Abstract
The Hippo pathway plays critical roles in cell growth, differentiation, organ development and tissue homeostasis, whereas its dysregulation can lead to tumorigenesis. YAP and TAZ are transcription co-activators and represent the main downstream effectors of the Hippo pathway. Here, we show that heat stress induces a strong and rapid YAP dephosphorylation and activation. The effect of heat shock on YAP is dominant to other signals known to modulate the Hippo pathway. Heat shock inhibits LATS kinase by promoting HSP90-dependent LATS interaction with and inactivation by protein phosphatase 5. Heat shock also induces LATS ubiquitination and degradation. YAP and TAZ are crucial for cellular heat shock responses, including the heat shock transcriptome and cell viability. This study uncovers previously unknown mechanisms of Hippo regulation by heat shock, as well as physiological functions of YAP, in the heat stress response. Our observations also reveal a potential combinational therapy involving hyperthermia and targeting of the Hippo pathway.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
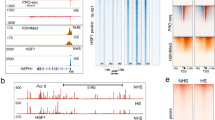
The RNA-seq data are available in the Gene Expression Omnibus (GEO) dataset with the accession number GSE133251. The available ChIP-seq data were obtained from ENCODE at the UCSC Genome Browser (http://www.genome.ucsc.edu/ENCODE/; UCSC accession numbers wgEncodeEH002333, wgEncodeEH002345 and wgEncodeEH000754). The consensus sequence of known motifs was downloaded from the Homer Motif database (http://homer.ucsd.edu/homer/motif/HomerMotifDB/homerResults.html). All other data that support the findings of this study are available upon request from the corresponding author. Source data are provided with this paper.
Change history
11 December 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41556-020-00623-4
References
Yu, F.-X., Zhao, B. & Guan, K.-L. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163, 811–828 (2015).
Pan, D. The Hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 (2010).
Lin, K. C., Park, H. W. & Guan, K.-L. Deregulation and therapeutic potential of the Hippo pathway in cancer. Annu. Rev. Cancer Biol. 2, 59–79 (2018).
Harvey, K. F., Zhang, X. & Thomas, D. M.The Hippo pathway and human cancer. Nat. Rev. Cancer 13, 246–257 (2013).
Meng, Z., Moroishi, T. & Guan, K.-L. Mechanisms of Hippo pathway regulation. Genes Dev. 30, 1–17 (2016).
Dong, J. et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 (2007).
Zhao, B. et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971 (2008).
Åkerfelt, M., Morimoto, R. I. & Sistonen, L.Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 11, 545–555 (2010).
Wust, P. et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. 3, 487–497 (2002).
Roti Roti, J. L. Cellular responses to hyperthermia (40–46 °C): cell killing and molecular events. Int. J. Hyperth. 24, 3–15 (2008).
Zhang, H.-G., Mehta, K., Cohen, P. & Guha, C. Hyperthermia on immune regulation: a temperature’s story. Cancer Lett. 271, 191–204 (2008).
Hansen, C. G., Moroishi, T. & Guan, K. L. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 25, 499–513 (2015).
Zhao, B. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 (2007).
Budzyński, M. A., Puustinen, M. C., Joutsen, J. & Sistonen, L. Uncoupling stress-inducible phosphorylation of heat shock factor 1 from its activation. Mol. Cell. Biol. 35, 2530–2540 (2015).
Chan, E. H. et al. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 24, 2076–2086 (2005).
Hergovich, A., Schmitz, D. & Hemmings, B. A. The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochem. Biophys. Res. Commun. 345, 50–58 (2006).
Ni, L., Zheng, Y., Hara, M., Pan, D. & Luo, X. Structural basis for Mob1-dependent activation of the core Mst–Lats kinase cascade in Hippo signaling. Genes Dev. 29, 1416–1431 (2015).
Wang, W. et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat. Cell Biol. 17, 490–499 (2015).
Mo, J.-S. et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat. Cell Biol. 17, 500–510 (2015).
