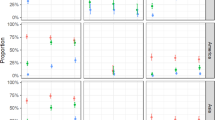
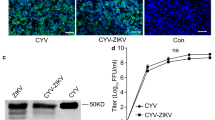
Abstract
Aedes aegypti and A. albopictus mosquitoes are the main vectors for dengue virus (DENV) and other arboviruses, including Zika virus (ZIKV). Understanding the factors that affect transmission of arboviruses from mosquitoes to humans is a priority because it could inform public health and targeted interventions. Reasoning that interactions among viruses in the vector insect might affect transmission, we analysed the viromes of 815 urban Aedes mosquitoes collected from 12 countries worldwide. Two mosquito-specific viruses, Phasi Charoen-like virus (PCLV) and Humaita Tubiacanga virus (HTV), were the most abundant in A. aegypti worldwide. Spatiotemporal analyses of virus circulation in an endemic urban area revealed a 200% increase in chances of having DENV in wild A. aegypti mosquitoes when both HTV and PCLV were present. Using a mouse model in the laboratory, we showed that the presence of HTV and PCLV increased the ability of mosquitoes to transmit DENV and ZIKV to a vertebrate host. By transcriptomic analysis, we found that in DENV-infected mosquitoes, HTV and PCLV block the downregulation of histone H4, which we identify as an important proviral host factor in vivo.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Small RNA and transcriptome libraries from this study have been deposited in the Sequence Read Archive (SRA) at NCBI (project accession PRJNA722589). Sequences spanning the RdRP region from newly discovered viruses were deposited in GenBank under accession MZ556103-MZ556111. Accession numbers for small RNA libraries are provided in Supplementary Table 1. Source data are provided with this paper.
Code availability
All scripts used in this work were deposited in GitHub and can be accessed at https://github.com/ericgdp/sRNA-virome code version 1.0.
References
Weaver, S. C., Charlier, C., Vasilakis, N. & Lecuit, M. Zika, chikungunya, and other emerging vector-borne viral diseases. Annu. Rev. Med 69, 395–408 (2018).
Bhatt, S. et al. The global distribution and burden of dengue. Nature 496, 504–507 (2013).
Messina, J. P. et al. Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol. 22, 138–146 (2014).
Franklinos, L. H. V., Jones, K. E., Redding, D. W. & Abubakar, I. The effect of global change on mosquito-borne disease. Lancet Infect. Dis. 19, e302–e312 (2019).
Kraemer, M. U. G. et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 4, 854–863 (2019).
Cromwell, E. A. et al. The relationship between entomological indicators of Aedes aegypti abundance and dengue virus infection. PLoS Negl. Trop. Dis. 11, e0005429 (2017).
de Almeida, J. P., Aguiar, E. R., Armache, J. N., Olmo, R. P. & Marques, J. T. The virome of vector mosquitoes. Curr. Opin. Virol. 49, 7–12 (2021).
Aguiar, E. R. G. R. et al. Sequence-independent characterization of viruses based on the pattern of viral small RNAs produced by the host. Nucleic Acids Res. 43, 6191–6206 (2015).
Boyles, S. M. et al. Under-the-radar dengue virus infections in natural populations of Aedes aegypti mosquitoes. mSphere 5, e00316–20 (2020).
Ramos-Nino, M. E. et al. High prevalence of Phasi Charoen-like virus from wild-caught Aedes aegypti in Grenada, W.I. as revealed by metagenomic analysis. PLoS ONE 15, e0227998 (2020).
Shi, C. et al. Stable distinct core eukaryotic viromes in different mosquito species from Guadeloupe, using single mosquito viral metagenomics. Microbiome 7, 121 (2019).
Zakrzewski, M. et al. Mapping the virome in wild-caught Aedes aegypti from Cairns and Bangkok. Sci. Rep. 8, 4690 (2018).
