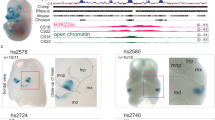
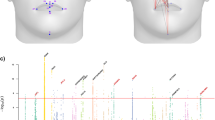
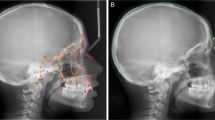
Abstract
Major differences in facial morphology distinguish vertebrate species. Variation of facial traits underlies the uniqueness of human individuals, and abnormal craniofacial morphogenesis during development leads to birth defects that significantly affect quality of life. Studies during the past 40 years have advanced our understanding of the molecular mechanisms that establish facial form during development, highlighting the crucial roles in this process of a multipotent cell type known as the cranial neural crest cell. In this Review, we discuss recent advances in multi-omics and single-cell technologies that enable genes, transcriptional regulatory networks and epigenetic landscapes to be closely linked to the establishment of facial patterning and its variation, with an emphasis on normal and abnormal craniofacial morphogenesis. Advancing our knowledge of these processes will support important developments in tissue engineering, as well as the repair and reconstruction of the abnormal craniofacial complex.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sheehan, M. J. & Nachman, M. W. Morphological and population genomic evidence that human faces have evolved to signal individual identity. Nat. Commun. 5, 4800 (2014).
Guo, J. et al. Variation and signatures of selection on the human face. J. Hum. Evol. 75, 143–152 (2014).
Santagati, F. & Rijli, F. M. Cranial neural crest and the building of the vertebrate head. Nat. Rev. Neurosci. 4, 806–818 (2003).
Tang, W. & Bronner, M. E. Neural crest lineage analysis: from past to future trajectory. Development 147, dev193193 (2020).
Minoux, M. & Rijli, F. M. Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development 137, 2605–2621 (2010).
le Douarin, N. M. & Dupin, E. The “beginnings” of the neural crest. Dev. Biol. 444, S3–S13 (2018).
Dash, S. & Trainor, P. A. The development, patterning and evolution of neural crest cell differentiation into cartilage and bone. Bone 137, 115409 (2020).
Martik, M. L. & Bronner, M. E. Riding the crest to get a head: neural crest evolution in vertebrates. Nat. Rev. Neurosci. 22, 616–626 (2021).
Chong-Morrison, V. & Sauka-Spengler, T. The cranial neural crest in a multiomics era. Front. Physiol. 12, 634440 (2021).
Candido-Ferreira, I. L., Lukoseviciute, M. & Sauka-Spengler, T. Multi-layered transcriptional control of cranial neural crest development. Semin. Cell Dev. Biol. 138, 1–14 (2022).
Schock, E. N., York, J. R. & LaBonne, C. The developmental and evolutionary origins of cellular pluripotency in the vertebrate neural crest. Semin. Cell Dev. Biol. 138, 36–44 (2023).
Piacentino, M. L., Li, Y. & Bronner, M. E. Epithelial-to-mesenchymal transition and different migration strategies as viewed from the neural crest. Curr. Opin. Cell Biol. 66, 43–50 (2020).
Milmoe, N. J. & Tucker, A. S. Craniofacial transitions: the role of EMT and MET during head development. Development 148, dev196030 (2021).
Zhao, R. & Trainor, P. A. Epithelial to mesenchymal transition during mammalian neural crest cell delamination. Semin. Cell Dev. Biol. 138, 54–67 (2023).
Hartmann, J. & Mayor, R. Self-organized collective cell behaviors as design principles for synthetic developmental biology. Semin. Cell Dev. Biol. 141, 63–73 (2023).
Kalcheim, C. The neural crest: a remarkable model system for studying development and disease. Methods Mol. Biol. 1976, 1–19 (2019).
Weiner, A. M. J., Coux, G., Armas, P. & Calcaterra, N. Insights into vertebrate head development: from cranial neural crest to the modelling of neurocristopathies. Int. J. Dev. Biol. 65, 215–225 (2021).
Som, P. M. & Naidich, T. P. Illustrated review of the embryology and development of the facial region, part 1: early face and lateral nasal cavities. Am. J. Neuroradiol. 34, 2233–2240 (2013).
Marcucio, R., Hallgrimsson, B. & Young, N. M. Facial morphogenesis. physical and molecular interactions between the brain and the face. Curr. Top. Dev. Biol. 115, 299–320 (2015).
Jiang, R., Bush, J. O. & Lidral, A. C. Development of the upper lip: morphogenetic and molecular mechanisms. Dev. Dyn. 235, 1152–1166 (2006).
