Abstract
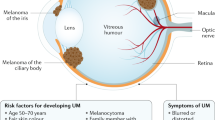
Melanomas arising in the uveal tract of the eye are a rare form of the disease with a biology and clinical phenotype distinct from their more common cutaneous counterparts. Treatment of primary uveal melanoma with radiotherapy, enucleation or other modalities achieves local control in more than 90% of patients, although 40% or more ultimately develop distant metastases, most commonly in the liver. Until January 2022, no systemic therapy had received regulatory approval for patients with metastatic uveal melanoma, and these patients have historically had a dismal prognosis owing to the limited efficacy of the available treatments. A series of seminal studies over the past two decades have identified highly prevalent early, tumour-initiating oncogenic genomic aberrations, later recurring prognostic alterations and immunological features that characterize uveal melanoma. These advances have driven the development of a number of novel emerging treatments, including tebentafusp, the first systemic therapy to achieve regulatory approval for this disease. In this Review, our multidisciplinary and international group of authors summarize the biology of uveal melanoma, management of primary disease and surveillance strategies to detect recurrent disease, and then focus on the current standard and emerging regional and systemic treatment approaches for metastatic uveal melanoma.
Key points
-
Advances in the understanding of the biology and immune microenvironment of uveal melanoma have improved prognostication and led to promising therapeutic strategies being tested in the adjuvant and metastatic settings.
-
Stratification of patients by risk of metastatic disease following treatment of primary disease using anatomical, clinical and molecular features has enabled the development of individualized radiographic surveillance strategies.
-
Tebentafusp, a first-in-class Immune-mobilizing monoclonal T cell receptor Against Cancer (ImmTAC), is the first therapy demonstrated to improve overall survival in patients with advanced-stage uveal melanoma.
-
The successful development of tebentafusp highlights the clinical efficacy that can be achieved with appropriate modulation of the antitumour immune response in this disease historically considered immune-resistant.
-
Novel regional therapeutic strategies focused on uveal melanoma liver metastases, systemic targeted, epigenetic and immunological treatments, and combinatorial approaches are being studied, providing hope for continued progress.
-
Advances in the metastatic setting are driving the development of novel adjuvant therapies that might reduce the risk of metastatic spread and increase cure rates for patients with uveal melanoma.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Shields, C. L. et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch. Ophthalmol. 127, 989–998 (2009).
Hu, D. N., Yu, G. P., McCormick, S. A., Schneider, S. & Finger, P. T. Population-based incidence of uveal melanoma in various races and ethnic groups. Am. J. Ophthalmol. 140, 612–617 (2005).
Virgili, G. et al. Incidence of uveal melanoma in Europe. Ophthalmology 114, 2309–2315 (2007).
Vajdic, C. M. et al. Incidence of ocular melanoma in Australia from 1990 to 1998. Int. J. Cancer 105, 117–122 (2003).
Aronow, M. E., Topham, A. K. & Singh, A. D. Uveal melanoma: 5-year update on incidence, treatment, and survival (SEER 1973-2013). Ocul. Oncol. Pathol. 4, 145–151 (2018).
Robertson, A. G. et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell 32, 204–220.e15 (2017).
Royer-Bertrand, B. et al. Comprehensive genetic landscape of uveal melanoma by whole-genome sequencing. Am. J. Hum. Genet. 99, 1190–1198 (2016).
Johansson, P. A. et al. Whole genome landscapes of uveal melanoma show an ultraviolet radiation signature in iris tumours. Nat. Commun. 11, 2408 (2020).
Nayman, T., Bostan, C., Logan, P. & Burnier, M. N. Jr Uveal melanoma risk factors: a systematic review of meta-analyses. Curr. Eye Res. 42, 1085–1093 (2017).
Robinson, S. et al. Protection against UVR involves MC1R-mediated non-pigmentary and pigmentary mechanisms in vivo. J. Invest. Dermatol. 130, 1904–1913 (2010).
Böhm, M. et al. α-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J. Biol. Chem. 280, 5795–5802 (2005).
Hauser, J. E. et al. Melanin content and MC1R function independently affect UVR-induced DNA damage in cultured human melanocytes. Pigment. Cell Res. 19, 303–314 (2006).
Shields, C. L. et al. Association of ocular and oculodermal melanocytosis with the rate of uveal melanoma metastasis: analysis of 7872 consecutive eyes. JAMA Ophthalmol. 131, 993–1003 (2013).
Shields, J. A., Demirci, H., Mashayekhi, A. & Shields, C. L. Melanocytoma of optic disc in 115 cases: the 2004 Samuel Johnson Memorial Lecture, part 1. Ophthalmology 111, 1739–1746 (2004).
Singh, A. D. et al. Lifetime prevalence of uveal melanoma in white patients with oculo(dermal) melanocytosis. Ophthalmology 105, 195–198 (1998).
Abdel-Rahman, M. H. et al. Whole exome sequencing identifies candidate genes associated with hereditary predisposition to uveal melanoma. Ophthalmology 127, 668–678 (2020).
Harbour, J. W. The genetics of uveal melanoma: an emerging framework for targeted therapy. Pigment. Cell Melanoma Res. 25, 171–181 (2012).
Singh, N., Singh, R., Bowen, R. C., Abdel-Rahman, M. H. & Singh, A. D. Uveal melanoma in BAP1 tumor predisposition syndrome: estimation of risk. Am. J. Ophthalmol. 224, 172–177 (2021).
Honavar, S. G., Singh, A. D., Shields, C. L., Shields, J. A. & Eagle, R. C. Jr. Iris melanoma in a patient with neurofibromatosis. Surv. Ophthalmol. 45, 231–236 (2000).