Patterson, E. I., Villinger, J., Muthoni, J. N., Dobel-Ober, L. & Hughes, G. L. Exploiting insect-specific viruses as a novel strategy to control vector-borne disease. Curr. Opin. Insect Sci. 39, 50–56 (2020).
Vasilakis, N. & Tesh, R. B. Insect-specific viruses and their potential impact on arbovirus transmission. Curr. Opin. Virol. 15, 69–74 (2015).
Aguiar, E. R. G. R., Olmo, R. P. & Marques, J. T. Virus-derived small RNAs: molecular footprints of host-pathogen interactions. Wiley Interdiscip. Rev. RNA 7, 824–837 (2016).
Morazzani, E. M., Wiley, M. R., Murreddu, M. G., Adelman, Z. N. & Myles, K. M. Production of virus-derived ping-pong-dependent piRNA-like small RNAs in the mosquito soma. PLoS Pathog. 8, e1002470 (2012).
Myles, K. M., Wiley, M. R., Morazzani, E. M. & Adelman, Z. N. Alphavirus-derived small RNAs modulate pathogenesis in disease vector mosquitoes. Proc. Natl Acad. Sci. USA 105, 19938–19943 (2008).
Frangeul, L., Blanc, H., Saleh, M.-C. & Suzuki, Y. Differential small RNA responses against co-infecting insect-specific viruses in Aedes albopictus mosquitoes. Viruses 12, 468 (2020).
Olmo, R. P. et al. Control of dengue virus in the midgut of Aedes aegypti by ectopic expression of the dsRNA-binding protein Loqs2. Nat. Microbiol. 3, 1385–1393 (2018).
Sedda, L. et al. The spatial and temporal scales of local dengue virus transmission in natural settings: a retrospective analysis. Parasit. Vectors 11, 79 (2018).
Schultz, M. J., Frydman, H. M. & Connor, J. H. Dual insect specific virus infection limits Arbovirus replication in Aedes mosquito cells. Virology 518, 406–413 (2018).
Fredericks, A. C. et al. Aedes aegypti (Aag2)-derived clonal mosquito cell lines reveal the effects of pre-existing persistent infection with the insect-specific bunyavirus Phasi Charoen-like virus on arbovirus replication. PLoS Negl. Trop. Dis. 13, e0007346 (2019).
Sedda, L., Taylor, B. M., Eiras, A. E., Marques, J. T. & Dillon, R. J. Using the intrinsic growth rate of the mosquito population improves spatio-temporal dengue risk estimation. Acta Trop. 208, 105519 (2020).
Lin, J.-J. et al. Aggressive organ penetration and high vector transmissibility of epidemic dengue virus-2 cosmopolitan genotype in a transmission mouse model. PLoS Pathog. 17, e1009480 (2021).
Sun, P. et al. A mosquito salivary protein promotes flavivirus transmission by activation of autophagy. Nat. Commun. 11, 260 (2020).
Lourenço, J. & Recker, M. Natural, persistent oscillations in a spatial multi-strain disease system with application to dengue. PLoS Comput. Biol. 9, e1003308 (2013).
Lourenço, J. et al. Epidemiological and ecological determinants of Zika virus transmission in an urban setting. eLife 6, e29820 (2017).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Flaus, A., Downs, J. A. & Owen-Hughes, T. Histone isoforms and the oncohistone code. Curr. Opin. Genet. Dev. 67, 61–66 (2021).
Lyons, S. M. et al. A subset of replication-dependent histone mRNAs are expressed as polyadenylated RNAs in terminally differentiated tissues. Nucleic Acids Res. 44, 9190–9205 (2016).
Baidaliuk, A. et al. Cell-fusing agent virus reduces arbovirus dissemination in Aedes aegypti mosquitoes in vivo. J. Virol. 93, e00705–e00719 (2019).
Blitvich, B. J. & Firth, A. E. Insect-specific flaviviruses: a systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses 7, 1927–1959 (2015).
