Abstract
Periodic fever is a characteristic clinical feature of human malaria, but how parasites survive febrile episodes is not known. Although the genomes of Plasmodium species encode a full set of chaperones, they lack the conserved eukaryotic transcription factor HSF1, which activates the expression of chaperones following heat shock. Here, we show that PfAP2-HS, a transcription factor in the ApiAP2 family, regulates the protective heat-shock response in Plasmodium falciparum. PfAP2-HS activates the transcription of hsp70-1 and hsp90 at elevated temperatures. The main binding site of PfAP2-HS in the entire genome coincides with a tandem G-box DNA motif in the hsp70-1 promoter. Engineered parasites lacking PfAP2-HS have reduced heat-shock survival and severe growth defects at 37 °C but not at 35 °C. Parasites lacking PfAP2-HS also have increased sensitivity to imbalances in protein homeostasis (proteostasis) produced by artemisinin, the frontline antimalarial drug, or the proteasome inhibitor epoxomicin. We propose that PfAP2-HS contributes to the maintenance of proteostasis under basal conditions and upregulates specific chaperone-encoding genes at febrile temperatures to protect the parasite against protein damage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The microarray data presented in Fig. 2 and Extended Data Figs. 1,5,6,8 has been deposited to the Gene Expression Omnibus (GEO) database under the accession code GSE149394. The genome sequencing and ChIP–seq data presented in Figs. 1b, 2e and Extended Data Fig. 7 have been deposited to the Sequence Read Archive (SRA) database with the accession codes PRJNA626524 and PRJNA670721, respectively. The authors declare that all other relevant data generated or analysed during this study are included in the Article, the Extended Data or the Supplementary Information files. We used data from the Pf3k pilot data release 5 (www.malariagen.net/pf3k) and different releases of PlasmoDB (www.plasmodb.org) databases. The materials described in this article, including the P. falciparum transgenic lines, are available from the corresponding author on reasonable request. Source data are provided with this paper.
Code availability
The scripts used for the analysis of microarray and next generation sequencing data are available at github (https://github.com/CortesMalariaLab/PfAP2-HS_Tinto_etal_NatMicrobiol_2021, with https://doi.org/10.5281/zenodo.4775988).
References
Richter, K., Haslbeck, M. & Buchner, J. The heat shock response: life on the verge of death. Mol. Cell 40, 253–266 (2010).
Hartl, F. U., Bracher, A. & Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 475, 324–332 (2011).
Anckar, J. & Sistonen, L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu. Rev. Biochem 80, 1089–1115 (2011).
Mahat, D. B., Salamanca, H. H., Duarte, F. M., Danko, C. G. & Lis, J. T. Mammalian heat shock response and mechanisms underlying its genome-wide transcriptional regulation. Mol. Cell 62, 63–78 (2016).
Solis, E. J. et al. Defining the essential function of yeast Hsf1 reveals a compact transcriptional program for maintaining eukaryotic proteostasis. Mol. Cell 63, 60–71 (2016).
Gomez-Pastor, R., Burchfiel, E. T. & Thiele, D. J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 19, 4–19 (2018).
Milner, D. A. Jr. Malaria Pathogenesis. Cold Spring Harb. Perspect. Med. 8, a025569 (2018).
Kwiatkowski, D. Malarial toxins and the regulation of parasite density. Parasitol. Today 11, 206–212 (1995).
Oakley, M. S., Gerald, N., McCutchan, T. F., Aravind, L. & Kumar, S. Clinical and molecular aspects of malaria fever. Trends Parasitol. 27, 442–449 (2011).
Gravenor, M. B. & Kwiatkowski, D. An analysis of the temperature effects of fever on the intra-host population dynamics of Plasmodium falciparum. Parasitology 117, 97–105 (1998).
Kwiatkowski, D. Febrile temperatures can synchronize the growth of Plasmodium falciparum in vitro. J. Exp. Med 169, 357–361 (1989).
