pacific Blue anti-Ly6G, APC-conjugated anti-F4/80 and anti-CD8b. Subset gating and analysis were performed using FLowJo10.2.
Data availability
All the relevant data supporting the findings of this study are available within the paper, its Supplementary Information and from the corresponding authors upon reasonable request.
Additional information
How to cite this article: Dondossola, E. et al. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat. Biomed. Eng. 1, 0007 (2016).
References
Babensee, J. E., Anderson, J. M., McIntire, L. V. & Mikos, A. G. Host response to tissue engineered devices. Adv. Drug Deliver. Rev. 33, 111–139 (1998).
Ward, W. K. A review of the foreign-body response to subcutaneously-implanted devices: the role of macrophages and cytokines in biofouling and fibrosis. J. Diabetes Sci. Technol. 2, 768–777 (2008).
Morais, J. M., Papadimitrakopoulos, F. & Burgess, D. J. Biomaterials/tissue interactions: possible solutions to overcome foreign body response. AAPS J. 12, 188–196 (2010).
Sheikh, Z., Brooks, P. J., Barzilay, O., Fine, N. & Glogauer, M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials 8, 5671–5701 (2015).
Sussman, E. M., Halpin, M. C., Muster, J., Moon, R. T. & Ratner, B. D. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann. Biomed. Eng. 42, 1508–1516 (2014).
Anderson, J. M. Inflammatory response to implants. ASAIO Trans. 34, 101–107 (1988).
Anderson, J. M., Rodriguez, A. & Chang, D. T. Foreign body reaction to biomaterials. Semin. Immunol. 20, 86–100 (2008).
Yu, T., Tutwiler, V. J. & Spiller, K. in Biomaterials in Regenerative Medicine and the Immune System (ed. Santambrogio, L. ) 17–34 (Springer, 2015).
Spiller, K. L. et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 35, 4477–4488 (2014).
Moore, L. B. & Kyriakides, T. R. Molecular characterization of macrophage-biomaterial interactions. Adv. Exp. Med. Biol. 865, 109–122 (2015).
Mantovani, A., Sozzani, S., Locati, M., Allavena, P. & Sica, A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 (2002).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 (2012).
Martinez, F. O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13 (2014).
Ferrante, C. J. & Leibovich, S. J. Regulation of macrophage polarization and wound healing. Adv. Wound Care 1, 10–16 (2012).
Rostam, H. M. et al. The impact of surface chemistry modification on macrophage polarisation. Immunobiology 221, 1237–1246 (2016).
Palmer, J. A., Abberton, K. M., Mitchell, G. M. & Morrison, W. A. Macrophage phenotype in response to implanted synthetic scaffolds: an immunohistochemical study in the rat. Cells Tissues Organs 199, 169–183 (2014).
Yu, T. et al. Temporal and spatial distribution of macrophage phenotype markers in the foreign body response to glutaraldehyde-crosslinked gelatin hydrogels. J. Biomat. Sci.-Polym. E. 27, 721–742 (2016).
Miron, R. J. & Bosshardt, D. D. OsteoMacs: key players around bone biomaterials. Biomaterials 82, 1–19 (2016).
Veiseh, O. et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 14, 643–651 (2015).
Major, M. R., Wong, V. W., Nelson, E. R., Longaker, M. T. & Gurtner, G. C. The foreign body response: at the interface of surgery and bioengineering. Plast. Reconstr. Surg. 135, 1489–1498 (2015).
Zeplin, P. H., Larena-Avellaneda, A. & Schmidt, K. Surface modification of silicone breast implants by binding the antifibrotic drug halofuginone reduces capsular fibrosis. Plast. Reconstr. Surg. 126, 266–274 (2010).
Klueh, U., Dorsky, D. I. & Kreutzer, D. L. Enhancement of implantable glucose sensor function in vivo using gene transfer-induced neovascularization. Biomaterials 26, 1155–1163 (2005).
Kastellorizios, M., Papadimitrakopoulos, F. & Burgess, D. J. Multiple tissue response modifiers to promote angiogenesis and prevent the foreign body reaction around subcutaneous implants. J. Control. Release 214, 103–111 (2015).