Rodrigues, M. et al. Outlier response to anti-PD1 in uveal melanoma reveals germline MBD4 mutations in hypermutated tumors. Nat. Commun. 9, 1866 (2018).
Derrien, A. C. et al. Germline MBD4 mutations and predisposition to uveal melanoma. J. Natl Cancer Inst. 113, 80–87 (2021).
Garg, G. et al. Patients presenting with metastases: stage IV uveal melanoma, an international study. Br. J. Ophthalmol. 106, 510–517 (2022).
Eskelin, S. & Kivela, T. Mode of presentation and time to treatment of uveal melanoma in Finland. Br. J. Ophthalmol. 86, 333–338 (2002).
Collaborative Ocular Melanoma Study Group. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch. Ophthalmol. 119, 670–676 (2001).
Khoja, L. et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular melanoma study. Ann. Oncol. 30, 1370–1380 (2019).
Rantala, E. S., Hernberg, M. & Kivela, T. T. Overall survival after treatment for metastatic uveal melanoma: a systematic review and meta-analysis. Melanoma Res. 29, 561–568 (2019).
Carvajal, R. D. et al. Phase I study of safety, tolerability, and efficacy of tebentafusp using a step-up dosing regimen and expansion in patients with metastatic uveal melanoma. J. Clin. Oncol. 40, 1939–1948 (2022).
Nathan, P. et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N. Engl. J. Med. 385, 1196–1206 (2021).
McLean, M. J., Foster, W. D. & Zimmerman, L. E. Prognostic factors in small malignant melanomas of choroid and ciliary body. Arch. Ophthalmol. 95, 48–58 (1977).
Bronkhorst, I. H. et al. Detection of M2-macrophages in uveal melanoma and relation with survival. Invest. Ophthalmol. Vis. Sci. 52, 643–650 (2011).
Folberg, R. et al. The prognostic value of tumor blood vessel morphology in primary uveal melanoma. Ophthalmology 100, 1389–1398 (1993).
Jager, M. J. et al. Uveal melanoma. Nat. Rev. Dis. Prim. 6, 24 (2020).
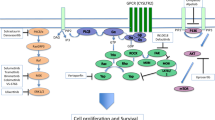
Van Raamsdonk, C. D. et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457, 599–602 (2009).
Van Raamsdonk, C. D. et al. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 363, 2191–2199 (2010).
Moore, A. R. et al. Recurrent activating mutations of G-protein-coupled receptor CYSLTR2 in uveal melanoma. Nat. Genet. 48, 675–680 (2016).
Johansson, P. et al. Deep sequencing of uveal melanoma identifies a recurrent mutation in PLCB4. Oncotarget 7, 4624–4631 (2016).
Chen, X. et al. RasGRP3 mediates MAPK pathway activation in GNAQ mutant uveal melanoma. Cancer Cell 31, 685–696.e6 (2017).
Ambrosini, G., Musi, E., Ho, A. L., de Stanchina, E. & Schwartz, G. K. Inhibition of mutant GNAQ signaling in uveal melanoma induces AMPK-dependent autophagic cell death. Mol. Cancer Ther. 12, 768–776 (2013).
Feng, X. et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell 25, 831–845 (2014).
Harbour, J. W. et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 330, 1410–1413 (2010).
Carbone, M. et al. BAP1 and cancer. Nat. Rev. Cancer 13, 153–159 (2013).
Maat, W. et al. Monosomy of chromosome 3 and an inflammatory phenotype occur together in uveal melanoma. Invest. Ophthalmol. Vis. Sci. 49, 505–510 (2008).
de Lange, M. J. et al. Heterogeneity revealed by integrated genomic analysis uncovers a molecular switch in malignant uveal melanoma. Oncotarget 6, 37824–37835 (2015).
Gezgin, G. et al. Genetic evolution of uveal melanoma guides the development of an inflammatory microenvironment. Cancer Immunol. Immunother. 66, 903–912 (2017).
Brouwer, N. J. et al. Ischemia is related to tumour genetics in uveal melanoma. Cancers https://doi.org/10.3390/cancers11071004 (2019).
Bronkhorst, I. H. et al. Different subsets of tumor-infiltrating lymphocytes correlate with macrophage influx and monosomy 3 in uveal melanoma. Invest. Ophthalmol. Vis. Sci. 53, 5370–5378 (2012).
Makitie, T., Summanen, P., Tarkkanen, A. & Kivela, T. Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest. Ophthalmol. Vis. Sci. 42, 1414–1421 (2001).
Krishna, Y., McCarthy, C., Kalirai, H. & Coupland, S. E. Inflammatory cell infiltrates in advanced metastatic uveal melanoma. Hum. Pathol. 66, 159–166 (2017).
Tosi, A. et al. The immune cell landscape of metastatic uveal melanoma correlates with overall survival. J. Exp. Clin. Cancer Res. 40, 154 (2021).
Qin, Y. et al. Parallel profiling of immune infiltrate subsets in uveal melanoma versus cutaneous melanoma unveils similarities and differences: a pilot study. Oncoimmunology 6, e1321187 (2017).
Rothermel, L. D. et al. Identification of an immunogenic subset of metastatic uveal melanoma. Clin. Cancer Res. 22, 2237–2249 (2016).
Yu, J. et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 27, 152–164 (2021).
Karlsson, J. et al. Molecular profiling of driver events in metastatic uveal melanoma. Nat. Commun. 11, 1894 (2020).
Durante, M. A. et al. Single-cell analysis reveals new evolutionary complexity in uveal melanoma. Nat. Commun. 11, 496 (2020).
Souri, Z. et al. LAG3 and its ligands show increased expression in high-risk uveal melanoma. Cancers https://doi.org/10.3390/cancers13174445 (2021).
Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report no. 28. Arch. Ophthalmol. 124, 1684–1693 (2006).
Jampol, L. M. et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report no. 19. Ophthalmology 109, 2197–2206 (2002).
Ophthalmic Oncology Task Force. Local recurrence significantly increases the risk of metastatic uveal melanoma. Ophthalmology 123, 86–91 (2016).
Caujolle, J. P. et al. Local recurrence after uveal melanoma proton beam therapy: recurrence types and prognostic consequences. Int. J. Radiat. Oncol. Biol. Phys. 85, 1218–1224 (2013).
Gragoudas, E. S., Lane, A. M., Munzenrider, J., Egan, K. M. & Li, W. Long-term risk of local failure after proton therapy for choroidal/ciliary body melanoma. Trans. Am. Ophthalmol. Soc. 100, 43–48 (2002). discussion 48-49.
Augsburger, J. J., Correa, Z. M., Freire, J. & Brady, L. W. Long-term survival in choroidal and ciliary body melanoma after enucleation versus plaque radiation therapy. Ophthalmology 105, 1670–1678 (1998).
Mosci, C. et al. Comparison of clinical outcomes for patients with large choroidal melanoma after primary treatment with enucleation or proton beam radiotherapy. Ophthalmologica 227, 190–196 (2012).
Dinca, E. B. et al. Survival and complications following Gamma Knife radiosurgery or enucleation for ocular melanoma: a 20-year experience. Acta Neurochir. 154, 605–610 (2012).
Chang, M. Y. & McCannel, T. A. Local treatment failure after globe-conserving therapy for choroidal melanoma. Br. J. Ophthalmol. 97, 804–811 (2013).
Shields, C. L. et al. Iris melanoma outcomes based on the American Joint Committee on cancer classification (eighth edition) in 432 patients. Ophthalmology 125, 913–923 (2018).
Force, A. O. O. T. International validation of the American Joint Committee on cancer’s 7th edition classification of uveal melanoma. JAMA Ophthalmol. 133, 376–383 (2015).
Kivelä, T. et al. in AJCC Cancer Staging Manual 8th edn (eds Amin, M. B. et al.) 805–817 (Springer, 2016).
Dogrusoz, M. et al. The prognostic value of AJCC staging in uveal melanoma is enhanced by adding chromosome 3 and 8q status. Invest. Ophthalmol. Vis. Sci. 58, 833–842 (2017).
Stacey, A. W., Dedania, V. S., Materin, M. & Demirci, H. Improved prognostic precision in uveal melanoma through a combined score of clinical stage and molecular prognostication. Ocul. Oncol. Pathol. 8, 35–41 (2022).
National Comprehensive Cancer Network. Melanoma: Uveal (version 2.2022) NCCN https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1488 (2022).
Nathan, P. et al. Uveal melanoma UK national guidelines. Eur. J. Cancer 51, 2404–2412 (2015).
Singh, A. D. et al. Fine-needle aspiration biopsy of uveal melanoma: outcomes and complications. Br. J. Ophthalmol. 100, 456–462 (2016).
Sellam, A. et al. Fine needle aspiration biopsy in uveal melanoma: technique, complications, and outcomes. Am. J. Ophthalmol. 162, 28–34.e1 (2016).
Prescher, G. et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet 347, 1222–1225 (1996).
Damato, B. et al. Cytogenetics of uveal melanoma: a 7-year clinical experience. Ophthalmology 114, 1925–1931 (2007).
Scholes, A. G. et al. Monosomy 3 in uveal melanoma: correlation with clinical and histologic predictors of survival. Invest. Ophthalmol. Vis. Sci. 44, 1008–1011 (2003).
Sisley, K. et al. Abnormalities of chromosomes 3 and 8 in posterior uveal melanoma correlate with prognosis. Genes Chromosomes Cancer 19, 22–28 (1997).
Ewens, K. G. et al. Genomic profile of 320 uveal melanoma cases: chromosome 8p-loss and metastatic outcome. Invest. Ophthalmol. Vis. Sci. 54, 5721–5729 (2013).
Damato, B., Dopierala, J. A. & Coupland, S. E. Genotypic profiling of 452 choroidal melanomas with multiplex ligation-dependent probe amplification. Clin. Cancer Res. 16, 6083–6092 (2010).
Shields, C. L. et al. Ten-year outcomes of uveal melanoma based on The Cancer Genome Atlas (TCGA) classification in 1001 cases. Indian J. Ophthalmol. 69, 1839–1845 (2021).
Gelmi, M. C. et al. Adding The Cancer Genome Atlas chromosome classes to American Joint Committee on cancer system offers more precise prognostication in uveal melanoma. Ophthalmology 129, 431–437 (2022).
Shao, Y. F., Echegaray, J. J., Singh, N. & Singh, A. D. Variability of bad prognosis in uveal melanoma. Ophthalmol. Retin. 3, 186–193 (2019).
Cunha Rola, A. et al. Multicenter external validation of the Liverpool uveal melanoma prognosticator online: an OOG collaborative study. Cancers 12, 477 (2020).
Impactgenetics. Uveal melanoma: prognostic genetic test. Impactgenetics https://impactgenetics.com/wp-content/uploads/2019/03/UM-Test-Description-11Mar2019.pdf (2019).
Farquhar, N. et al. Patterns of BAP1 protein expression provide insights into prognostic significance and the biology of uveal melanoma. J. Pathol. Clin. Res. 4, 26–38 (2018).
Yavuzyigitoglu, S. et al. Uveal melanomas with SF3B1 mutations: a distinct subclass associated with late-onset metastases. Ophthalmology 123, 1118–1128 (2016).
Castle Biosciences. DecisionDx-UMSeq overview. Castle Biosciences https://castletestinfo.com/products/decisiondx-um-overview/decisiondx-umseq/ (2022).