Long, H. Y., Lell, B., Dietz, K. & Kremsner, P. G. Plasmodium falciparum: in vitro growth inhibition by febrile temperatures. Parasitol. Res. 87, 553–555 (2001).
Portugaliza, H. P. et al. Artemisinin exposure at the ring or trophozoite stage impacts Plasmodium falciparum sexual conversion differently. eLife 9, e60058 (2020).
Pavithra, S. R., Kumar, R. & Tatu, U. Systems analysis of chaperone networks in the malarial parasite Plasmodium falciparum. PLoS Comput Biol. 3, 1701–1715 (2007).
Muralidharan, V., Oksman, A., Pal, P., Lindquist, S. & Goldberg, D. E. Plasmodium falciparum heat shock protein 110 stabilizes the asparagine repeat-rich parasite proteome during malarial fevers. Nat. Commun. 3, 1310 (2012).
Silva, M. D. et al. A role for the Plasmodium falciparum RESA protein in resistance against heat shock demonstrated using gene disruption. Mol. Microbiol 56, 990–1003 (2005).
Kudyba, H. M. et al. The endoplasmic reticulum chaperone PfGRP170 is essential for asexual development and is linked to stress response in malaria parasites. Cell. Microbiol. 21, e13042 (2019).
Lu, K. Y. et al. Phosphatidylinositol 3-phosphate and Hsp70 protect Plasmodium falciparum from heat-induced cell death. eLife 9, e56773 (2020).
Zhang, M. et al. The apicoplast link to fever-survival and artemisinin-resistance in the malaria parasite. Preprint at BioRxiv https://doi.org/10.1101/2020.12.10.419788 (2021).
Oakley, M. S. et al. Molecular factors and biochemical pathways induced by febrile temperature in intraerythrocytic Plasmodium falciparum parasites. Infect. Immun. 75, 2012–2025 (2007).
Rovira-Graells, N. et al. Transcriptional variation in the malaria parasite Plasmodium falciparum. Genome Res. 22, 925–938 (2012).
Balaji, S., Babu, M. M., Iyer, L. M. & Aravind, L. Discovery of the principal specific transcription factors of Apicomplexa and their implication for the evolution of the AP2-integrase DNA binding domains. Nucleic Acids Res 33, 3994–4006 (2005).
Campbell, T. L., De Silva, E. K., Olszewski, K. L., Elemento, O. & Llinas, M. Identification and genome-wide prediction of DNA binding specificities for the ApiAP2 family of regulators from the malaria parasite. PLoS Pathog. 6, e1001165 (2010).
Jeninga, M. D., Quinn, J. E. & Petter, M. ApiAP2 transcription factors in apicomplexan parasites. Pathogens 8, 47 (2019).
Militello, K. T., Dodge, M., Bethke, L. & Wirth, D. F. Identification of regulatory elements in the Plasmodium falciparum genome. Mol. Biochem. Parasitol. 134, 75–88 (2004).
Dobson, C. M., Sali, A. & Karplus, M. Protein folding: a perspective from theory and experiment. Angew. Chem. Int. Ed. 37, 868–893 (1998).
Masterton, R. J., Roobol, A., Al-Fageeh, M. B., Carden, M. J. & Smales, C. M. Post-translational events of a model reporter protein proceed with higher fidelity and accuracy upon mild hypothermic culturing of Chinese hamster ovary cells. Biotechnol. Bioeng. 105, 215–220 (2010).
Bozdech, Z. et al. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 1, E5 (2003).
Dogovski, C. et al. Targeting the cell stress response of Plasmodium falciparum to overcome artemisinin resistance. PLoS Biol. 13, e1002132 (2015).
Claessens, A., Affara, M., Assefa, S. A., Kwiatkowski, D. P. & Conway, D. J. Culture adaptation of malaria parasites selects for convergent loss-of-function mutants. Sci. Rep. 7, 41303 (2017).
