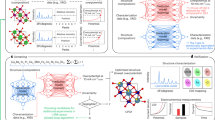
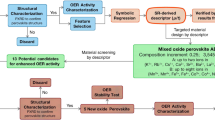
Abstract
Improved, highly active cathode materials are needed to promote the commercialization of ceramic fuel cell technology. However, the conventional trial-and-error process of material design, characterization and testing can make for a long and complex research cycle. Here we demonstrate an experimentally validated machine-learning-driven approach to accelerate the discovery of efficient oxygen reduction electrodes, where the ionic Lewis acid strength (ISA) is introduced as an effective physical descriptor for the oxygen reduction reaction activity of perovskite oxides. Four oxides, screened from 6,871 distinct perovskite compositions, are successfully synthesized and confirmed to have superior activity metrics. Experimental characterization reveals that decreased A-site and increased B-site ISAs in perovskite oxides considerably improve the surface exchange kinetics. Theoretical calculations indicate such improved activity is mainly attributed to the shift of electron pairs caused by polarization distribution of ISAs at sites A and B, which greatly reduces oxygen vacancy formation energy and migration barrier.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All relevant data are included in the paper and its Supplementary Information. Source Data are provided with this paper.
Code availability
The Python script for machine-learning model training and material screening are available at https://github.com/nlpcui/MLORR.
References
Shao, Z. & Haile, S. M. A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 431, 170–173 (2004).
Wachsman, E. D. & Lee, K. T. Lowering the temperature of solid oxide fuel cells. Science 334, 935–939 (2011).
Duan, C. et al. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 4, 230–240 (2019).
Ni, M. & Shao, Z. Fuel cells that operate at 300° to 500 °C. Science 369, 138–139 (2020).
Xie, H. et al. Cu-modified Ni foams as three-dimensional outer anodes for high-performance hybrid direct coal fuel cells. Chem. Eng. J. 410, 128239 (2021).
Wachsman, E. D., Marlowe, C. A. & Lee, K. T. Role of solid oxide fuel cells in a balanced energy strategy. Energy Environ. Sci. 5, 5498–5509 (2012).
Duan, C. et al. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 349, 1321–1326 (2015).
Song, Y. et al. A cobalt‐free multi‐phase nanocomposite as near‐ideal cathode of intermediate‐temperature solid oxide fuel cells developed by smart self‐assembly. Adv. Mater. 32, 1906979 (2020).
Jun, A., Kim, J., Shin, J. & Kim, G. Perovskite as a cathode material: a review of its role in solid‐oxide fuel cell technology. ChemElectroChem 3, 511–530 (2016).
Bello, I. T., Zhai, S., He, Q., Xu, Q. & Ni, M. Scientometric review of advancements in the development of high-performance cathode for low and intermediate temperature solid oxide fuel cells: three decades in retrospect. Int. J. Hydrog. Energy 46, 26518–26536 (2021).
Sunarso, J., Hashim, S. S., Zhu, N. & Zhou, W. Perovskite oxides applications in high temperature oxygen separation, solid oxide fuel cell and membrane reactor: a review. Prog. Energy Combust. Sci. 61, 57–77 (2017).
Suntivich, J. et al. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 3, 546–550 (2011).
Schmidt, J., Marques, M. R., Botti, S. & Marques, M. A. Recent advances and applications of machine learning in solid-state materials science. npj Comput. Mater. 5, 1–36 (2019).
Lee, Y.-L., Kleis, J., Rossmeisl, J., Shao-Horn, Y. & Morgan, D. Prediction of solid oxide fuel cell cathode activity with first-principles descriptors. Energy Environ. Sci. 4, 3966–3970 (2011).
Calle-Vallejo, F., Díaz-Morales, O. A., Kolb, M. J. & Koper, M. T. Why is bulk thermochemistry a good descriptor for the electrocatalytic activity of transition metal oxides? ACS Catal. 5, 869–873 (2015).
Hong, W. T. et al. Charge-transfer-energy-dependent oxygen evolution reaction mechanisms for perovskite oxides. Energy Environ. Sci. 10, 2190–2200 (2017).
Jacobs, R., Mayeshiba, T., Booske, J. & Morgan, D. Material discovery and design principles for stable, high activity perovskite cathodes for solid oxide fuel cells. Adv. Energy Mater. 8, 1702708 (2018).
Guan, D. et al. Screening highly active perovskites for hydrogen-evolving reaction via unifying ionic electronegativity descriptor. Nat. Commun. 10, 1–8 (2019).
Calle-Vallejo, F. et al. Number of outer electrons as descriptor for adsorption processes on transition metals and their oxides. Chem. Sci. 4, 1245–1249 (2013).
Stoerzinger, K. A., Risch, M., Han, B. & Shao-Horn, Y. Recent insights into manganese oxides in catalyzing oxygen reduction kinetics. ACS Catal. 5, 6021–6031 (2015).
Hu, S. & Li, W.-X. Sabatier principle of metal-support interaction for design of ultrastable metal nanocatalysts. Science 374, eabi9828 (2021).
Weng, B. et al. Simple descriptor derived from symbolic regression accelerating the discovery of new perovskite catalysts. Nat. Commun. 11, 1–8 (2020).
Shannon, R. T. & Prewitt, C. T. Effective ionic radii in oxides and fluorides. Acta Crystallogr. B 25, 925–946 (1969).
Lide, D. R. CRC Handbook of Chemistry and Physics Vol. 85 (CRC, 2004).
Wei, Y. et al. Activation strategies of perovskite-type structure for applications in oxygen-related electrocatalysts. Small Methods 5, 2100012 (2021).