Afshar, A. R. et al. Next-generation sequencing of uveal melanoma for detection of genetic alterations predicting metastasis. Transl. Vis. Sci. Technol. 8, 18 (2019).
Demirci, H. et al. Do largest basal tumor diameter and the American Joint Committee on Cancer’s cancer staging influence prognostication by gene expression profiling in choroidal melanoma. Am. J. Ophthalmol. 195, 83–92 (2018).
Onken, M. D., Worley, L. A., Ehlers, J. P. & Harbour, J. W. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 64, 7205–7209 (2004).
Onken, M. D. et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology 119, 1596–1603 (2012).
Aaberg, T. M. et al. Gene expression profiling in uveal melanoma: five-year prospective outcomes and meta-analysis. Ocul. Oncol. Pathol. 6, 360–367 (2020).
Schefler, A. C. et al. Design, methods, and rationale for the Collaborative Ocular Oncology Group 2 (COOG2) study [abstract]. Investig. Ophthalmol. Vis. Sci. 62, 2870 (2021).
Binkley, E. M. et al. Gene expression profiling prognostication of posterior uveal melanoma: does size matter? Ophthalmol. Retin. 4, 620–629 (2020).
Wong, A. J. et al. Three-year outcomes of uveal melanoma treated with intra-operative ultrasound-guided iodine-125 brachytherapy using custom-built eye plaques. J. Contemp. Brachytherapy 14, 130–139 (2022).
Decatur, C. L. et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 134, 728–733 (2016).
Field, M. G. et al. PRAME as an independent biomarker for metastasis in uveal melanoma. Clin. Cancer Res. 22, 1234–1242 (2016).
Ballhausen, A. et al. Metastatic risk factors associated with class 1A uveal melanoma patients. Cancers https://doi.org/10.3390/cancers13133292 (2021).
Field, M. G. et al. Epigenetic reprogramming and aberrant expression of PRAME are associated with increased metastatic risk in class 1 and class 2 uveal melanomas. Oncotarget 7, 59209–59219 (2016).
Castle Biosciences. Uveal melanoma: DecisionDx-PRAME. Castle Biosciences https://castlebiosciences.com/products/decisiondx-prame-testing/ (2022).
Kujala, E., Makitie, T. & Kivela, T. Very long-term prognosis of patients with malignant uveal melanoma. Invest. Ophthalmol. Vis. Sci. 44, 4651–4659 (2003).
Diener-West, M. et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group report no. 26. Arch. Ophthalmol. 123, 1639–1643 (2005).
Rietschel, P. et al. Variates of survival in metastatic uveal melanoma. J. Clin. Oncol. 23, 8076–8080 (2005).
Wei, A. Z. et al. Characterizing metastatic uveal melanoma patients who develop symptomatic brain metastases. Front. Oncol. https://doi.org/10.3389/fonc.2022.961517 (2022).
Servois, V. et al. Preoperative staging of liver metastases from uveal melanoma by magnetic resonance imaging (MRI) and fluorodeoxyglucose-positron emission tomography (FDG-PET). Eur. J. Surg. Oncol. 36, 189–194 (2010).
Francis, J. H. et al. Hepatic abnormalities identified by staging MRI and accuracy of MRI of patients with uveal melanoma. Br. J. Ophthalmol. 103, 1266–1271 (2019).
Hicks, C., Foss, A. J. & Hungerford, J. L. Predictive power of screening tests for metastasis in uveal melanoma. Eye 12, 945–948 (1998).
Rantala, E. S., Peltola, E., Helminen, H., Hernberg, M. & Kivela, T. T. Hepatic ultrasonography compared with computed tomography and magnetic resonance imaging at diagnosis of metastatic uveal melanoma. Am. J. Ophthalmol. 216, 156–164 (2020).
Orcurto, V. et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography and magnetic resonance imaging in patients with liver metastases from uveal melanoma: results from a pilot study. Melanoma Res. 22, 63–69 (2012).
Marshall, E. et al. MRI in the detection of hepatic metastases from high-risk uveal melanoma: a prospective study in 188 patients. Br. J. Ophthalmol. 97, 159–163 (2013).
Diener-West, M. et al. Screening for metastasis from choroidal melanoma: the Collaborative Ocular Melanoma Study Group report 23. J. Clin. Oncol. 22, 2438–2444 (2004).
Gombos, D. S., Van Quill, K. R., Uusitalo, M. & O’Brien, J. M. Geographic disparities in diagnostic screening for metastatic uveal melanoma. Ophthalmology 111, 2254–2258 (2004).
Daniels, A. B. et al. Impact of different initial systemic staging imaging strategies on metastasis detection in uveal melanoma patients: the Melanoma of the Uvea Staging Imaging Consortium (MUSIC) study [abstract]. Invest. Ophthalmol. Vis. Sci. 61, 4027 (2020).
McLean, I. W. et al. A randomized study of methanol-extraction residue of bacille Calmette-Guerin as postsurgical adjuvant therapy of uveal melanoma. Am. J. Ophthalmol. 110, 522–526 (1990).
Desjardins, L. et al. Etude randomisée de chimiothérapie adjuvante par le Déticène dans le mélanome choroïdien [French]. Ophtalmologie 12, 168–173 (1998).
Piperno-Neumann, S. et al. A randomized multicenter phase 3 trial of adjuvant fotemustine versus surveillance in high risk uveal melanoma (UM) patients (FOTEADJ) [abstract]. J. Clin. Oncol. 35 (Suppl. 15), 9502 (2017).
Seedor, R. S. et al. Randomized phase II study of adjuvant sunitinib or valproic acid in high-risk patients with uveal melanoma: the final analysis of cohort 1 [abstract]. J. Clin. Oncol. 40 (Suppl. 16), 9586 (2022).