Hong, A. W. et al. Osmotic stress-induced phosphorylation by NLK at Ser128 activates YAP. EMBO Rep. 18, 72–86 (2017).
Trinklein, N. D., Murray, J. I., Hartman, S. J., Botstein, D. & Myers, R. M. The role of heat shock transcription factor 1 in the genome-wide regulation of the mammalian heat shock response. Mol. Biol. Cell 15, 1254–1261 (2004).
Huntoon, C. J. et al. Heat shock protein 90 inhibition depletes LATS1 and LATS2, two regulators of the mammalian Hippo tumor suppressor pathway. Cancer Res. 70, 8642–8650 (2010).
Raghunathan, V. K. et al. Involvement of YAP, TAZ and HSP90 in contact guidance and intercellular junction formation in corneal epithelial cells. PLoS ONE 9, e109811 (2014).
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Papp, E. & Csermely, P. in Molecular Chaperones in Health and Disease (eds. Starke, K. & Gaestel, M.) 405–416 (Springer, 2006).
Sreedhar, A. S., Kalmár, É., Csermely, P. & Shen, Y.-F. Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett. 562, 11–15 (2004).
Russell, L. C., Whitt, S. R., Chen, M.-S. & Chinkers, M. Identification of conserved residues required for the binding of a tetratricopeptide repeat domain to heat shock protein 90. J. Biol. Chem. 274, 20060–20063 (1999).
Das, A. K., Cohen, P. T. & Barford, D. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein–protein interactions. EMBO J. 17, 1192–1199 (1998).
Yu, F.-X. et al. Regulation of the Hippo–YAP pathway by G-protein-coupled receptor signaling. Cell 150, 780–791 (2012).
Chen, R., Xie, R., Meng, Z., Ma, S. & Guan, K. L. STRIPAK integrates upstream signals to initiate the Hippo kinase cascade. Nat. Cell Biol. 21, 1565–1577 (2019).
Jolly, C. & Morimoto, R. I. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J. Natl Cancer Inst. 92, 1564–1572 (2000).
Kregel, K. C. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. 92, 2177–2186 (2002).
Ma, B. et al. Hypoxia regulates Hippo signalling through the SIAH2 ubiquitin E3 ligase. Nat. Cell Biol. 17, 95–103 (2015).
Salah, Z., Melino, G. & Aqeilan, R. I. Negative regulation of the Hippo pathway by E3 ubiquitin ligase ITCH is sufficient to promote tumorigenicity. Cancer Res. 71, 2010–2020 (2011).
Ho, K. C. et al. Itch E3 ubiquitin ligase regulates large tumor suppressor 1 stability. Proc. Natl Acad. Sci. USA 108, 4870–4875 (2011).
Richter, K., Haslbeck, M. & Buchner, J. The heat shock response: life on the verge of death. Mol. Cell 40, 253–266 (2010).
Iwasa, H. et al. Yes-associated protein homolog, YAP-1, is involved in the thermotolerance and aging in the nematode Caenorhabditis elegans. Exp. Cell Res. 319, 931–945 (2013).
Meng, Z. et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat. Commun. 6, 8357 (2015).
Moroishi, T. et al. The Hippo pathway kinases LATS1/2 suppress cancer immunity. Cell 167, 1525–1539 (2016).
Acknowledgements
This work was supported by grants from the National Institutes of Health (CA196878 and DE015694) to K.-L.G. This study was supported by grants from the National Natural Science Foundation of China (31800773), China Postdoctoral Science Foundation (2018M633369), Sichuan Science and Technology Program (2019YJ0063) and Youth Science Foundation of West China Hospital of Stomatology (WCHS-201703) to M.L. This work was supported by the National Major Scientific and Technological Special Project for ‘Significant New Drugs Development’ (2018ZX09733001), Development Program of China (2016YFA0201402) and Excellent Youth Foundation of Sichuan Scientific Committee Grant in China (N2019JDJQ008) to X.W. This research was also partly funded by the China Scholarship Council (201506240035). We thank F. Yu from Fudan University and B. Zhao from Zhejiang University for reagents.