Stewart, L. B. et al. Intrinsic multiplication rate variation and plasticity of human blood stage malaria parasites. Commun. Biol. 3, 624 (2020).
Manske, M. et al. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature 487, 375–379 (2012).
Lamech, L. T. & Haynes, C. M. The unpredictability of prolonged activation of stress response pathways. J. Cell Biol. 209, 781–787 (2015).
Blasco, B., Leroy, D. & Fidock, D. A. Antimalarial drug resistance: linking Plasmodium falciparum parasite biology to the clinic. Nat. Med. 23, 917–928 (2017).
Haldar, K., Bhattacharjee, S. & Safeukui, I. Drug resistance in Plasmodium. Nat. Rev. Microbiol. 16, 156–170 (2018).
Ariey, F. et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505, 50–55 (2014).
Birnbaum, J. et al. A Kelch13-defined endocytosis pathway mediates artemisinin resistance in malaria parasites. Science 367, 51–59 (2020).
Bridgford, J. L. et al. Artemisinin kills malaria parasites by damaging proteins and inhibiting the proteasome. Nat. Commun. 9, 3801 (2018).
Mok, S. et al. Drug resistance. Population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance. Science 347, 431–435 (2015).
Lindner, S. E., De Silva, E. K., Keck, J. L. & Llinas, M. Structural determinants of DNA binding by a P. falciparum ApiAP2 transcriptional regulator. J. Mol. Biol. 395, 558–567 (2010).
Kafsack, B. F. et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature 507, 248–252 (2014).
Modrzynska, K. et al. A knockout screen of ApiAP2 genes reveals networks of interacting transcriptional regulators controlling the Plasmodium life cycle. Cell Host Microbe 21, 11–22 (2017).
Zhang, C. et al. Systematic CRISPR–Cas9-mediated modifications of Plasmodium yoelii ApiAP2 genes reveal functional insights into parasite development. mBio 8, e01986-17 (2017).
Llora-Batlle, O., Tinto-Font, E. & Cortes, A. Transcriptional variation in malaria parasites: why and how. Brief. Funct. Genomics 18, 329–341 (2019).
Cortés, A., Benet, A., Cooke, B. M., Barnwell, J. W. & Reeder, J. C. Ability of Plasmodium falciparum to invade Southeast Asian ovalocytes varies between parasite lines. Blood 104, 2961–2966 (2004).
Cortés, A. A chimeric Plasmodium falciparum Pfnbp2b/Pfnbp2a gene originated during asexual growth. Int. J. Parasitol. 35, 125–130 (2005).
Cortés, A. et al. Epigenetic silencing of Plasmodium falciparum genes linked to erythrocyte invasion. PLoS Pathog. 3, e107 (2007).
Walliker, D. et al. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science 236, 1661–1666 (1987).
Anders, R. F., Brown, G. V. & Edwards, A. Characterization of an S antigen synthesized by several isolates of Plasmodium falciparum. Proc. Natl Acad. Sci. USA 80, 6652–6656 (1983).
Ghorbal, M. et al. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR–Cas9 system. Nat. Biotechnol. 32, 819–821 (2014).
Lim, M. Y. et al. UDP-galactose and acetyl-CoA transporters as Plasmodium multidrug resistance genes. Nat. Microbiol 1, 16166 (2016).
Bancells, C. et al. Revisiting the initial steps of sexual development in the malaria parasite Plasmodium falciparum. Nat. Microbiol. 4, 144–154 (2019).
Knuepfer, E., Napiorkowska, M., van Ooij, C. & Holder, A. A. Generating conditional gene knockouts in Plasmodium—a toolkit to produce stable DiCre recombinase-expressing parasite lines using CRISPR/Cas9. Sci. Rep. 7, 3881 (2017).
Rovira-Graells, N., Aguilera-Simon, S., Tinto-Font, E. & Cortes, A. New assays to characterise growth-related phenotypes of Plasmodium falciparum reveal variation in density-dependent growth inhibition between parasite lines. PLoS ONE 11, e0165358 (2016).