Colpitts, T. M., Barthel, S., Wang, P. & Fikrig, E. Dengue virus capsid protein binds core histones and inhibits nucleosome formation in human liver cells. PLoS ONE 6, e24365 (2011).
Mourão, D. et al. A histone-like motif in yellow fever virus contributes to viral replication. Preprint at bioRxiv https://doi.org/10.1101/2020.05.05.078782 (2020).
Girardi, E. et al. Histone-derived piRNA biogenesis depends on the ping-pong partners Piwi5 and Ago3 in Aedes aegypti. Nucleic Acids Res. 45, 4881–4892 (2017).
Varjak, M. et al. Aedes aegypti Piwi4 is a noncanonical PIWI protein involved in antiviral responses. mSphere 2, e00144-17 (2017).
Parry, R. & Asgari, S. Aedes anphevirus: an insect-specific virus distributed worldwide in Aedes aegypti mosquitoes that has complex interplays with Wolbachia and dengue virus infection in cells. J. Virol. 92, e00224-18 (2018).
Zhang, G., Asad, S., Khromykh, A. A. & Asgari, S. Cell fusing agent virus and dengue virus mutually interact in Aedes aegypti cell lines. Sci. Rep. 7, 6935 (2017).
Nasar, F., Erasmus, J. H., Haddow, A. D., Tesh, R. B. & Weaver, S. C. Eilat virus induces both homologous and heterologous interference. Virology 484, 51–58 (2015).
Kenney, J. L., Solberg, O. D., Langevin, S. A. & Brault, A. C. Characterization of a novel insect-specific flavivirus from Brazil: potential for inhibition of infection of arthropod cells with medically important flaviviruses. J. Gen. Virol. 95, 2796–2808 (2014).
Romo, H., Kenney, J. L., Blitvich, B. J. & Brault, A. C. Restriction of Zika virus infection and transmission in Aedes aegypti mediated by an insect-specific flavivirus. Emerg. Microbes Infect. 7, 1–13 (2018).
Goenaga, S. et al. Potential for co-infection of a mosquito-specific flavivirus, nhumirim virus, to block West Nile virus transmission in mosquitoes. Viruses 7, 5801–5812 (2015).
Alefelder, S., Patel, B. K. & Eckstein, F. Incorporation of terminal phosphorothioates into oligonucleotides. Nucleic Acids Res. 26, 4983–4988 (1998).
Marques, J. T. et al. Loqs and R2D2 act sequentially in the siRNA pathway in Drosophila. Nat. Struct. Mol. Biol. 17, 24–30 (2010).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet j. 17, 10–12 (2011).
Matthews, B. J. et al. Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature 563, 501–507 (2018).
Chen, X.-G. et al. Genome sequence of the Asian Tiger mosquito, Aedes albopictus, reveals insights into its biology, genetics, and evolution. Proc. Natl Acad. Sci. USA 112, E5907–E5915 (2015).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Zerbino, D. R. Using the Velvet de novo assembler for short-read sequencing technologies. Curr. Protoc. Bioinform. https://doi.org/10.1002/0471250953.bi1105s31 (2010).
Bankevich, A. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477 (2012).
O’Leary, N. A. et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 44, D733–D745 (2016).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Fu, L., Niu, B., Zhu, Z., Wu, S. & Li, W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28, 3150–3152 (2012).
Aguiar, E. R. G. R. et al. A single unidirectional piRNA cluster similar to the flamenco locus is the major source of EVE-derived transcription and small RNAs in Aedes aegypti mosquitoes. RNA 26, 581–594 (2020).
Whitfield, Z. J. et al. The diversity, structure, and function of heritable adaptive immunity sequences in the Aedes aegypti genome. Curr. Biol. 27, 3511–3519.e7 (2017).
Palatini, U. et al. Comparative genomics shows that viral integrations are abundant and express piRNAs in the arboviral vectors Aedes aegypti and Aedes albopictus. BMC Genomics 18, 512 (2017).
