Abstract
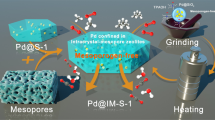
Metal–zeolite composites with metal (oxide) and acid sites are promising catalysts for integrating multiple reactions in tandem to produce a wide variety of wanted products without separating or purifying the intermediates. However, the conventional design of such materials often leads to uncontrolled and non-ideal spatial distributions of the metal inside/on the zeolites, limiting their catalytic performance. Here we demonstrate a simple strategy for synthesizing double-shelled, contiguous metal oxide@zeolite hollow spheres (denoted as MO@ZEO DSHSs) with controllable structural parameters and chemical compositions. This involves the self-assembly of zeolite nanocrystals onto the surface of metal ion-containing carbon spheres followed by calcination and zeolite growth steps. The step-by-step formation mechanism of the material is revealed using mainly in situ Raman spectroscopy and X-ray diffraction and ex situ electron microscopy. We demonstrate that it is due to this structure that an Fe2O3@H-ZSM-5 DSHSs-showcase catalyst exhibits superior performance compared with various conventionally structured Fe2O3-H-ZSM-5 catalysts in gasoline production by the Fischer–Tropsch synthesis. This work is expected to advance the rational synthesis and research of hierarchically hollow, core–shell, multifunctional catalyst materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information files. Source data are provided with this paper.
References
Yang, P. & Tarascon, J.-M. Towards systems materials engineering. Nat. Mater. 11, 560–563 (2012).
Zhu, Y. et al. Structural engineering of 2D nanomaterials for energy storage and catalysis. Adv. Mater. 30, 1706347 (2018).
Parlett, C. M. A. et al. Spatially orthogonal chemical functionalization of a hierarchical pore network for catalytic cascade reactions. Nat. Mater. 15, 178–182 (2016).
Isaacs, M. A. et al. A spatially orthogonal hierarchically porous acid-base catalyst for cascade and antagonistic reactions. Nat. Catal. 3, 921–931 (2020).
Cho, H. J., Kim, D., Li, J., Su, D. & Xu, B. Zeolite-encapsulated Pt nanoparticles for tandem catalysis. J. Am. Chem. Soc. 140, 13514–13520 (2018).
Cheng, K. et al. Impact of the spatial organization of bifunctional metal-zeolite catalysts for hydroisomerization of light alkanes. Angew. Chem. Int. Ed. 59, 3592–3600 (2020).
Climent, M. J., Corma, A., Iborra, S. & Sabater, M. J. Heterogeneous catalysis for tandem reactions. ACS Catal. 4, 870–891 (2014).
Behr, A., Vorholt, A. J., Ostrowski, K. A. & Seidensticker, T. Towards resource efficient chemistry: tandem reactions with renewables. Green Chem. 16, 982–1006 (2014).
Lohr, T. L. & Marks, T. J. Orthogonal tandem catalysis. Nat. Chem. 7, 477–482 (2015).
Védrine, J. C. Metal oxides in heterogeneous oxidation catalysis: state of the art and challenges for a more sustainable world. ChemSusChem 12, 577–588 (2019).
De Jong, K. P. in Synthesis of Solid Catalysts (ed. De Jong, K. P.) 3–12 (Wiley-VCH, 2009).
Chen, S., Takata, T. & Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2, 17050 (2017).
Hong, W. T. et al. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 8, 1404–1427 (2015).
Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 97, 2373–2420 (1997).
Vogt, E. T. C. & Weckhuysen, B. M. Fluid catalytic cracking: recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 44, 7342–7370 (2015).
Li, Y., Li, L. & Yu, J. Applications of zeolites in sustainable chemistry. Chem 3, 928–949 (2017).
Jiao, F. et al. Selective conversion of syngas to light olefins. Science 351, 1065–1068 (2016).
Cheng, K. et al. Bifunctional catalysts for one-step conversion of syngas into aromatics with excellent selectivity and stability. Chem 3, 334–347 (2017).
Gao, P. et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 9, 1019–1024 (2017).
Wang, Y. et al. Rationally designing bifunctional catalysts as an efficient strategy to boost CO2 hydrogenation producing value-added aromatics. ACS Catal. 9, 895–901 (2019).
Takeuchi, M., Kimura, T., Hidaka, M., Rakhmawaty, D. & Anpo, M. Photocatalytic oxidation of acetaldehyde with oxygen on TiO2/ZSM-5 photocatalysts: effect of hydrophobicity of zeolites. J. Catal. 246, 235–240 (2007).
Huang, H. et al. Catalytic oxidation of benzene over Mn modified TiO2/ZSM-5 under vacuum UV irradiation. Appl. Catal. B 203, 870–878 (2017).
Wang, Q., Li, H., Chen, L. & Huang, X. Monodispersed hard carbon spherules with uniform nanopores. Carbon 39, 2211–2214 (2001).
Tosheva, L. & Valtchev, V. P. Nanozeolites: synthesis, crystallization mechanism, and applications. Chem. Mater. 17, 2494–2513 (2005).
Israelachvili, J. N. in Intermolecular and Surface Forces 3rd edn (ed. Israelachvili, J. N.) 253–340 (Academic Press, 2011).
Lee, J. S., Kim, J. H., Lee, Y. J., Jeong, N. C. & Yoon, K. B. Manual assembly of microcrystal monolayers on substrates. Angew. Chem. Int. Ed. 46, 3087–3090 (2007).
Kudin, K. N. et al. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 8, 36–41 (2008).