Lane, A. M. et al. Adjuvant interferon therapy for patients with uveal melanoma at high risk of metastasis. Ophthalmology 116, 2206–2212 (2009).
Voelter, V. et al. Adjuvant intra-arterial hepatic fotemustine for high-risk uveal melanoma patients. Melanoma Res. 18, 220–224 (2008).
Fountain, E. et al. Adjuvant ipilimumab in high-risk uveal melanoma. Cancers https://doi.org/10.3390/cancers11020152 (2019).
Binkley, E. et al. A prospective trial of adjuvant therapy for high-risk uveal melanoma: assessing 5-year survival outcomes. Br. J. Ophthalmol. 104, 524–528 (2020).
Khan, S. et al. Adjuvant crizotinib in high-risk uveal melanoma following definitive therapy. Front. Oncol. 12, 976837 (2022).
Abdel-Rahman, M. H., Boru, G., Massengill, J., Salem, M. M. & Davidorf, F. H. MET oncogene inhibition as a potential target of therapy for uveal melanomas. Investig. Ophthalmol. Vis. Sci. 51, 3333–3339 (2010).
Surriga, O. et al. Crizotinib, a c-Met inhibitor, prevents metastasis in a metastatic uveal melanoma model. Mol. Cancer Ther. 12, 2817–2826 (2013).
Valsecchi, M. E. et al. Adjuvant sunitinib in high-risk patients with uveal melanoma: comparison with institutional controls. Ophthalmology 125, 210–217 (2018).
Landreville, S. et al. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin. Cancer Res. 18, 408–416 (2012).
Souri, Z. et al. HDAC inhibition increases HLA class I expression in uveal melanoma. Cancers https://doi.org/10.3390/cancers12123690 (2020).
Kuznetsoff, J. N. et al. Dual screen for efficacy and toxicity identifies HDAC inhibitor with distinctive activity spectrum for BAP1-mutant uveal melanoma. Mol. Cancer Res. 19, 215–222 (2021).
Tawbi, H. A. et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N. Engl. J. Med. 386, 24–34 (2022).
Furney, S. J. et al. SF3B1 mutations are associated with alternative splicing in uveal melanoma. Cancer Discov. 3, 1122–1129 (2013).
Saint-Ghislain, M. et al. MBD4 deficiency is predictive of response to immune checkpoint inhibitors in metastatic uveal melanoma patients. Eur. J. Cancer 173, 105–112 (2022).
Mariani, P. et al. Surgical management of liver metastases from uveal melanoma: 16 years’ experience at the Institut Curie. Eur. J. Surg. Oncol. 35, 1192–1197 (2009).
Sterbis, E. et al. Safety and efficacy of microwave ablation for uveal melanoma metastatic to the liver [abstract 184]. J. Vasc. Interv. Radiol. 30 (Suppl. 3), S84 (2019).
Mariani, P. et al. Radiofrequency ablation and surgical resection of liver metastases from uveal melanoma. Eur. J. Surg. Oncol. 42, 706–712 (2016).
Rivoire, M. et al. Treatment of liver metastases from uveal melanoma. Ann. Surg. Oncol. 12, 422–428 (2005).
Rowcroft, A., Loveday, B. P. T., Thomson, B. N. J., Banting, S. & Knowles, B. Systematic review of liver directed therapy for uveal melanoma hepatic metastases. HPB 22, 497–505 (2020).
Leyvraz, S. et al. Hepatic intra-arterial versus intravenous fotemustine in patients with liver metastases from uveal melanoma (EORTC 18021): a multicentric randomized trial. Ann. Oncol. 25, 742–746 (2014).
Gonsalves, C. F. et al. A prospective phase II trial of radioembolization for treatment of uveal melanoma hepatic metastasis. Radiology 293, 223–231 (2019).
Peuker, C.-A. A. et al. First interim analysis of the SirTac trial: a randomized phase II study of SIRT and DSM-TACE in patients with liver metastases from uveal melanoma [abstract]. J. Clin. Oncol. 40 (Suppl. 16), 9511 (2022).
Valsecchi, M. E. et al. Double-blinded, randomized phase II study using embolization with or without granulocyte-macrophage colony-stimulating factor in uveal melanoma with hepatic metastases. J. Vasc. Interv. Radiol. 26, 523–532.e2 (2015).
Yamamoto, A. et al. High-dose immunoembolization: survival benefit in patients with hepatic metastases from uveal melanoma. Radiology 252, 290–298 (2009).
Gonsalves, C. F., Eschelman, D. J., Thornburg, B., Frangos, A. & Sato, T. Uveal melanoma metastatic to the liver: chemoembolization with 1,3-bis-(2-chloroethyl)-1-nitrosourea. AJR Am. J. Roentgenol. 205, 429–433 (2015).
Tan, A., Eschelman, D., Gonsalves, C., Frangos, A. & Sato, T. Treatment of bulky uveal melanoma (UM) hepatic metastases with doxorubicin eluting beads (DEBDOX) followed by 1,3-bis(2-chloroethyl)-1-nitrosurea (BCNU) TACE: an initial experience [abstract 84]. J. Vasc. Interv. Radiol. 25 (Suppl. 3), S45 (2014).
Bagge, R. O. et al. Isolated hepatic perfusion as a treatment for uveal melanoma liver metastases, first results from a phase III randomized controlled multicenter trial (the SCANDIUM trial) [abstract]. J. Clin. Oncol. 40 (Suppl. 17), LBA9509 (2022).