Author information
Authors and Affiliations
Contributions
M.L. and K.-L.G. conceived the project, designed the experiments, analysed the data and wrote the manuscript. M.L. performed most of the experiments and data analysis in the laboratories of K.-L.G., P.Z. and Y.W. Z.M. and T.M. provided parts of the knockout cell lines and plasmids. Z.M., T.M. and K.C.L. provided technical support. M.L. performed the syngeneic murine experiments with assistance from G.S. and F.M., as well as the RNA-seq analysis with help from B.S. and X.W.
Corresponding author
Ethics declarations
Competing interests
K.-L.G. is a co-founder of, and has an equity interest in, Vivace Therapeutics. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Heat shock activates YAP.
a, Heat shock induces YAP dephosphorylation in multiple cell lines: human colon cancer HCT116; human uveal melanoma MEL270; mouse fibroblast NIH3T3; mouse squamous cell carcinoma SCC7; mouse melanoma B16-OVA. Various tumor and non-tumor cell lines were seeded onto six well plates to achieve high density (100% confluence). After 24 h, cells were heat shocked at 43 °C for the indicated times and collected for YAP phos-tag gel analyses. b, Heat shock induced a sustained YAP dephosphorylation. c, Mild heat shock-induced YAP dephosphorylation is cell type-dependent. High density HEK293A and A549 cells were heat shocked at 40 °C for the indicated times and collected for YAP phos-tag gel analyses. d, Cold stress does not affect YAP phosphorylation. High density HEK293A cells were placed at 25 °C or 4 °C for 30 min and 1 h; then samples were collected for YAP phos-tag gel analyses. Immunoblotting in panels a-d has been performed two times with similar results. Source data are available online.
Extended Data Fig. 2 MST1/2 and MAP4Ks are not required for heat shock-induced YAP regulation.
a, HEK293A cells were cultured under high density (upper panel) or in the absence of serum (lower panel), and were subjected to heat shock for the indicated times. YAP phosphorylation was detected by the phos-tag gel. b, Heat shock does not affect MST1 phosphorylation. HEK293A cells were transiently transfected with GST-MST1. 24 h after transfection, cells were subjected to heat shock for the indicated times. Glutathione Sepharose 4B beads (GE Healthcare) were used to purify GTS-MST1. Phosphorylation of the purified GST-MST1 was analyzed by Western blot with pMST1 (Thr183) antibody. c, YAP dephosphorylation time course in MST1-rescued or MAP4K4-rescued MM8KO cells upon heat shock. Plasmids for HA-YAP and FLAG-MST1 or FLAG-MAP4K4 were transiently co-transfected into HEK293A MM8 KO cells. 24 h after transfection, cells were subcultured to new plate and reached a medium confluence the next day, treated with serum starvation for 2 h, then subjected to heat shock for indicated durations. YAP phosphorylation was detected by the phos-tag gel. d, Heat shock does not affect the LATS1-MOB1 interaction. HEK293A cells were transiently co-transfected with FLAG-LATS1 and 3×HA-MOB1. 24 h after transfection, cells were subjected to heat shock for the indicated times. FLAG antibodies were used for immunoprecipitation and the co-precipitated proteins were detected by Western blot. The uppermost panel were normalized against FLAG-LATS1 protein levels. Immunoblotting in panels a-d has been performed two times with similar results. Source data are available online.