Li, Y., Guo, Q., Kalb, J. & Thompson, C. Matching glass-forming ability with the density of the amorphous phase. Science 322, 1816–1819 (2008).
Yin, Y. et al. Formation of hollow nanocrystals through the nanoscale Kirkendall effect. Science 304, 711–714 (2004).
Yang, L.-P. et al. General synthetic strategy for hollow hybrid microspheres through a progressive inward crystallization process. J. Am. Chem. Soc. 138, 5916–5922 (2016).
Cao, L., Chen, D. & Caruso, R. A. Surface-metastable phase-initiated seeding and ostwald ripening: a facile fluorine-free process towards spherical fluffy core/shell, yolk/shell, and hollow anatase nanostructures. Angew. Chem. Int. Ed. 52, 10986–10991 (2013).
Lai, X. et al. General synthesis and gas-sensing properties of multiple-shell metal oxide hollow microspheres. Angew. Chem. Int. Ed. 50, 2738–2741 (2011).
Wang, J. Y., Wan, J. W. & Wang, D. Hollow multishelled structures for promising applications: understanding the structure–performance correlation. Acc. Chem. Res. 52, 2169–2178 (2019).
Xu, Y. et al. Selective conversion of syngas to aromatics over Fe3O4@MnO2 and hollow HZSM-5 bifunctional catalysts. ACS Catal. 9, 5147–5156 (2019).
Zhao, B. et al. Direct transformation of syngas to aromatics over Na-Zn-Fe5C2 and hierarchical HZSM-5 tandem catalysts. Chem 3, 323–333 (2017).
Xu, Y., Wang, J., Ma, G., Lin, J. & Ding, M. Designing of hollow ZSM-5 with controlled mesopore sizes to boost gasoline production from syngas. ACS Sustain. Chem. Eng. 7, 18125–18132 (2019).
Selvin, R., Hsu, H. L., Roselin, L. S. & Bououdina, M. Effect of aging on the precursor sol for the synthesis of nanocrystalline ZSM-5. Synth. React. Inorg. Met. Org. Nano. Met. Chem. 41, 1028–1032 (2011).
Roselin, L. S., Selvin, R. & Bououdina, M. Nanocrystalline ZSM-5: an efficient catalyst for regioselective acetolysis of epichlorohydrin. Chem. Eng. Commun. 199, 221–230 (2012).
Yordanov, I. et al. Elucidation of Pt clusters in the micropores of zeolite nanoparticles assembled in thin films. J. Phys. Chem. C 114, 20974–20982 (2010).
Hartman, T., Geitenbeek, R. G., Whiting, G. T. & Weckhuysen, B. M. Operando monitoring of temperature and active species at the single catalyst particle level. Nat. Catal. 2, 986–996 (2019).
Acknowledgements
B.M.W. acknowledges financial support from the Netherlands Organization for Scientific Research (NWO) in the frame of a Gravitation Program MCEC (Netherlands Center for Multiscale Catalytic Energy Conversion, www.mcec-researchcenter.nl). X.X. (Utrecht University) acknowledges financial support from the EU H2020-MSCA-ITN-2015 project ‘MULTIMAT’ (project no. 676045). K.C. and Y.W. acknowledge financial support from the National Natural Science Foundation of China (grant nos. 91945301, 22121001 and 22072120). J.X. thanks D. Wezendonk (Utrecht University) for his help with the in situ XRD measurements.
Author information
Authors and Affiliations
Contributions
J.X., B.M.W., K.C. and Y.W. conceived and designed the experiments. B.M.W. and Y.W. supervised the project. J.X. synthesized and characterized the materials, analysed the data and wrote the initial manuscript. K.C. and M.W. performed the catalytic reactions, analysed the resulting data and drafted the catalytic reaction part. X.X. and M.A.v.H. performed the (S)TEM-related measurements. Y.L. and T.H. performed the ex situ IR and in situ Raman measurements, respectively. S.X. performed a part of the SEM measurements and the ex situ Raman measurements. D.F. and K.B. contributed to the exploration of the zeolite secondary growth conditions. A.v.B. helped with the mechanistic understanding of the self-assembly process. J.X., B.M.W., K.C. and Y.W. revised the paper with contributions from all the other co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Michael Claeys and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–39, Tables 1–7 and refs. 1–17.
Source data
Source Data Fig. 2
Source data for Fig. 2a,b.
Source Data Fig. 3
Source data for Fig. 3a–c,f,g.
Source Data Fig. 4
Source data for Fig. 4d,e–h,l,m–s.
Source Data Fig. 5
Source data for Fig. 5a–d.
Rights and permissions
About this article
Cite this article
Xiao, J., Cheng, K., Xie, X. et al. Tandem catalysis with double-shelled hollow spheres. Nat. Mater. 21, 572–579 (2022). https://doi.org/10.1038/s41563-021-01183-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-021-01183-0
This article is cited by
-
Nanoparticle proximity controls selectivity in benzaldehyde hydrogenation
Nature Catalysis (2024)
-
Morphology and optical properties of wet chemistry synthesized submicron CePO4:Tb3+ hollow spheres
Chemical Papers (2024)
-
Tandem-biocatalysis reactors constructed by topological evolution of CaCO3 particles into hollow metal hydroxide spheres
Nature Communications (2023)
-
Giving oxygenates a new spin
Nature Catalysis (2022)
-
Imaging Cu2O nanocube hollowing in solution by quantitative in situ X-ray ptychography
Nature Communications (2022)