Prasad, R. et al. Blocking Plasmodium falciparum development via dual inhibition of hemoglobin degradation and the ubiquitin proteasome system by MG132. PLoS ONE 8, e73530 (2013).
Crowley, V. M., Rovira-Graells, N., de Pouplana, L. R. & Cortés, A. Heterochromatin formation in bistable chromatin domains controls the epigenetic repression of clonally variant Plasmodium falciparum genes linked to erythrocyte invasion. Mol. Microbiol. 80, 391–406 (2011).
Casas-Vila, N., Pickford, A. K., Portugaliza, H. P., Tintó-Font, E. & Cortés, A. Transcriptional analysis of tightly synchronized Plasmodium falciparum intraerythrocytic stages by RT–qPCR. Methods Mol. Biol. https://doi.org/10.1007/978-1-0716-1681-9_10 (2021).
Kafsack, B. F., Painter, H. J. & Llinas, M. New Agilent platform DNA microarrays for transcriptome analysis of Plasmodium falciparum and Plasmodium berghei for the malaria research community. Malar. J. 11, 187 (2012).
Painter, H. J., Altenhofen, L. M., Kafsack, B. F. & Llinas, M. Whole-genome analysis of Plasmodium spp. utilizing a new agilent technologies DNA microarray platform. Methods Mol. Biol. 923, 213–219 (2013).
Llorà-Batlle, O. et al. Conditional expression of PfAP2-G for controlled massive sexual conversion in Plasmodium falciparum. Sci. Adv. 6, eaaz5057 (2020).
Lemieux, J. E. et al. Statistical estimation of cell-cycle progression and lineage commitment in Plasmodium falciparum reveals a homogeneous pattern of transcription in ex vivo culture. Proc. Natl Acad. Sci. USA 106, 7559–7564 (2009).
Saeed, A. I. et al. TM4 microarray software suite. Methods Enzymol. 411, 134–193 (2006).
Bauer, S., Grossmann, S., Vingron, M. & Robinson, P. N. Ontologizer 2.0–a multifunctional tool for GO term enrichment analysis and data exploration. Bioinformatics 24, 1650–1651 (2008).
Alexa, A., Rahnenfuhrer, J. & Lengauer, T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22, 1600–1607 (2006).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Josling, G. A. et al. Dissecting the role of PfAP2-G in malaria gametocytogenesis. Nat. Commun. 11, 1503 (2020).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Acknowledgements
We thank J. J. López-Rubio (University of Montpellier) for the plasmid pL6-egfp, M. Lee (Wellcome Sanger Institute) for the plasmid pDC2-Cas9-U6-hdhfr and E. Knuepfer (The Francis Crick Institute) for the plasmid pDC2-Cas9-U6-hDHFRyFCU. We also thank O. Llorà-Batlle and C. Sànchez-Guirado (ISGlobal) for their assistance with the generation of the plasmids used in this study, N. Rovira-Graells (ISGlobal) and A. Gupta. (Nanyang Technological University) for assistance with the 3D7-A and 3D7-A-HS microarray experiments, O. Billker (Wellcome Sanger Institute) for the experiments attempted in P. berghei and H. Ginsburg (The Hebrew University of Jerusalem) for providing data from the Malaria Parasite Metabolic Pathways. This publication uses data generated by the Pf3k project (www.malariagen.net/pf3k). This work was supported by grants from the Spanish Ministry of Economy and Competitiveness (MINECO)/Agencia Estatal de Investigación (AEI) (grant nos SAF2013-43601-R, SAF2016-76190-R and PID2019-107232RB-I00 to A.C.), co-funded by the European Regional Development Fund (ERDF, European Union), and from the NIH/NIAID (grant no. 1R01 AI125565 to M.L.). E.T.-F. and L.M.-T. were supported by fellowships from the Spanish Ministry of Economy and Competitiveness (grant nos BES-2014-067901 and BES-2017-081079, respectively), co-funded by the European Social Fund (ESF). T.J.R. was supported by a training grant from the NIH/NIGMS (grant no. T32 GM125592-01). This research is part of ISGlobal’s Program on the Molecular Mechanisms of Malaria, which is partially supported by the Fundación Ramón Areces. We acknowledge support from the Spanish Ministry of Science and Innovation through the ‘Centro de Excelencia Severo Ochoa 2019–2023’ Program (grant no. CEX2018-000806-S) and support from the Generalitat de Catalunya through the CERCA Program.