Katoh, K., Misawa, K., Kuma, K. & Miyata, T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002).
Miller, M. A., Pfeiffer, W. & Schwartz, T. The CIPRES science gateway: a community resource for phylogenetic analyses. In Towns J. Proc. 2011 TeraGrid Conference on Extreme Digital Discovery - TG ’11 41, 1-8 (ACM Press, 2011).
Darriba, D. et al. ModelTest-NG: a new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evol. 37, 291–294 (2020).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 47, W256–W259 (2019).
Barletta, A. B. F. et al. Microbiota activates IMD pathway and limits Sindbis infection in Aedes aegypti. Parasit. Vectors 10, 103 (2017).
Donald, C. L. et al. Full genome sequence and sfRNA interferon antagonist activity of Zika virus from Recife, Brazil. PLoS Negl. Trop. Dis. 10, e0005048 (2016).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Korotkevich, G. et al. Fast gene set enrichment analysis. Preprint at bioRxiv https://doi.org/10.1101/060012 (2016).
Di Tommaso, P. et al. T-Coffee: a web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res. 39, W13–W17 (2011).
Guindon, S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321 (2010).
Lefort, V., Longueville, J.-E. & Gascuel, O. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 34, 2422–2424 (2017).
Lambert, D. Zero-inflated Poisson regression, with an application to defects in manufacturing. Technometrics 34, 1–14 (1992).
Hilbe, J. M. Negative Binomial Regression (2007).
Cameron, A. C. & Trivedi, P. K. Regression Analysis of Count Data (Cambridge Univ. Press, 1998).
Zeileis, A., Kleiber, C. & Jackman, S. Regression models for count data in R. J. Stat. Softw. 27, 8 (2008).
Acknowledgements
We thank all members of the ZIKAlliance consortium, especially A.-B. Failloux and A. Kohl for helping with establishing a network of collaborators and contributing fruitful discussions; current and former members of the Marques laboratory and the M3i unit - Insect Models of Innate Immunity, especially S. Blandin and N. Martins for suggestions and discussions. This work of the Interdisciplinary Thematic Institute IMCBio, as part of the ITI 2021-2028 programme of the University of Strasbourg, CNRS and Inserm, was supported by IdEx Unistra (ANR-10-IDEX-0002), by the SFRI-STRAT’US project (ANR 20-SFRI-0012), and EUR IMCBio (IMCBio ANR-17-EURE-0023) under the framework of the French Investments for the Future Program as well as by the previous Labex NetRNA (ANR-10-LABX-0036) to J.T.M and J.-L.I. This work was also supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to J.T.M. and E.R.G.R.A.; Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG), Rede Mineira de Imunobiológicos (grant no. REDE-00140-16), Rede Mineira de Biomoléculas (grant no. REDE-00125-16), Instituto Nacional de Ciência e Tecnologia de Vacinas (INCTV), Fonds Régional de Coopération pour la Recherche FRCT2020 Région Grand-Est – ViroMod and Institute for Advanced Studies of the University of Strasbourg (USIAS fellowship 2019 to J.T.M.); Google Latin American Research Award (LARA 2019) to J.T.M. and J.P.P.d.A.; FAPESP (Grant No. 13/21719-3) to M.L.N.; the European Union’s Horizon 2020 research and innovation programme under ZIKAlliance grant agreement no. 734548 and Investissement d’Avenir Programs (ANR-10-LABX-0036 and ANR- 11-EQPX-0022) to J.-L.I. and J.T.M.; and ANR PRC TIGERBRIDGE, grant no. 16-CE35-0010-01 to C.P. J.T.M., B.P.D., E.G.K and M.L.N. are CNPq Research Fellows. M.L.N. was partly funded by the Centers for Research in Emerging Infectious Diseases (CREID), the Coordinating Research on Emerging Arboviral Threats Encompassing the Neotropics (CREATE-NEO) grant U01 AI151807 by the National Institutes of Health (NIH/USA). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 to J.T.M. and V.A.-S. R.P.O. received a post-doctoral fellowship from CAPES.