Mooney, J. E. et al. Cellular plasticity of inflammatory myeloid cells in the peritoneal foreign body response. Am. J. Pathol. 176, 369–380 (2010).
Cao, H., McHugh, K., Chew, S. Y. & Anderson, J. M. The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J. Biomed. Mater. Res. A 93, 1151–1159 (2010).
Chen, S. et al. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials 31, 3479–3491 (2010).
Damanik, F. F., Rothuizen, T. C., van Blitterswijk, C., Rotmans, J. I. & Moroni, L. Towards an in vitro model mimicking the foreign body response: tailoring the surface properties of biomaterials to modulate extracellular matrix. Sci. Rep. 4, 6325 (2014).
Woodruff, M. A. & Hutmacher, D. W. The return of a forgotten polymer—polycaprolactone in the 21st century. Prog. Polym. Sci. 35, 1217–1256 (2010).
Holzapfel, B. M. et al. Tissue engineered humanized bone supports human hematopoiesis in vivo . Biomaterials 61, 103–114 (2015).
Holzapfel, B. M. et al. Species-specific homing mechanisms of human prostate cancer metastasis in tissue engineered bone. Biomaterials 35, 4108–4115 (2014).
Andresen, V. et al. Infrared multiphoton microscopy: subcellular-resolved deep tissue imaging. Curr. Opin. Biotech. 20, 54–62 (2009).
Weigelin, B., Bakker, G. J. & Friedl, P. Third harmonic generation microscopy of cells and tissue organization. J. Cell Sci. 129, 245–255 (2016).
Ho, R. M., Chiang, Y. W., Lin, C. C. & Huang, B. H. Crystallization and melting behavior of poly(epsilon-caprolactone) under physical confinement. Macromolecules 38, 4769–4779 (2005).
Rodriguez, A., Macewan, S. R., Meyerson, H., Kirk, J. T. & Anderson, J. M. The foreign body reaction in T-cell-deficient mice. J. Biomed. Mater. Res. A 90, 106–113 (2009).
Rodriguez, A., Voskerician, G., Meyerson, H., MacEwan, S. R. & Anderson, J. M. T cell subset distributions following primary and secondary implantation at subcutaneous biomaterial implant sites. J. Biomed. Mater. Res. A 85, 556–565 (2008).
Marques, S. M. et al. Genetic background determines mouse strain differences in inflammatory angiogenesis. Microvasc. Res. 82, 246–252 (2011).
Rohan, R. M., Fernandez, A., Udagawa, T., Yuan, J. & D’Amato, R. J. Genetic heterogeneity of angiogenesis in mice. FASEB J. 14, 871–876 (2000).
Middleton, J. C. & Tipton, A. J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 21, 2335–2346 (2000).
Gunatillake, P. A. & Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 5, 1–16 (2003).
Wang, P. et al. Quantitative analysis of tumor vascular structure after drug treatment. In Ann. Int. Conf. IEEE Engineer. Med. Biol. Soc. IEEE Engineering in Medicine and Biology Society. Annual Conference 726–729 (IEEE, 2010).
Wild, R., Ramakrishnan, S., Sedgewick, J. & Griffioen, A. W. Quantitative assessment of angiogenesis and tumor vessel architecture by computer-assisted digital image analysis: effects of VEGF-toxin conjugate on tumor microvessel density. Microvasc. Res. 59, 368–376 (2000).
Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000).
Van Rooijen, N. & Sanders, A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174, 83–93 (1994).
Ferrara, N., Gerber, H. P. & LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 9, 669–676 (2003).
Holash, J. et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc. Natl Acad. Sci. USA 99, 11393–11398 (2002).
Wang, T. F. & Lockhart, A. C. Aflibercept in the treatment of metastatic colorectal cancer. Clin. Med. Ins. Oncol. 6, 19–30 (2012).
Stewart, M. W. Aflibercept (VEGF Trap-eye): the newest anti-VEGF drug. Br. J. Ophthalmol. 96, 1157–1158 (2012).