Depew, M. J. & Compagnucci, C. Tweaking the hinge and caps: testing a model of the organization of jaws. J. Exp. Zool. B. Mol. Dev. Evol. 310, 315–335 (2008).
Tamarin, A. & Boyde, A. Facial and visceral arch development in the mouse embryo: a study by scanning electron microscopy. J. Anat. 124, 563–580 (1977).
Takahashi, Y., Sipp, D. & Enomoto, H. Tissue interactions in neural crest cell development and disease. Science 341, 860–863 (2013).
Simões-Costa, M. & Bronner, M. E. Establishing neural crest identity: a gene regulatory recipe. Development 142, 242–257 (2015).
Jeong, J., Mao, J., Tenzen, T., Kottmann, A. H. & McMahon, A. P. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 18, 937–951 (2004).
Iwata, J., Parada, C. & Chai, Y. The mechanism of TGF-β signaling during palate development. Oral Dis. 17, 733–744 (2011).
Neben, C. L. & Merrill, A. E. Signaling pathways in craniofacial development. Insights from rare skeletal disorders. Curr. Top. Dev. Biol. 115, 493–542 (2015).
Ornitz, D. M. & Marie, P. J. Fibroblast growth factors in skeletal development. Curr. Top. Dev. Biol. 133, 195–234 (2019).
van Otterloo, E. et al. AP-2α and AP-2β cooperatively function in the craniofacial surface ectoderm to regulate chromatin and gene expression dynamics during facial development. eLife 11, e70511 (2022).
Noden, D. M. & Trainor, P. A. Relations and interactions between cranial mesoderm and neural crest populations. J. Anat. 207, 575–601 (2005).
Marcucio, R. S., Cordero, D. R., Hu, D. & Helms, J. A. Molecular interactions coordinating the development of the forebrain and face. Dev. Biol. 284, 48–61 (2005).
Cordero, D. R. et al. Cranial neural crest cells on the move: their roles in craniofacial development. Am. J. Med. Genet. A 155, 270–279 (2011).
Hooper, J. E. et al. Systems biology of facial development: contributions of ectoderm and mesenchyme. Dev. Biol. 426, 97–114 (2017).
LaMantia, A. S. Why Does the face predict the brain? Neural crest induction, craniofacial morphogenesis, and neural circuit development. Front. Physiol. 11, 610970 (2020).
Ferretti, E. et al. A conserved Pbx-Wnt-p63-Irf6 regulatory module controls face morphogenesis by promoting epithelial apoptosis. Dev. Cell. 21, 627–641 (2011). This study reports a HOX-independent, PBX-dependent Wnt–p63–Irf6 regulatory network in midfacial ectoderm that is conserved in mammals, dysregulation of which leads to suppression of midfacial apoptosis and cleft lip with or without cleft palate.
Losa, M. et al. Face morphogenesis is promoted by Pbx-dependent EMT via regulation of snail1 during frontonasal prominence fusion. Development 145, dev157628 (2018).
Welsh, I. C. et al. Pbx loss in cranial neural crest, unlike in epithelium, results in cleft palate only and a broader midface. J. Anat. 233, 222–242 (2018).
Lumsden, A. & Krumlauf, R. Patterning the vertebrate neuraxis. Science 274, 1109–1115 (1996).
Gendron-Maguire, M., Mallo, M., Zhang, M. & Gridley, T. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell 75, 1317–1331 (1993).
Rijli, F. M. et al. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell 75, 1333–1349 (1993).
Köntges, G. & Lumsden, A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development 122, 3229–3242 (1996).
Couly, G., Creuzet, S., Bennaceur, S., Vincent, C. & le Douarin, N. M. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development 129, 1061–1073 (2002).
Kitazawa, T., Minoux, M., Ducret, S. & Rijli, F. M. Different ectopic Hoxa2 expression levels in mouse cranial neural crest cells result in distinct craniofacial anomalies and homeotic phenotypes. J. Dev. Biol. 10, 9 (2022).
Minoux, M., Antonarakis, G. S., Kmita, M., Duboule, D. & Rijli, F. M. Rostral and caudal pharyngeal arches share a common neural crest ground pattern. Development 136, 637–645 (2009).
Vieux-Rochas, M., Mascrez, B., Krumlauf, R. & Duboule, D. Combined function of HoxA and HoxB clusters in neural crest cells. Dev. Biol. 382, 293–301 (2013).