Author information
Authors and Affiliations
Contributions
R.P.O., E.R.G.R.A., J.-L.I. and J.T.M conceived the project. R.P.O., Y.M.H.T., E.R.G.R.A., J.P.P.d.A., J.N.A., L.S. and J.T.M. designed the experiments and performed computational analysis. R.P.O., Y.M.H.T., I.J.S.d.F., F.V.F., A.G.A.F., S.C.G.A., A.T.S.S., K.P.R.d.S., A.P.P.V. and A.B. performed experiments. C.H.T., M.D., A.G., C.P., J.O.-N., T.M.V., C.J.M.K., M.A.W., A.L.C.C., M.T.P., M.C.P.P., M.L.N., V.A.-S., R.N.M., M.A.Z.B., B.P.D., E.G.K. and E.M. provided samples and reagents. M.R. performed the mathematical modelling. R.P.O., Y.M.H.T., E.R.G.R.A., J.-L.I. and J.T.M. wrote the manuscript. All authors read and contributed to manuscript editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Co-occurrence of 139 viral contig clusters identified in A. aegypti and A. albopictus mosquitoes.
Heatmap represents the small RNA abundance for each of the 139 viral contigs in our 91 small RNA libraries from A. aegypti and A. albopictus mosquitoes. White indicates absence of small RNAs mapping to that contig. Contig clusters were defined using the dendrogram shown on the heatmap. Clusters that had a RdRp sequence were classified as a putative virus. Virus presence was considered if >50% of contigs belonging to a cluster were represented.
Extended Data Fig. 2 Phylogeny of viruses identified in A. aegypti mosquitoes.
Phylogenetic trees were generated using the RdRp amino acid (aa) or nucleotide (nt) sequences and the substitution models as indicated: a, Aslam narnavirus (aa - LG + G); b, Nyamuk partiti-like virus (aa - BLOSUM 62); c, Orbis virgavirus (aa - BLOSUM62 + F); d, Bahianus rhabdovirus (aa - BLOSUM62); e, Lactea totivirus (nt - Tamura-Nei 93). Bootstrap confidence is shown close to each clade and values under 60% were omitted.
Extended Data Fig. 3 Virus-derived small RNA profiles in mosquitoes.
Small RNA size distribution and 5’ base preference is shown on the left while the density of small RNAs (coverage) is shown on the right for representative contig(s) of each of the 12 viruses identified in this study.
Extended Data Fig. 4 Burden of viruses in mosquitoes from different collection sites.
a, Abundance of small RNA sequences in pooled libraries from each location. Each dot represents the small RNA abundance in a contig, and violin plots represent contig clusters (see Extended Data Fig. 1) at different locations with colors matching the mosquito species. Error bars represent the standard deviation of the mean for each location. The number of contigs analyzed per location is indicated above each graph. b, Detection of representative contigs of newly detected viruses by RT-qPCR (black bars) in comparison to the detection of small RNAs (20-30 nt length) to the same given contig (gray bars). RT-qPCR detection is normalized against the endogenous constitutive gene RpL32. *, indicates detection by conventional RT-PCR. c, viral contig detection by conventional RT-PCR using independent sets of primers pairs. Conventional PCR and qPCR were repeated twice on the same samples. The expected size of viral contigs is shown. ns indicates a non-specific band. d, Sequence variation between viral contigs of Orbis virgavirus in RNA samples originated from Suriname along the region that is complementary to RT-qPCR primers. e, Ratio between relative RT-qPCR and small RNA abundance for each virus. The number of independent mosquito samples analyzed per virus is indicated above each graph. f, Combined incidence of DENV and ZIKV for each mosquito capture location in the previous, current, and subsequent years of collection (represented by −1, 0, and +1, respectively). Data were obtained from public sources for each location.