Author information
Authors and Affiliations
Contributions
E.T.-F. performed all of the experiments, except for those presented in Extended Data Fig. 1 and the western blot and ChIP–seq experiments. L.M.-T., E.T.-F., T.J.R. and A.C. performed the bioinformatics analyses. N.C.-V. performed the western blot experiments. T.J.R. performed, and M.L. supervised, the ChIP–seq experiments. Z.B. provided microarray hybridizations for the experiments presented in Extended Data Fig. 1. D.J.C. provided advice on clinical isolates and provided Line 1 from The Gambia. E.T.-F. and A.C. conceived the project, designed and interpreted the experiments and wrote the manuscript (with input from all authors and major input from M.L. and D.J.C.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
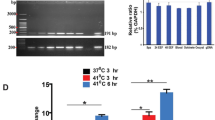
Extended Data Fig. 1 Transcriptional analysis of heat-shock-adapted and control lines.
a, Microarray-based transcriptomic comparison across the asexual blood cycle of 3D7-A cultures adapted to heat shock after five cycles of selection with a 3 h heat shock at 41.5 °C at the trophozoite stage (3D7-A-HS r1 and r2) and control parasite lines maintained in parallel without heat shock (3D7-A r1 and r2). Values are the log2 of the maximum expression fold-change (from the average of all lines compared) across a time interval corresponding to half the length of the asexual cycle, calculated using the aMAFC score as previously described21. Genes with a > 1.5-fold-change in expression in the two independent 3D7-A heat-shock-adapted lines (3D7-A-HS r1 and r2) relative to their respective controls (3D7-A r1 and r2) are shown. Data for parasite lines 10 G (heat-shock-sensitive subclone), 1.2B (heat-shock-resistant subclone) and 3D7-A (right panel) is from Rovira-Graells et al21. b, Time-course expression of genes in panel a that showed a concordant change in expression between heat-shock-adapted and control cultures, and between the heat-shock-resistant subclone 1.2B and the heat-shock-sensitive subclone 10 G. Based on the predicted function of the three genes, clag2 was considered the most plausible candidate to play a role in heat-shock resistance. c, Expression of clag2 is neither necessary nor sufficient for heat-shock resistance. RT-qPCR analysis of clag2 transcript levels (normalized against rhoph2) in schizonts of heat-shock sensitive (S) and heat-shock resistant (R) 3D7-A subclones (see Fig. 1f), and of the heat-shock-adapted and control lines (n = 1 biological replicates).
Extended Data Fig. 2 Generation and characterization of transgenic parasite lines edited at the pfap2-hs locus.