Brown, K. K. et al. HOXA2 haploinsufficiency in dominant bilateral microtia and hearing loss. Hum. Mutat. 34, 1347–1351 (2013).
Santagati, F., Minoux, M., Ren, S. Y. & Rijli, F. M. Temporal requirement of Hoxa2 in cranial neural crest skeletal morphogenesis. Development 132, 4927–4936 (2005).
Minoux, M. et al. Mouse Hoxa2 mutations provide a model for microtia and auricle duplication. Development 140, 4386–4397 (2013). This study reports that the mouse external ear (pinna) derives from the Hoxa2-expressing, neural crest-derived mesenchyme of BA2, and that Hoxa2 is necessary and sufficient to drive pinna morphogenesis.
Moens, C. B. & Selleri, L. Hox cofactors in vertebrate development. Dev. Biol. 291, 193–206 (2006).
Selleri, L., Zappavigna, V. & Ferretti, E. ‘Building a perfect body’: control of vertebrate organogenesis by PBX-dependent regulatory networks. Genes Dev. 33, 258–275 (2019).
Capellini, T. D. et al. Pbx1/Pbx2 requirement for distal limb patterning is mediated by the hierarchical of Hox gene spatial distribution and Shh expression. Development 133, 2263–2273 (2006).
Mariani, L. et al. A TALE/HOX code unlocks WNT signalling response towards paraxial mesoderm. Nat. Commun. 12, 5136 (2021).
Garcia, D. A. et al. Power-law behavior of transcription factor dynamics at the single-molecule level implies a continuum affinity model. Nucleic Acids Res. 49, 6605–6620 (2021).
Hansen, J. L., Loell, K. J. & Cohen, B. A. A test of the pioneer factor hypothesis using ectopic liver gene activation. eLife 11, e73358 (2022).
Selleri, L. et al. Requirement for Pbx1 in skeletal patterning and programming chondrocyte proliferation and differentiation. Development 128, 3543–3557 (2001).
Amin, S. et al. Hoxa2 selectively enhances meis binding to change a branchial arch ground state. Dev. Cell. 32, 265–277 (2015).
Slavotinek, A. et al. De novo, deleterious sequence variants that alter the transcriptional activity of the homeoprotein PBX1 are associated with intellectual disability and pleiotropic developmental defects. Hum. Mol. Genet. 26, 4849–4860 (2017).
Depew, M. J., Simpson, C. A., Morasso, M. & Rubenstein, J. L. R. Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development. J. Anat. 207, 501–561 (2005).
Acampora, D. et al. Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development 126, 3795–3809 (1999).
Depew, M. J. et al. Dlx5 regulates regional development of the branchial arches and sensory capsules. Development 126, 3831–3846 (1999).
Beverdam, A. et al. Jaw transformation with gain of symmetry after Dlx5/Dlx6 inactivation: mirror of the past? Genesis 34, 221–227 (2002).
Depew, M. J., Lufkin, T. & Rubenstein, J. L. R. Specification of jaw subdivisions by Dlx genes. Science 298, 381–385 (2002).
Jeong, J. et al. Dlx genes pattern mammalian jaw primordium by regulating both lower jaw-specific and upper jaw-specific genetic programs. Development 135, 2905–2916 (2008).
Sauka-Spengler, T. & Bronner-Fraser, M. A gene regulatory network orchestrates neural crest formation. Nat. Rev. Mol. Cell. Biol. 9, 557–568 (2008).
Schock, E. N. & LaBonne, C. Sorting Sox: diverse roles for Sox transcription factors during neural crest and craniofacial development. Front. Physiol. 11, 606889 (2020).
Betancur, P., Bronner-Fraser, M. & Sauka-Spengler, T. Genomic code for Sox10 activation reveals a key regulatory enhancer for cranial neural crest. Proc. Natl Acad. Sci. USA 107, 3570–3575 (2010).
Simoes-Costa, M. & Bronner, M. E. Reprogramming of avian neural crest axial identity and cell fate. Science 352, 1570–1573 (2016).
McGonnell, I. M. & Graham, A. Trunk neural crest has skeletogenic potential. Curr. Biol. 12, 767–771 (2002).
Gilbert, S. F., Bender, G., Betters, E., Yin, M. & Cebra-Thomas, J. A. The contribution of neural crest cells to the nuchal bone and plastron of the turtle shell. Integr. Comp. Biol. 47, 401–408 (2007).
Rada-Iglesias, A. et al. Epigenomic annotation of enhancers predicts transcriptional regulators of human neural crest. Cell Stem Cell 11, 633–648 (2012).