Zager, J. S. et al. FOCUS phase 3 trial results: percutaneous hepatic perfusion (PHP) with melphalan for patients with ocular melanoma liver metastases (PHP-OCM-301/301A) [abstract]. J. Clin. Oncol. 40 (Suppl. 16), 9510 (2022).
Olofsson, R. et al. Isolated hepatic perfusion for ocular melanoma metastasis: registry data suggests a survival benefit. Ann. Surg. Oncol. 21, 466–472 (2014).
Hughes, M. S. et al. Results of a randomized controlled multicenter phase III trial of percutaneous hepatic perfusion compared with best available care for patients with melanoma liver metastases. Ann. Surg. Oncol. 23, 1309–1319 (2016).
Schmittel, A. et al. A randomized phase II trial of gemcitabine plus treosulfan versus treosulfan alone in patients with metastatic uveal melanoma. Ann. Oncol. 17, 1826–1829 (2006).
Sacco, J. J. et al. Sunitinib versus dacarbazine as first-line treatment in patients with metastatic uveal melanoma [abstract]. J. Clin. Oncol. 31 (Suppl. 15), 9031 (2013).
Carvajal, R. D. et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA 311, 2397–2405 (2014).
Shoushtari, A. N. et al. A randomized phase 2 study of trametinib with or without GSK2141795 in patients with advanced uveal melanoma [abstract]. J. Clin. Oncol. 34(Suppl. 15), 9511 (2016).
Carvajal, R. D. et al. Selumetinib in combination with dacarbazine in patients with metastatic uveal melanoma: a phase III, multicenter, randomized trial (SUMIT). J. Clin. Oncol. 36, 1232–1239 (2018).
Nathan, P. et al. SELPAC: A 3 arm randomised phase II study of the MEK inhibitor selumetinib alone or in combination with paclitaxel (PT) in metastatic uveal melanoma (UM) [abstract LBA73]. Ann. Oncol. 30 (Suppl. 5), v908–v910 (2019).
Luke, J. J. et al. Randomized phase II trial and tumor mutational spectrum analysis from cabozantinib versus chemotherapy in metastatic uveal melanoma (Alliance A091201). Clin. Cancer Res. 26, 804–811 (2020).
Bedikian, A. Y., Papadopoulos, N., Plager, C., Eton, O. & Ring, S. Phase II evaluation of temozolomide in metastatic choroidal melanoma. Melanoma Res. 13, 303–306 (2003).
Schmidt-Hieber, M., Schmittel, A., Thiel, E. & Keilholz, U. A phase II study of bendamustine chemotherapy as second-line treatment in metastatic uveal melanoma. Melanoma Res. 14, 439–442 (2004).
O’Neill, P. A., Butt, M., Eswar, C. V., Gillis, P. & Marshall, E. A prospective single arm phase II study of dacarbazine and treosulfan as first-line therapy in metastatic uveal melanoma. Melanoma Res. 16, 245–248 (2006).
Bhatia, S. et al. Phase II trial of sorafenib in combination with carboplatin and paclitaxel in patients with metastatic uveal melanoma: SWOG S0512. PLoS ONE 7, e48787 (2012).
Guenterberg, K. D. et al. A pilot study of bevacizumab and interferon-α2b in ocular melanoma. Am. J. Clin. Oncol. 34, 87–91 (2011).
Tarhini, A. A. et al. Aflibercept (VEGF Trap) in inoperable stage III or stage IV melanoma of cutaneous or uveal origin. Clin. Cancer Res. 17, 6574–6581 (2011).
Piperno-Neumann, S. et al. Phase II trial of bevacizumab in combination with temozolomide as first-line treatment in patients with metastatic uveal melanoma. Oncologist 21, 281–282 (2016).
Penel, N. et al. O-Mel-Inib: a Cancero-pole Nord-Ouest multicenter phase II trial of high-dose imatinib mesylate in metastatic uveal melanoma. Invest. N. Drugs 26, 561–565 (2008).
Hofmann, U. B., Kauczok-Vetter, C. S., Houben, R. & Becker, J. C. Overexpression of the KIT/SCF in uveal melanoma does not translate into clinical efficacy of imatinib mesylate. Clin. Cancer Res. 15, 324–329 (2009).
Mattei, J. et al. A phase II study of the insulin-like growth factor type I receptor inhibitor IMC-A12 in patients with metastatic uveal melanoma. Melanoma Res. 30, 574–579 (2020).
Hasanov, M. et al. A phase II study of glembatumumab vedotin for metastatic uveal melanoma. Cancers https://doi.org/10.3390/cancers12082270 (2020).
Patel, S. P. et al. A phase II study of gefitinib in patients with metastatic melanoma. Melanoma Res. 21, 357–363 (2011).
Shoushtari, A. N. et al. A phase 2 trial of everolimus and pasireotide long-acting release in patients with metastatic uveal melanoma. Melanoma Res. 26, 272–277 (2016).
Shoushtari, A. N. et al. A phase Ib study of sotrastaurin, a PKC inhibitor, and alpelisib, a PI3Kα inhibitor, in patients with metastatic uveal melanoma. Cancers https://doi.org/10.3390/cancers13215504 (2021).
Kraehenbuehl, L. et al. Pilot trial of arginine deprivation plus nivolumab and ipilimumab in patients with metastatic uveal melanoma. Cancers https://doi.org/10.3390/cancers14112638 (2022).
Ambrosini, G. et al. Identification of unique MEK-dependent genes in GNAQ mutant uveal melanoma involved in cell growth, tumor cell invasion, and MEK resistance. Clin. Cancer Res. 18, 3552–3561 (2012).
Khalili, J. S. et al. Combination small molecule MEK and PI3K inhibition enhances uveal melanoma cell death in a mutant GNAQ- and GNA11-dependent manner. Clin. Cancer Res. 18, 4345–4355 (2012).