a, Schematic of the CRISPR–Cas9 strategy used to knockout pfap2-hs, using two guide RNAs. b–d, Tagging of endogenous PfAP2-HS using CRISPR–Cas9 technology. The tags used were a C-terminal 3xHA (b), a C-terminal eYFP (c) and an N-terminal eYFP (d). e, C-terminal tagging of endogenous PfAP2-HS by single homologous recombination with a tag consisting of a 2xHA epitope and an FKBP destabilization domain (DD domain). In all panels, the position of the primers used for analytical PCR (arrowheads), guide RNA and AP2 domains (blue vertical bars) is indicated. The electrophoresis images at the right are the analytical PCR validation of the genetic edition (single genomic DNA extraction and PCR analysis), showing correct edition and absence of wild-type locus in all cases except for the 8 A subclone of 1.2B-ddFKBP (8 A and 12E are subclones obtained after drug cycling). The bar charts at the right show the level of survival (mean of n = 2 independent biological replicates) of sorbitol-synchronized cultures of the transgenic lines upon heat shock (HS) exposure at the trophozoite stage, with heat-shock-resistant (10E) and heat-shock-sensitive (10 G, expressing PfAP2-HSΔD3) subclones as controls. Addition of a C-terminal eYFP or HA-FKBP tag did not affect growth at 37 °C but resulted in high heat-shock sensitivity, similar to the 10 G line. In contrast, C-terminal addition of the smaller 3xHA tag or addition of an N-terminal eYFP did not affect growth at 37 °C or heat-shock sensitivity. In all cases, tagged PfAP2-HS was not detectable by immunofluorescence or Western blot analysis, probably as a consequence of the very low abundance of this transcription factor (see proteomic data in www.PlasmoDB.org).
Extended Data Fig. 3 Validation of the transcriptomic changes upon heat shock and distribution of the tandem G-box motif.
a, RT-qPCR analysis of transcript levels (normalized against serine–tRNA ligase) of the genes selected for validation, using biological samples independent from the samples used for microarray analysis. Values are the average of triplicate reactions. The log2 expression fold-change [heat shock (HS) relative to control (35 °C) conditions] for these genes in the microarray analysis (Fig. 2a) is shown in a heatmap to facilitate comparison. b, Genes in the P. falciparum genome containing tandem arrangements (maximum distance between the two: 9 nucleotides) of the G-box [(A/G)NGGGG(C/A)] motif in their regulatory regions (defined as the 2 kb upstream of the start codon or until the neighbour gene, when it is closer). The sequence of the G-box in each gene is shown in blue, and the level of concordance with the consensus G-box motif is expressed as the P value of the match (determined using the FIMO v5.0.5 function in the MEME suite). Expression changes upon heat shock for these genes are shown as in panel a.
Extended Data Fig. 4 Changes in hsp70-1, hsp90 and PF3D7_1421800 transcript levels in parasites lacking the entire PfAP2-HS or D3.
Fold increase in transcript levels (determined by RT-qPCR, normalized against serine–tRNA ligase) during and after heat shock (HS) starting at 33-35 (a) or 30-35 (b) h.p.i., relative to cultures maintained in parallel without heat shock (37 or 35 °C). In panel a, values for three individual 3D7-A subclones carrying or not the Q3417X mutation are shown as dotted lines, whereas the average of the three subclones is shown as a continuous line. In panel b, the mean of n = 2 independent biological replicates is shown.
Extended Data Fig. 5 Transcript level changes upon heat shock in chaperone-encoding genes.
Log2 expression fold-change [heat shock (HS) relative to control (35 °C) conditions, as in Fig. 2a] for all chaperone-encoding genes described by Pavithra and colleagues14. Columns at the left indicate presence of the G-box23 or tandem G-box (TdGbox) in the upstream region, and log2 fold-change during heat shock in a previous study20 (Oakley).
Extended Data Fig. 6 Transcriptomic characterization of the heat-shock response in parasites expressing complete PfAP2-HS (10E line).
Log2 expression fold-change [heat shock (HS) relative to control (35 °C) conditions, as in Fig. 2a] in the wild-type 10E line determined by microarray analysis. Genes with a fold-change ≥2 at any of the time points analysed are shown. The mean log2 expression fold-change (with 95% confidence interval) and representative enriched GO terms are shown for each cluster. Columns at the left indicate fold-change during heat shock in a previous study20 (Oakley), and annotation as chaperone14. Ten genes had values out of the range displayed (actual range: -3.89 to +4.03).