Krishnakumar, R. et al. FOXD3 regulates pluripotent stem cell potential by simultaneously initiating and repressing enhancer activity. Cell Stem Cell 18, 104–117 (2016).
Respuela, P. et al. Foxd3 promotes exit from naive pluripotency through enhancer decommissioning and inhibits germline specification. Cell Stem Cell 18, 118–133 (2016).
Lukoseviciute, M. et al. From pioneer to repressor: bimodal foxd3 activity dynamically remodels neural crest regulatory landscape in vivo. Dev. Cell. 47, 608–628 (2018).
Simões-Costa, M. S., McKeown, S. J., Tan-Cabugao, J., Sauka-Spengler, T. & Bronner, M. E. Dynamic and differential regulation of stem cell factor FoxD3 in the neural crest is encrypted in the genome. PLoS Genet. 8, e1003142 (2012).
Attanasio, C. et al. Fine tuning of craniofacial morphology by distant-acting enhancers. Science 342, 1241006 (2013). This work identifies mouse craniofacial developmental enhancers that fine-tune gene expression during facial assembly, and suggests that variation of enhancer sequence or copy number might contribute to facial variation in human populations.
Uslu, V. V. et al. Long-range enhancers regulating Myc expression are required for normal facial morphogenesis. Nat. Genet. 46, 753–758 (2014).
Bronner-Fraser, M. & Fraser, S. E. Cell lineage analysis reveals multipotency of some avian neural crest cells. Nature 335, 161–164 (1988).
Baggiolini, A. et al. Premigratory and migratory neural crest cells are multipotent in vivo. Cell Stem Cell 16, 314–322 (2015).
Zalc, A. et al. Reactivation of the pluripotency program precedes formation of the cranial neural crest. Science 371, eabb4776 (2021).
Tiana, M. et al. Pluripotency factors regulate the onset of Hox cluster activation in the early embryo. Sci. Adv. 8, eabo3583 (2022).
Buitrago-Delgado, E., Nordin, K., Rao, A., Geary, L. & Labonne, C. Shared pluripotency programs suggest derivation of vertebrate neural crest from blastula cells. Science 348, 1332–1335 (2015).
Briggs, J. A. et al. The dynamics of gene expression in vertebrate embryogenesis at single-cell resolution. Science 360, eaar5780 (2018).
Scerbo, P. & Monsoro-Burq, A. H. The vertebrate-specific VENTX/NANOG gene empowers neural crest with ectomesenchyme potential. Sci. Adv. 6, eaaz1469 (2020).
Hovland, A. S. et al. Pluripotency factors are repurposed to shape the epigenomic landscape of neural crest cells. Dev. Cell. 57, 2257–2272 (2022). This work suggests that OCT4 and SOX2 pluripotency factors are co-opted from the ESC circuit and repurposed to generate distinct chromatin landscapes by interaction with TFAP2A during neural crest development.
Pajanoja, C. et al. Maintenance of pluripotency in the entire ectoderm enables neural crest formation. Preprint at Res. Sq. https://doi.org/10.21203/rs.3.rs-2285117/v1 (2023).
Betancur, P., Bronner-Fraser, M. & Sauka-Spengler, T. Assembling neural crest regulatory circuits into a gene regulatory network. Annu. Rev. Cell Dev. Biol. 26, 581–603 (2010).
Soldatov, R. et al. Spatiotemporal structure of cell fate decisions in murine neural crest. Science 364, eaas9536 (2019). This study shows that neural crest progenitor cells choose between possible fates by first transcriptionally coactivating competing gene expression programmes that instruct distinct cellular fates, and then, following a decision point (bifurcation), upregulating one programme and downregulating another to transition towards a specific fate.
Dupin, E., Calloni, G. W., Coelho-Aguiar, J. M. & le Douarin, N. M. The issue of the multipotency of the neural crest cells. Dev. Biol. 444, S47–S59 (2018).
Barriga, E. H., Franze, K., Charras, G. & Mayor, R. Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature 554, 523–527 (2018).
Hu, M. et al. Multilineage gene expression precedes commitment in the hemopoietic system. Genes Dev. 11, 774–785 (1997).
Kelsh, R. N. et al. Cyclical fate restriction: a new view of neural crest cell fate specification. Development 148, dev176057 (2021).
le Douarin, N. M., Creuzet, S., Couly, G. & Dupin, E. Neural crest cell plasticity and its limits. Development 131, 4637–4650 (2004).
Noden, D. M. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev. Biol. 96, 144–165 (1983).