Extended Data Fig. 5 Characterization of HTV and PCLV infection in wild and laboratory mosquitoes.
a, geographic distribution of mosquitoes carrying HTV and PCLV in the city of Caratinga, Brazil. Maps show the density of adult A. aegypti mosquitoes captured from July 2010 until August 2011 estimated from the number of mosquitoes captured in individual traps. All mosquitoes, HTV positive, PCLV-positive and double positive individuals are shown. Virus detection was performed by RT-qPCR. Map source: OpenStreetMap. b,c tissue tropism of HTV and PCLV upon natural and artificial infections in A. aegypti mosquitoes. b, Scheme of mosquito dissection and tissues tested for virus infection by RT-qPCR. Pie charts show the prevalence of HTV and PCLV infection, assessed in tissues of naturally infected wild mosquitoes or laboratory mosquitoes injected with HTV and PCLV. Individual tissues were tested for virus presence upon dissection at 2-, 4-, 6- and 8-days post injection (d.p.i.) by RT-qPCR. c, Detection of HTV and PCLV in eggs by RT-PCR. Eggs were either rinsed with distilled water (no treatment group) or washed with bleach (2,5% active chlorine) prior to RNA extraction. The endogenous constitutive gene RpL32 was used as amplification control. Results are representative of two independent experiments. d-e, HTV and PCLV do not grow in mammalian cell culture. VERO cells were exposed to mosquito extracts containing HTV (d) and PCLV (e), and supernatants were collected at 1-, 3- and 5-days post exposure. A spike containing 105 pfu of vesicular stomatitis virus (VSV) was added prior RNA extraction and used to normalize the quantification of HTV and PCLV in the supernatant. No statistically significant difference was observed in HTV and PCLV levels at 1-, 3- and 5-days post infection as determined by two-sided one-way ANOVA with Dunns’ correction for multiple comparisons. Dots and error bars indicate the mean and the standard error of the mean, respectively. n indicates the number of independent tissue culture wells tested for each virus at each time point.
Extended Data Fig. 6 HTV and PCLV facilitate systemic ZIKV infection in mosquitoes.
a-c, Strategy to evaluate the interference of HTV and PCLV for ZIKV infection and replication in natural populations of mosquitoes. (a) HTV/PCLV-infected and virus-free wild mosquito populations were infected with ZIKV by intrathoracic injection. Viral loads and prevalence of infection were measured in the (b) midgut and (c) carcass of mosquitoes at 2-, 4- and 8-days post feeding. The prevalence of infection in each group is shown below plots. d-f, Laboratory mosquitoes (d) were infected artificially with HTV and PCLV and 7 days later were fed on ZIKV-infected mice. Viral loads and prevalence of infection were measured in the (e) midgut and (f) carcass of mosquitoes at 4- and 8-days post injection. The prevalence of infection in each group is shown below the plots. g-i, Laboratory mosquitoes were infected artificially with HTV and PCLV or control (mock) and 7 days later infected with ZIKV by intrathoracic injection. Viral loads and prevalence of infection were measured in the (h) midgut and (i) carcass of mosquitoes at 2-, 4- and 8-days post injection. The prevalence of infection in each group is shown below plots. j-n, Wild mosquito populations naturally infected with HTV and PCLV were allowed to feed in mice infected with ZIKV or mock-infected controls. Viral loads of HTV and PCLV were measured in the midgut (k,l) and in the carcass (m,n) of mosquitoes at 4-, 8- and 14-days post feeding. o-p, HTV/PCLV-infected or control mosquitoes were exposed to ZIKV-infected mice (o). Viral loads and prevalence of infection were measured in salivary glands (p) of mosquitoes at the indicated time points. Pie charts below each group indicate the prevalence of ZIKV infection. d.p.f. – days post feeding, d.p.i. – days post injection, NS – non-significant. In box plots of b, c, e, f, h, i, k, l, m, n, and p, boxes show the second and third interquartile ranges divided by the median while whiskers represent maximum and minimum values. Statistical significance was determined by two-tailed Mann–Whitney U-test. Numbers of infected samples over the total number tested are indicated above each column. Each dot represents an individual sample. Statistical significance of prevalence was determined by two-tailed Fisher’s exact test.