Extended Data Fig. 7 ChIP analysis of the chromosomal distribution of PfAP2-HS.
a, ChIP–seq analysis of HA-tagged PfAP2-HS. Number of reads of ChIP (IP) and input (in) tracks, and log2-transformed ChIP/input ratio tracks (IP/in) for five independent biological replicates [three including heat shock (HS) and 37 °C conditions, two including only the 37 °C condition]. Snapshots are shown for the three genes in cluster I (Fig. 2a) and snoR04. Binding at the hsp70-1 and hsp90 promoters coincides with the position of a tandem G-box motif, whereas PF3D7_1421800 and snoR04 lack a G-box. The positions of the relevant peaks are indicated by an arrowhead. b, Peaks present in ≥3 out of 5 replicate ChIP–seq experiments (37 °C) or ≥2 out of 3 replicate experiments (heat shock) and with a MACS score >100 in each positive replicate. c, ChIP–qPCR analysis of HA-tagged PfAP2-HS binding at selected loci, in cultures exposed to heat shock (HS) or control (37 °C) conditions (mean and s.e.m. of % input in n = 3 independent biological replicates). No significant difference (P < 0.05) was observed between 37 °C and heat shock using a two-sided unpaired Student’s t-test.
Extended Data Fig. 8 Transcriptional changes associated with PfAP2-HS deletion under basal (no heat shock) conditions.
a, Changes in transcript levels in the absence of heat shock for genes with an average expression fold-change >2 between 10E_Δpfap2-hs and 10E. Values are the log2 of the average expression fold-change relative to 10E across the time period compared (~27–30.5 hpi). Genes artificially modified or introduced in the knockout line, which serve as controls, are shown at the bottom (their values are out of the range displayed). The column at the left indicates the presence of the G-box23. b, Expression plots for selected genes under basal conditions. Expression values are plotted against statistically estimated parasite age, expressed in h post-invasion (h.p.i.). Grey shading marks the interval used to calculate the average expression fold-change. c, RT-qPCR analysis of hsp70-1 and hsp90 transcript levels in pfap2-hs knockout (KO) lines compared to their wild-type (WT) controls (the parental line for each knockout line) under basal conditions. Expression values are normalized against serine–tRNA ligase, and expressed as the fold-change (FC) in the knockout versus control lines. The mean of n = 3 (10E, 30-35 hpi) or n = 2 (others) independent biological replicates is shown.
Extended Data Fig. 9 Analysis of proteome stress and UPR markers in pfap2-hs mutants.
a, b, Western blot analysis (representative of n = 4) of polyubiquitinated proteins (Ub) (a) or phosphorylated eIF2α (eIF2α-P) (b) immediately after a 3 h heat shock (3 h HS) and 2 h later (2 h post HS). Histone H3 is a loading control. DHA was used as a positive control, as it is a known inducer of the UPR29,38. The Log2 of histone H3-normalized signal in heat shock or DHA-treated cultures versus control cultures is shown at the bottom (mean and s.e.m. of n = 4, except for the DHA control mean of n = 2 independent biological replicates). P values were calculated using a two-sided unpaired Student’s t-test. Only significant P values (P < 0.05) are shown. The position of molecular weight markers is shown (in kDa).
Extended Data Fig. 10 Sequence alignment of the three AP2 domains (D1–3) present in AP2-HS orthologues in Plasmodium spp.
Dots indicate identity with the amino acid in the first sequence.
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Supplementary Table 1
Genetic changes between 3D7-A-HS and control 3D7-A lines identified by whole-genome sequencing. Frequency of SNPs and indels (percentage of reads containing the mutation) in two heat-shock-adapted 3D7-A lines (3D7-A-HS r1 and r2) and their respective controls (3D7-A r1 and r2).