Kwee, B. J. & Mooney, D. J. Manipulating the intersection of angiogenesis and inflammation. Ann. Biomed. Eng. 43, 628–640 (2015).
Jones, J. A. et al. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J. Biomed. Mater. Res. A 83, 585–596 (2007).
Hristodorov, D. et al. Targeting CD64 mediates elimination of M1 but not M2 macrophages in vitro and in cutaneous inflammation in mice and patient biopsies. MAbs 7, 853–862 (2015).
Kennel, K. A. & Drake, M. T. Adverse effects of bisphosphonates: implications for osteoporosis management. Mayo Clin. Proc. 84, 632–637 (2009).
McClung, M. et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am. J. Med. 126, 13–20 (2013).
Do, D. V. et al. The DA VINCI Study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology 118, 1819–1826 (2011).
Thibaudeau, L. et al. A tissue-engineered humanized xenograft model of human breast cancer metastasis to bone. Dis. Model. Mech. 7, 299–309 (2014).
Alexander, S., Koehl, G. E., Hirschberg, M., Geissler, E. K. & Friedl, P. Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem. Cell Biol. 130, 1147–1154 (2008).
Acknowledgements
We thank M. Starbuck, C. Johnston, Y. Xiaoqing, R. Jimenez and J. Douglas for histological processing of the samples; and E. De Juan Pardo for the manufacturing of the mPCL-CaP scaffolds. E.D. was supported by the Cancer Prevention and Research Institute of Texas (RP140482) and the Prostate Cancer Foundation (16YOUN24). P.F. was supported by the Netherlands Science Organization (NWO-VICI 918.11.626), the European Research Council (ERC-CoG DEEPINSIGHT, Project No. 617430) and the Cancer Genomics Center, The Netherlands. This work was further supported by the National Health and Medical Research Council (NHMRC Project Grant 1082313), the National Breast Cancer Foundation (NBCF IN-15-047) and the Worldwide Cancer Research (WWCR 15-11563) to B.M.H. and D.W.H. and the German Research Foundation (DFG HO 5068/1-1) to B.M.H. The Genitourinary Cancers Program of the CCSG shared resources at the MD Anderson Cancer Center was supported by the National Institute of Health/National Cancer Institute award number P30 CA016672.
Author information
Authors and Affiliations
Contributions
E.D., B.M.H., S.A., D.W.H. and P.F. designed the research. E.D., B.M.H., S.A., S.F. and D.W.H. performed the research. E.D., S.F., D.W.H. and P.F. analysed the data. E.D., B.M.H., D.W.H. and P.F. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures and video captions. (PDF 13926 kb)
Video 1
Kinetics of infiltrate cells at day 4 post-scaffold implantation. (AVI 5426 kb)
Video 2
Kinetics of infiltrate cells at day 7 post-scaffold implantation. (AVI 12122 kb)
Video 3
Kinetics of infiltrate cells at day 10 post-scaffold implantation. (AVI 15468 kb)
Video 4
Cytoplasmic dynamics of scaffold-associated giant cells. (AVI 1036 kb)
Rights and permissions
About this article
Cite this article
Dondossola, E., Holzapfel, B., Alexander, S. et al. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat Biomed Eng 1, 0007 (2017). https://doi.org/10.1038/s41551-016-0007
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-016-0007
This article is cited by
-
Polymer clip granuloma mimicking lymph node recurrence: a case report
Surgical Case Reports (2024)
-
Tissue Remodeling After Implantation with Polymethylmethacrylate: An Experimental Study in Mice
Aesthetic Plastic Surgery (2023)
-
Transfer learning in a biomaterial fibrosis model identifies in vivo senescence heterogeneity and contributions to vascularization and matrix production across species and diverse pathologies
GeroScience (2023)
-
Engineering the next generation of cell-based therapeutics
Nature Reviews Drug Discovery (2022)
-
Controlled release of growth factors using synthetic glycosaminoglycans in a modular macroporous scaffold for tissue regeneration
Communications Biology (2022)