Extended Data Fig. 3 Related to Fig. 4.
a, Deletion of HSF-1 abolishes expression of heat shock responsive genes. High density HEK293A WT and HSF-1 KO cells were subjected to heat shock for 1 or 2 h, and mRNA expression of downstream target genes was measured by RT-PCR. Data are presented as mean ± s.d.; n = 3 biologically independent samples. Two-way ANOVA test, ns, not significant. Two independent KO clones are shown. b, Chemical chaperones, which can prevent protein misfolding, cannot block YAP dephosphorylation by heat shock. HEK2933A cells were pretreated with indicated chemical chaperones sodium 4-phenylbutyrate (4-PBA) or DMSO for 24 h and were then subjected to heat shock as indicated. The phosphorylation of YAP was detected by a phos-tag gel. c, Knockout of HSP90, but not HSF-1 or HSP70, delays YAP dephosphorylation in response to heat shock. HEK293A WT, HSF-1 KO, HSP70-1/2 DKO, and HSP90α/β DKO cells were subjected to heat shock. Samples were analyzed by western blot on phos-tag gel for YAP phosphorylation and regular gel for YAP protein levels. d, Quantification of the Western blots in Extended Data Fig. 3c. e, The verification of HSP90α and HSP90β knockdown efficiency by Western blot. Two independent siRNAs were used. Immunoblotting in panels b, c and e has been performed two times with similar results. Source data are available online.
Extended Data Fig. 4 Verification of YAP/TAZ knockout in B16-OVA cells.
a, The DNA sequence confirms inactivation of YAP1 and WWTR1 genes in the #2 clone of B16-OVA YAP/TAZ DKO cells. b, Functional verification of YAP/TAZ knockout. LPA induced expression of YAP/TAZ target genes is abolished in the YAP/TAZ knockout B16-OVA cells. RT-PCR results are presented as mean ± s.d.; n = 3 biologically independent samples. Two-way ANOVA test, ns, not significant. Source data are available online.
Extended Data Fig. 5 YAP/TAZ silencing sensitizes tumor cells to heat shock.
a, Verification of YAP/TAZ siRNA knockdown in B16-OVA cells by Western blot and RT-PCR. Presented are mean ± s.d., n = 3 biologically independent samples. Two-way ANOVA test. b, YAP/TAZ knockdown reduces B16-OVA cell viability in response to heat shock. Cells were transfected with control or two independent YAP and TAZ siRNAs, subjected to heat shock at 45 °C or 46 °C for 1 h, then recovered at 37 °C for 24 h. Cell viability was determined by CCK8 assays. Data are mean ± s.d.; n = 3 biologically independent experiments. Two-way ANOVA test. ns, not significant; NT, no treatment. c, YAP/TAZ knockdown promotes B16-OVA apoptosis. Images are representative of two independent experiments with similar results. d, More cell debris and apoptotic bodies in YAP/TAZ knockdown cells. B16-OVA cells transfected with siRNA, heat shocked at 45oC for 1 h, and recovered at 37oC for 6 h. Cells were subjected to microscopic examination for morphology. Representative pictures from three independent samples are shown. Scale bar, 100 μm. e, YAP/TAZ knockdown compromises HSP induction by heat shock. Cells were similarly treated as in (d). mRNAs were quantified by RT-PCR. Data are mean ± s.d.; n = 3 biologically independent samples. Two-way ANOVA test. ns, not significant. Only siRNA#2 was chosen in this experiment. f, g, YAP/TAZ knockdown reduces SCC7 cell viability upon heat shock. CCK8 assay (f) and Annexin V staining (g). Data presented as mean ± s.d.; n = 3 biologically independent experiments. One-way ANOVA test. h, Verification of YAP/TAZ siRNA knockdown in HCT116 tumor cells. Immunoblotting has been performed two times with similar results. i, YAP/TAZ knockdown HCT116 cells are more sensitive to heat shock stress. CCK8 assay was performed to measure cell viability. Data are presented as mean ± s.d.; n = 3 biologically independent experiments. One -way ANOVA test. Source data are available online.
Extended Data Fig. 6 YAP/TAZ contribute to the heat shock-induced transcriptome.
a, Top 10 enriched gene ontology analysis for biological processes (BP) of 396 up-regulated genes regulated by YAP/TAZ. b, Top 10 enriched KEGG pathway analysis of 396 up-regulated genes regulated by YAP/TAZ. c, Log2 Fold Change of representative HSPs in B16-OVA siCon or siYAP/TAZ#2 treated cells (Data are from RNA-SEQ). d, Heat map of representative heat shock up-regulated genes regulated by YAP/TAZ (Data are from RNA-SEQ).