Zimmer, L. et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naive patients with metastatic uveal melanoma. PLoS ONE 10, e0118564 (2015).
Joshua, A. M. et al. A phase 2 study of tremelimumab in patients with advanced uveal melanoma. Melanoma Res. 25, 342–347 (2015).
Algazi, A. P. et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 122, 3344–3353 (2016).
Karydis, I. et al. Clinical activity and safety of pembrolizumab in ipilimumab pre-treated patients with uveal melanoma. Oncoimmunology 5, e1143997 (2016).
Rossi, E. et al. Pembrolizumab as first-line treatment for metastatic uveal melanoma. Cancer Immunol. Immunother. 68, 1179–1185 (2019).
Ny, L. et al. The PEMDAC phase 2 study of pembrolizumab and entinostat in patients with metastatic uveal melanoma. Nat. Commun. 12, 5155 (2021).
Piulats, J. M. et al. Nivolumab plus ipilimumab for treatment-naive metastatic uveal melanoma: an open-label, multicenter, phase II trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402). J. Clin. Oncol. 39, 586–598 (2021).
Pelster, M. S. et al. Nivolumab and ipilimumab in metastatic uveal melanoma: results from a single-arm phase II study. J. Clin. Oncol. 39, 599–607 (2021).
Najjar, Y. G. et al. Ipilimumab plus nivolumab for patients with metastatic uveal melanoma: a multicenter, retrospective study. J. Immunother. Cancer 8, e000331 (2020).
Heppt, M. V. et al. Combined immune checkpoint blockade for metastatic uveal melanoma: a retrospective, multi-center study. J. Immunother. Cancer 7, 299 (2019).
Javed, A. et al. PD-L1 expression in tumor metastasis is different between uveal melanoma and cutaneous melanoma. Immunotherapy 9, 1323–1330 (2017).
Li, X. et al. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer 21, 541–557 (2021).
Middleton, M. R. et al. Tebentafusp, a TCR/anti-CD3 bispecific fusion protein targeting gp100, potently activated antitumor immune responses in patients with metastatic melanoma. Clin. Cancer Res. 26, 5869–5878 (2020).
Carvajal, R. D. et al. Clinical and molecular response to tebentafusp in previously treated patients with metastatic uveal melanoma: a phase 2 trial. Nat. Med. https://doi.org/10.1038/s41591-022-02015-7 (2022).
Milhem, M. M. et al. Phase 1b/2, open label, multicenter, study of the combination of SD-101 and pembrolizumab in patients with advanced melanoma who are naïve to anti-PD-1 therapy [abstract]. J. Clin. Oncol. 37 (Suppl. 15), 9534 (2019).
Wu, X., Li, J., Zhu, M., Fletcher, J. A. & Hodi, F. S. Protein kinase C inhibitor AEB071 targets ocular melanoma harboring GNAQ mutations via effects on the PKC/Erk1/2 and PKC/NF-κB pathways. Mol. Cancer Ther. 11, 1905–1914 (2012).
Chen, X. et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene 33, 4724–4734 (2014).
Piperno-Neumann, S. et al. Genomic profiling of metastatic uveal melanoma and clinical results of a phase I study of the protein kinase C inhibitor AEB071. Mol. Cancer Ther. 19, 1031 (2020).
Kapiteijn, E. et al. A phase I trial of LXS196, a novel PKC inhibitor for metastatic uveal melanoma [abstract]. Cancer Res. 79 (Suppl. 13), CT068 (2019).
Piperno-Neumann, S. et al. A phase I trial of LXS196, a protein kinase C (PKC) inhibitor, for metastatic uveal melanoma. Br. J. Cancer (in the press).
Wagle, M.-C., Ravindran, N., Pankajakshan, D., Lackner, M. & Mounir, Z. Preclinical evaluation of a PKC and MET inhibitor combination in metastatic uveal melanoma [abstract]. Cancer Res. 81 (Suppl. 13), 1343 (2021).
IDEAYA Biosciences. IDEAYA reports positive interim phase 2 clinical results for darovasertib and crizotinib synthetic lethal combination in metastatic uveal melanoma. IDEAYA Biosciences https://media.ideayabio.com/2022-09-11-IDEAYA-Reports-Positive-Interim-Phase-2-Clinical-Results-for-Darovasertib-and-Crizotinib-Synthetic-Lethal-Combination-in-Metastatic-Uveal-Melanoma (2022).
Yu, F. X. et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell 25, 822–830 (2014).
Feng, X. et al. A platform of synthetic lethal gene interaction networks reveals that the GNAQ uveal melanoma oncogene controls the Hippo pathway through FAK. Cancer Cell 35, 457–472.e5 (2019).
Paradis, J. S. et al. Synthetic lethal screens reveal cotargeting FAK and MEK as a multimodal precision therapy for GNAQ-driven uveal melanoma. Clin. Cancer Res. 27, 3190–3200 (2021).
Onken, M. D. et al. Targeting primary and metastatic uveal melanoma with a G protein inhibitor. J. Biol. Chem. 296, 100403 (2021).
Lapadula, D. et al. Effects of oncogenic Gαq and Gα11 inhibition by FR900359 in uveal melanoma. Mol. Cancer Res. 17, 963–973 (2019).
Ma, J., Weng, L., Bastian, B. C. & Chen, X. Functional characterization of uveal melanoma oncogenes. Oncogene 40, 806–820 (2021).
Hitchman, T. D. et al. Combined inhibition of Gαq and MEK enhances therapeutic efficacy in uveal melanoma. Clin. Cancer Res. 27, 1476–1490 (2021).