Extended Data Fig. 7 Differential gene expression in wild mosquitoes carrying HTV and PCLV.
a, Differential gene expression in the carcass of wild mosquitoes carrying HTV and PCLV or non-infected siblings during DENV infection at 4, 8 and 14 days post feeding. b, number of up or down regulated genes regarding the infection with HTV and PCLV at each time point as shown in a. Common genes across time points are shown. c, Immune genes regulated during infection with HTV and PCLV in comparison to virus-free siblings at different times after DENV infection. d-e, Intrathoracic injection of ZIKV in wild mosquitoes carrying HTV and PCLV or virus free siblings (d). Histone H4 levels were quantified in the midgut of mosquitoes at 2, 4, and 8 days post injection with ZIKV (e). Error bars represent mean and standard deviations of the mean, and statistical significance was determined by two-sided one-way ANOVA with Tukey’s correction for multiple comparisons. f-g, Artificial infection of laboratory mosquitoes with HTV and PCLV does not modulate levels of histone H4. g, laboratory mosquitoes were artificially infected with HTV and PCLV and histone H4 levels were analyzed at different time points. In box plots of e and g, boxes show the second and third interquartile ranges divided by the median while whiskers represent maximum and minimum values. Statistics were performed using two-sided one-way ANOVA with Tukey’s correction for multiple comparisons. Each dot represents an individual sample. CPM – counts per million, d.p.i. – days post infection, NS – non-significant.
Extended Data Fig. 8 Complexity of histone genes in the genome of A. aegypti.
a, Histone H4 gene copies in the A. aegypti genome (Vectorbase version 52) were reannotated using BLAST similarity search with further confirmation of RNA-seq reads mapping to each gene copy. Along with other histone genes currently annotated in Vectorbase, the number of copies in chromosomes or supercontigs are shown and the largest cluster of genes highlighted. b, Organization of the largest cluster of histone genes on chromosome 3 as indicated by the gray box. c, Weblogo showing the conservation of the amino acid sequence of histone H4 open reading frames, which only varied at the positions 36 and 98, indicated by a circle and an asterisk, respectively. The number of amino acid changes in each position is indicated. d, Histone H4 genes organized by nucleotide sequence similarity according to the dendrogram with the expression indicated by the heatmap in different A. aegypti tissues. Bootstrap values over 60 are shown. Histone H4 genes positioned in the cluster at chromosome 3 are indicated by gray boxes and the presence of a polyadenylation signal is indicated. e, Histone H4 gene expression in wild mosquito populations carrying HTV and PCLV or virus free siblings infected with DENV, quantified by RT-qPCR from cDNAs synthesized with random primers (hexamers) or anchored oligo dT22. d.p.i., days post injection. In d, SRA accession numbers in same order as shown in the heatmap: Female whole body (non-BF SRR1585314, SRR1585315, SRR1585316; 48 h post-BF SRR1532683, SRR1532684, SRR1532685, SRR1532693, SRR1532694, SRR1532695); Female brain (48 h post-BF SRR1166497; 96 h post-BF SRR1167481); Male (brain SRR1167543); Female salivary glands (SRR2659965, SRR2659966); Female midgut (SRR5288077, SRR5288080, SRR5288082, SRR5288087, SRR5288093, SRR5288100); Female malp. Tubules (non-BF SRR3680433, SRR3680434); Female carcass (12 h post-BF SRR923823; 24 h post-BF SRR923830; 36 h post-BF SRR923835; 48 h post-BF SRR923841; 60 h post-BF SRR923847; 72 h post-BF SRR923736); Fem. Carcass (no ovaries) 24 h post-BF (SRR388683); Fem. Low reprod. Tract (0 h post-mating SRR3213863, SRR3213864; 