Extended Data Fig. 7 The ITCH and SIAH2 E3 ubiquitin ligases do not mediate heat shock-induced LATS degradation.
a, Knockdown of ITCH and SIAH2 does not block the heat shock-induced LATS1 degradation. Immunoblotting has been performed two times with similar results. b, Verification of ITCH&SIAH2 siRNA knockdown efficiency by RT-PCR. Data are presented as mean ± s.d.; n = 3 biologically independent samples. Two-way ANOVA test. Source data are available online.
Extended Data Fig. 8 Heat shock does not induce a universal protein dephosphorylation and kinase degradation.
a-b, High density HEK293A cells were heat shocked at 43oC for the indicated times and cell lysates were collected for Western blot to detect the phosphorylation and protein levels of the indicated proteins. c, HEK293A cells were transiently transfected with GST-MST1. 24 h after transfection, cells were subjected to heat shock for the indicated times. Glutathione Sepharose 4B beads were used to purify GST-MST1. Phosphorylation of GST-MST1 was analyzed by Western blot with pMST1 (Thr183) antibody. Immunoblotting for a-c has been performed two times with similar results. Source data are available online.
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Supplementary Table 1
Genomic co-occupancy analyses of interested genes by ChIP-seq data from ENCODE.
Source data
Source Data Fig. 1
Statistical source data
Source Data Fig. 1
Unprocessed western blots
Source Data Fig. 2
Statistical source data
Source Data Fig. 2
Unprocessed western blots
Source Data Fig. 3
Statistical source data
Source Data Fig. 3
Unprocessed western blots
Source Data Fig. 4
Statistical source data
Source Data Fig. 4
Unprocessed western blots
Source Data Fig. 5
Statistical source data
Source Data Fig. 5
Unprocessed western blots
Source Data Fig. 6
Statistical source data
Source Data Fig. 6
Unprocessed western blots
Source Data Fig. 7
Statistical source data
Source Data Fig. 7
Unprocessed western blots
Source Data Extended Data Fig. 1
Unprocessed western blots
Source Data Extended Data Fig. 2
Unprocessed western blots
Source Data Extended Data Fig. 3
Statistical source data
Source Data Extended Data Fig. 3
Unprocessed western blots
Source Data Extended Data Fig. 4
Statistical source data
Source Data Extended Data Fig. 5
Statistical source data
Source Data Extended Data Fig. 5
Unprocessed western blots
Source Data Extended Data Fig. 7
Statistical source data
Source Data Extended Data Fig. 7
Unprocessed western blots
Source Data Extended Data Fig. 8
Unprocessed western blots
Rights and permissions
About this article
Cite this article
Luo, M., Meng, Z., Moroishi, T. et al. Heat stress activates YAP/TAZ to induce the heat shock transcriptome. Nat Cell Biol 22, 1447–1459 (2020). https://doi.org/10.1038/s41556-020-00602-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-020-00602-9
This article is cited by
-
Comprehensive multi-omics analysis reveals WEE1 as a synergistic lethal target with hyperthermia through CDK1 super-activation
Nature Communications (2024)
-
Engineering a triple-functional magnetic gel driving mutually-synergistic mild hyperthermia-starvation therapy for osteosarcoma treatment and augmented bone regeneration
Journal of Nanobiotechnology (2023)
-
Insights into recent findings and clinical application of YAP and TAZ in cancer
Nature Reviews Cancer (2023)
-
Novel sequential therapy with metformin enhances the effects of cisplatin in testicular germ cell tumours via YAP1 signalling
Cancer Cell International (2022)
-
Okicamelliaside targets the N-terminal chaperone pocket of HSP90 disrupts the chaperone protein interaction of HSP90-CDC37 and exerts antitumor activity
Acta Pharmacologica Sinica (2022)