Zhou, W. et al. Barium-and strontium-enriched (Ba0.5Sr0.5)1+xCo0.8Fe0.2O3−δ oxides as high-performance cathodes for intermediate-temperature solid-oxide fuel cells. Acta Mater. 56, 2687–2698 (2008).
Ciucci, F. & Chen, C. J. E. A. Analysis of electrochemical impedance spectroscopy data using the distribution of relaxation times: a Bayesian and hierarchical Bayesian approach. Electrochim. Acta 167, 439–454 (2015).
Song, Y. et al. Nanocomposites: a new opportunity for developing highly active and durable bifunctional air electrodes for reversible protonic ceramic cells.Adv. Energy Mater. 11, 2101899 (2021).
Jiang, Y. et al. Combination of hybrid CVD and cation exchange for upscaling Cs‐substituted mixed cation perovskite solar cells with high efficiency and stability. Adv. Funct. Mater. 28, 1703835 (2018).
Yang, X. et al. Improving stability and electrochemical performance of Ba0.5Sr0.5Co0.2Fe0.8O3–δ electrode for symmetrical solid oxide fuel cells by Mo doping. J. Alloy. Compd. 831, 154711 (2020).
Shen, L., Du, Z., Zhang, Y., Dong, X. & Zhao, H. Medium-entropy perovskites Sr(FeαTiβCoγMnζ)O3–δ as promising cathodes for intermediate temperature solid oxide fuel cell. Appl. Catal. B 295, 120264 (2021).
Zhang, Y. et al. Thermal-expansion offset for high-performance fuel cell cathodes. Nature 591, 246–251 (2021).
Li, X. et al. Ultrafast room-temperature synthesis of self-supported NiFe-layered double hydroxide as large-current-density oxygen evolution electrocatalyst. Small 18, 2104354 (2022).
Liang, M. et al. A new durable surface nanoparticles-modified perovskite cathode for protonic ceramic fuel cells from selective cation exsolution under oxidizing atmosphere. Adv. Mater. 34, 2106379 (2022).
Ramos, A. E., Maiti, D., Daza, Y. A., Kuhn, J. N. & Bhethanabotla, V. R. Co, Fe, and Mn in La-perovskite oxides for low temperature thermochemical CO2 conversion. Catal. Today 338, 52–59 (2019).
Sun, Y.-R. et al. Lattice contraction tailoring in perovskite oxides towards improvement of oxygen electrode catalytic activity. Chem. Eng. J. 421, 129698 (2021).
Hong, W. T., Welsch, R. E. & Shao-Horn, Y. Descriptors of oxygen-evolution activity for oxides: a statistical evaluation. J. Phys. Chem. C 120, 78–86 (2016).
Suntivich, J., May, K. J., Gasteiger, H. A., Goodenough, J. B. & Shao-Horn, Y. J. S. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 334, 1383–1385 (2011).
Liang, M. et al. Nickel-doped BaCo0.4Fe0.4Zr0.1Y0.1O3-δ as a new high-performance cathode for both oxygen-ion and proton conducting fuel cells. Chem. Eng. J. 420, 127717 (2021).
Guan, D. et al. Exceptionally robust face‐sharing motifs enable efficient and durable water oxidation. Advanced Materials 33, 2103392 (2021).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
This work is supported by National Natural Science Foundation of China (grant no. 51827901, H.X.), Project of Strategic Importance Program of The Hong Kong Polytechnic University (grant no P0035168, M.N.), Sichuan Science and Technology Department (grant no. 2020YFH0012, H.X.), Program for Guangdong Introducing Innovative and Entrepreneurial Teams (grant no. 2019ZT08G315, H.X.), as well as the Natural Science Foundation of Guangdong Province (grant no. 2020A1515010550, H.X.).
Author information
Authors and Affiliations
Contributions
S.Z., H.X., M.N. and Z.S. conceived the idea and designed the experiments. P.C. provided machine-learning codes. S.Z., S.Y.Z. and B.C. carried out the sample synthesis, characterization and measurements. Y.S, D.G. and J.W. participated in the discussion of the experimental results. S.Z., H.X., M.N. and Z.S. co-wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Yueh-Lin Lee, Steven McIntosh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Notes 1–5, Figs. 1–24 and Tables 1–8.
Supplementary Data 1
The nine ionic descriptor values for each perovskite in the dataset.
Supplementary Data 2
The ASR predicted values for 6,871 distinct perovskite oxide compositions.
Source data
Source Data Fig. 2
Model evaluation and descriptor importance degree analysis.
Source Data Fig. 3
Structure and electrochemical performance of the synthesized perovskite oxide sample.
Source Data Fig. 4
Symmetrical cell stability and single cell performance based on SCCN cathode.
Source Data Fig. 6
Oxygen-transfer related characterization.
Source Data Fig. 7
DFT calculation of electronic structure evolution.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhai, S., Xie, H., Cui, P. et al. A combined ionic Lewis acid descriptor and machine-learning approach to prediction of efficient oxygen reduction electrodes for ceramic fuel cells. Nat Energy 7, 866–875 (2022). https://doi.org/10.1038/s41560-022-01098-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-022-01098-3
This article is cited by
-
Prediction of electrode microstructure evolutions with physically constrained unsupervised image-to-image translation networks
npj Computational Materials (2024)
-
Deformable Catalytic Material Derived from Mechanical Flexibility for Hydrogen Evolution Reaction
Nano-Micro Letters (2024)
-
Bridging the complexity gap in computational heterogeneous catalysis with machine learning
Nature Catalysis (2023)
-
Lowering the operating temperature of protonic ceramic electrochemical cells to <450 °C
Nature Energy (2023)
-
Catalyst design with machine learning
Nature Energy (2022)