Supplementary Table 2
Global transcriptional alterations in the 10E, 10G and 10E-Δpfap2-hs parasite lines exposed to heat shock. Data are presented as the log2-transformed expression fold change in cultures exposed to heat shock (HS) versus control (35 °C, no heat shock) cultures during (1.5 and 3 h) or after (2 h post) heat shock, as in Fig. 2a. Only genes with a fold change ≥ 4 (at any of the time points analysed) are shown. Genes are ordered by hierarchical clustering. Annotation as chaperone14, presence of the G-box23 or tandem G-box (TdGbox) in the upstream region, log2-transformed fold change after heat shock in a previous study20 (Oakley) and GO-terms are provided for each gene. For each cluster, significantly enriched motifs and enriched GO terms are shown. Gene set enrichment analysis of the comparative transcriptomic analysis between the 10E and 10E_Δpfap2-hs lines under basal conditions is also included.
Supplementary Table 3
Transcriptomic characterization of the heat-shock response in parasites expressing complete PfAP2-HS (10E line). Data are presented as the log2-transformed expression fold change in cultures exposed to heat-shock (HS) relative to control cultures unexposed to heat shock (35 °C) in the wild-type 10E line, as in Extended Data Fig. 6. Only genes with a fold change ≥ 2 at any of the analysed time points are shown. Annotation as chaperone14, presence of the G-box23 or tandem G-box (TdGbox) in the upstream region, log2-transformed fold change during heat shock in a previous study20 (Oakley) and GO-terms annotation are shown for each gene. For each cluster, significantly enriched GO terms are shown.
Supplementary Table 4
PfAP2-HS ChIP–seq peaks in cultures exposed to heat shock or under control conditions. List of peaks with a MACS2 score greater than 100 in any of the replicates, with values for each replicate, and peaks identified in each individual sample with a q-value < 0.01.
Supplementary Table 5
List of oligonucleotides used in this study.
Source data
Source Data Fig. 1
Numerical data for the graphs in Fig. 1b,e–g.
Source Data Fig. 2
Numerical data for the heatmap and graphs in Fig. 2a,c,d.
Source Data Fig. 3
Numerical data for the graphs in Fig. 3.
Source Data Fig. 4
Numerical data for the graphs in Fig. 4b,c,e,f.
Source Data Fig. 5
Numerical data for the graphs in Fig. 5.
Source Data Fig. 6
Phylogenetic tree in text format and gene IDs, Fig. 6b,c.
Source Data Extended Data Fig. 1
Numerical data for the heatmap and graphs in Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Numerical data for the graphs in Extended Data Fig. 2.
Source Data Extended Data Fig. 2
Unprocessed agarose gels from Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Numerical data for the heatmaps and graphs in Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Numerical data for the graphs in Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Numerical data for the heatmap in Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Numerical data for the heatmaps in Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Numerical data for the graphs in Extended Data Fig. 7c.
Source Data Extended Data Fig. 8
Numerical data for the heatmap and graphs in Extended Data Fig. 8.
Source Data Extended Data Fig. 9
Numerical data for the graphs in Extended Data Fig. 9.
Source Data Extended Data Fig. 9
Unprocessed western blots from Extended Data Fig. 9.
Source Data Extended Data Fig. 10
Multiple sequence alignment from Extended Data Fig. 10 in text format.
Rights and permissions
About this article
Cite this article
Tintó-Font, E., Michel-Todó, L., Russell, T.J. et al. A heat-shock response regulated by the PfAP2-HS transcription factor protects human malaria parasites from febrile temperatures. Nat Microbiol 6, 1163–1174 (2021). https://doi.org/10.1038/s41564-021-00940-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-021-00940-w
This article is cited by
-
tRNA modification reprogramming contributes to artemisinin resistance in Plasmodium falciparum
Nature Microbiology (2024)
-
Distinct evolution of type I glutamine synthetase in Plasmodium and its species-specific requirement
Nature Communications (2023)
-
Protein KIC5 is a novel regulator of artemisinin stress response in the malaria parasite Plasmodium falciparum
Scientific Reports (2023)
-
DNA-binding protein PfAP2-P regulates parasite pathogenesis during malaria parasite blood stages
Nature Microbiology (2023)
-
Malaria parasite beats the heat
Nature Microbiology (2021)