6 h post-mating SRR3213865, SRR3213866; 24 h post-mating SRR3213867, SRR3213868); Male sperm (early SRR3554588; late SRR3554589); Male testis (SRR6311395, SRR6311396); Embryo (4-8 h SRR1578254, SRR1578255, SRR1578256); 1 day old female ovaries (SRR388680); Female ovaries (non-BF SRR1167515, SRR1167516, SRR1167517, SRR1167518, SRR1167519, SRR1167520; 24 h post-BF SRR388682; 96 h post-BF SRR1167538, SRR1167539). f-h, silencing of histone H4 by RNA interference in adult mosquitoes. f, strategy for dsRNA mediated gene silencing in adult mosquitoes. g-h, Histone H4 levels in the midgut of ISV free laboratory mosquitoes (g) or wild mosquitoes carrying HTV and PCLV (h) injected with dsRNA targeting GFP (dsGFP) as control or histone H4 (dsH4) at 4 days post feeding. I, AGO2 levels in the midgut of mosquitoes carrying HTV and PCLV injected with dsRNA targeting GFP (dsGFP) as control or Ago2 (dsAGO2). Each dot represents an individual sample. In box plots of e, g, h, and i, boxes show interquartile ranges divided by the median while whiskers represent maximum and minimum values. Statistical significance was determined using two-sided one-way ANOVA with Tukey’s correction for multiple comparisons.
Supplementary information
Supplementary Table
Supplementary Table 1Overview of small RNA libraries. Metadata of small RNA libraries used in our study (SRA deposit ID, species, mosquito capture location, number of mosquitoes per pool, RNA treatment, and sequencing method). Supplementary Table 2Small RNA assembly metrics. Detailed information of assembled contigs with length >50 nt and length >199 nt per small RNA library. Supplementary Table 3Overview of contigs with similarity to viral sequences deposited in GenBank. Detailed information of BLASTn or BLASTp hits from contigs matching viral sequences (length > 199 nt) per small RNA library. Supplementary Table 4Overview of CD-Hit clusters. Number of contigs that compose each CD-Hit cluster. Supplementary Table 5List of oligonucleotides used in this study. Supplementary Table 6Parameters used to model DENV transmission.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig./Table 1
Statistical source data.
Source Data Extended Data Fig./Table 2
Source data in fasta and Newick formats.
Source Data Extended Data Fig./Table 3
Source data of coverage plots in BED formats and bar plots in Excel table.
Source Data Extended Data Fig./Table 4
Statistical source data, unprocessed gel image.
Source Data Extended Data Fig./Table 5
Statistical source data, unprocessed gel image.
Source Data Extended Data Fig./Table 6
Statistical source data.
Source Data Extended Data Fig./Table 7
Statistical source data.
Source Data Extended Data Fig./Table 8
Statistical source data, annotation of histone genes in gff3 format.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Olmo, R.P., Todjro, Y.M.H., Aguiar, E.R.G.R. et al. Mosquito vector competence for dengue is modulated by insect-specific viruses. Nat Microbiol 8, 135–149 (2023). https://doi.org/10.1038/s41564-022-01289-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-022-01289-4
This article is cited by
-
Effects of climate change and human activities on vector-borne diseases
Nature Reviews Microbiology (2024)
-
Generating prophylactic immunity against arboviruses in vertebrates and invertebrates
Nature Reviews Immunology (2024)
-
Metagenomic analysis of individual mosquito viromes reveals the geographical patterns and drivers of viral diversity
Nature Ecology & Evolution (2024)
-
Characterizing viral species in mosquitoes (Culicidae) in the Colombian Orinoco: insights from a preliminary metagenomic study
Scientific Reports (2023)
-
Microbiota in disease-transmitting vectors
Nature Reviews Microbiology (2023)