Johnson, Z. et al. Preclinical development of a novel, highly selective PI3Kδ inhibitor, IOA-244, for the treatment of solid malignancies [abstract 93P]. Ann. Oncol. 30 (Suppl. 7), vii27 (2019).
Faiao-Flores, F. et al. HDAC inhibition enhances the in vivo efficacy of MEK inhibitor therapy in uveal melanoma. Clin. Cancer Res. 25, 5686–5701 (2019).
Rago, F. et al. The discovery of SWI/SNF chromatin remodeling activity as a novel and targetable dependency in uveal melanoma. Mol. Cancer Ther. 19, 2186–2195 (2020).
Bigot, J. et al. Splicing patterns in SF3B1-mutated uveal melanoma generate shared immunogenic tumor-specific neoepitopes. Cancer Discov. 11, 1938–1951 (2021).
Grimes, J. et al. Clinical characteristics of SF3B1 mutant (mut) uveal melanoma (UM) and response to immune checkpoint inhibition (ICI) [abstract]. J. Clin. Oncol. 39 (Suppl. 15), 9535 (2021).
Fong, J. Y. et al. Therapeutic targeting of RNA splicing catalysis through inhibition of protein arginine methylation. Cancer Cell 36, 194–209.e9 (2019).
Ito, K. et al. PRMT5 inhibition regulates alternative splicing and DNA damage repair pathways in SF3B1 R625G expressing uveal melanoma cells [abstract]. Cancer Res. 81(Suppl. 13), 1137 (2021).
Chandran, S. S. et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: a single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol. 18, 792–802 (2017).
Fang, D. D. et al. MDM2 inhibitor APG-115 synergizes with PD-1 blockade through enhancing antitumor immunity in the tumor microenvironment. J. Immunother. Cancer 7, 327 (2019).
Tolcher, A. W. et al. Preliminary results of a phase II study of alrizomadlin (APG-115), a novel, small-molecule MDM2 inhibitor, in combination with pembrolizumab in patients (pts) with unresectable or metastatic melanoma or advanced solid tumors that have failed immuno-oncologic (I-O) drugs [abstract]. J. Clin. Oncol. 39 (Suppl. 15), 2506 (2021).
Immunocore. Immunocore presents promising initial phase 1 data for first off-the-shelf TCR therapy targeting PRAME at the ESMO 2022 Congress. Immunocore https://ir.immunocore.com/news-releases/news-release-details/immunocore-presents-promising-initial-phase-1-data-first-shelf (2022).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
R.D.C. has acted as a consultant for Alkermes, Aura Biosciences, Bristol Myers Squibb, Castle Biosciences, Chimeron, Delcath, Eisai, Hengrui, Ideaya, Immunocore, InxMed, Iovance, Merck, Novartis, Oncosec, Pierre Fabre, PureTech Health, Regeneron, Rgenix, Sanofi Genzyme, Sorrento Therapeutics and TriSalus Life Sciences; holds stock options in Aura Biosciences, Chimeron and Rgenix; and has received research funding through his institution from Amgen, Astellis, AstraZeneca, BioMed Valley, Bolt, Bristol Myers Squibb, Corvus, Cstone, Foghorn, Ideaya, Immatics, Immunocore, InxMed, Iovance, Merck, Mirati, Novartis, Pfizer, Plexxikon, Regeneron and Roche/Genentech. J.J.S. has acted as a consultant for Bristol Myers Squibb, Delcath, Immunocore and Merck Sharp & Dohme; has received speaker’s honoraria from Pierre-Fabre and Immunocore; and research funding through his institution from AstraZeneca, Bristol Myers Squibb, Immunocore, Merck Sharp & Dohme and Replimune. D.J.E. has acted as a consultant for Delcath. R.O.B. has acted as a consultant for Amgen, BD/BARD, Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Roche and Sanofi Genzyme; has received speaker’s honoraria from Pfizer and Roche; is a shareholder of SATMEG Ventures AB; and has received research funding through his institution from Bristol Myers Squibb and SkyLineDx. J.W.H. has acted as a consultant for Castle Biosciences and Immunocore; and receives patent royalties from Washington University. S.P.P. has acted as a consultant for Advance Knowledge in Healthcare, Bristol Myers Squibb, Cardinal Health, Delcath, Immunocore, Novartis and TriSalus Life Sciences; and has received research funding through her institution from Bristol Myers Squibb, Foghorn Therapeutics, Ideaya, InxMed, Lvgen, Novartis, Provectus Biopharmaceuticals, Seagen, Syntrix Bio and TriSalus Life Sciences. A.M.J. has received research funding through his institution from Ideaya and Immunocore. M.J.J., N.D.C. and S.P.-N. declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks U. Pfeffer, J. Piulats, A. D. Singh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov/
Liverpool Uveal Melanoma Prognosticator Online (LUMPO): https://mpcetoolsforhealth.liverpool.ac.uk/LUMPONet/LUMPONet.html
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carvajal, R.D., Sacco, J.J., Jager, M.J. et al. Advances in the clinical management of uveal melanoma. Nat Rev Clin Oncol 20, 99–115 (2023). https://doi.org/10.1038/s41571-022-00714-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-022-00714-1
This article is cited by
-
PRAME induces genomic instability in uveal melanoma
Oncogene (2024)
-
Uveal melanoma immunogenomics predict immunotherapy resistance and susceptibility
Nature Communications (2024)
-
Identification and validation of a costimulatory molecule-related signature to predict the prognosis for uveal melanoma patients
Scientific Reports (2024)
-
1,4-dihydroxy quininib activates ferroptosis pathways in metastatic uveal melanoma and reveals a novel prognostic biomarker signature
Cell Death Discovery (2024)
-
The Future of Checkpoint Inhibitors in Uveal Melanoma: A Narrative Review
Ophthalmology and Therapy (2024)