Trainor, P. A., Ariza-McNaughton, L. & Krumlauf, R. Role of the isthmus and FGFs in resolving the paradox of neural crest plasticity and prepatterning. Science 295, 1288–1291 (2002).
Hu, D., Marcucio, R. S. & Helms, J. A. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development 130, 1749–1758 (2003).
Clouthier, D. E. et al. Signaling pathways crucial for craniofacial development revealed by endothelin-A receptor-deficient mice. Dev. Biol. 217, 10–24 (2000).
Ozeki, H., Kurihara, Y., Tonami, K., Watatani, S. & Kurihara, H. Endothelin-1 regulates the dorsoventral branchial arch patterning in mice. Mech. Dev. 121, 387–395 (2004).
Sato, T. et al. An endothelin-1 switch specifies maxillomandibular identity. Proc. Natl Acad. Sci. USA 105, 18806–18811 (2008).
Schneider, R. A. & Helms, J. A. The cellular and molecular origins of beak morphology. Science 299, 565–568 (2003).
Bernstein, B. E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006).
Minoux, M. et al. Gene bivalency at Polycomb domains regulates cranial neural crest positional identity. Science 355, eaal2913 (2017). This study found that poised, bivalent, Polycomb-dependent chromatin domains maintain the positional plasticity and broad developmental potential of CNCCs during development.
Schwarz, D. et al. Ezh2 is required for neural crest-derived cartilage and bone formation. Development 141, 867–877 (2014).
Gibson, W. T. et al. Mutations in EZH2 cause Weaver syndrome. Am. J. Hum. Genet. 90, 110–118 (2012).
Naqvi, S. et al. Decoding the human face: progress and challenges in understanding the genetics of craniofacial morphology. Annu. Rev. Genomics Hum. Genet. 23, 383–412 (2022).
Liu, F. et al. A genome-wide association study identifies five loci influencing facial morphology in Europeans. PLoS Genet. 8, e1002932 (2012).
Paternoster, L. et al. Genome-wide association study of three-dimensional facial morphology identifies a variant in PAX3 associated with nasion position. Am. J. Hum. Genet. 90, 478–485 (2012).
Shaffer, J. R. et al. Genome-wide association study reveals multiple loci influencing normal human facial morphology. PLoS Genet. 12, e1006149 (2016).
Cole, J. B. et al. Genomewide association study of African children identifies association of SCHIP1 and PDE8A with facial size and shape. PLoS Genet. 12, e1006174 (2016).
Adhikari, K. et al. A genome-wide association scan implicates DCHS2, RUNX2, GLI3, PAX1 and EDAR in human facial variation. Nat. Commun. 7, 11616 (2016).
Claes, P. et al. Genome-wide mapping of global-to-local genetic effects on human facial shape. Nat. Genet. 50, 414–423 (2018). This work used unsupervised hierarchical spectral clustering and canonical correlation analysis to identify genetic variants associated with craniofacial shape.
Pickrell, J. K. et al. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet. 48, 709–717 (2016).
White, J. D. et al. Insights into the genetic architecture of the human face. Nat. Genet. 53, 45–53 (2021). This work suggests that the genomic regions that surround the signals associated with variation of facial traits are enriched for enhancer activity in CNCCs and craniofacial tissues.
Liu, C. et al. Genome scans of facial features in East Africans and cross-population comparisons reveal novel associations. PLoS Genet. 17, e1009695 (2021).
Xiong, Z. et al. Novel genetic loci affecting facial shape variation in humans. eLife 8, e49898 (2019).
Indencleef, K. et al. The intersection of the genetic architectures of orofacial clefts and normal facial variation. Front. Genet. 12, 626403 (2021). This study suggests a shared genetic architecture of normal facial development and orofacial clefting.
Rada-Iglesias, A. et al. A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470, 279–283 (2011).
Creyghton, M. P. et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl Acad. Sci. USA 107, 21931–21936 (2010).
Weinberg, S. M. What’s shape got to do with it? Examining the relationship between facial shape and orofacial clefting. Front. Genet. 13, 891502 (2022).
Naqvi, S. et al. Shared heritability of human face and brain shape. Nat. Genet. 53, 830–839 (2021). This work identifies 472 genomic loci that influence brain shape, of which 76 are also associated with face shape, and suggests that during early development the face and brain shape each other.
Plummer, J. T., Gordon, A. J. & Levitt, P. The genetic intersection of neurodevelopmental disorders and shared medical comorbidities - relations that translate from bench to bedside. Front. Psychiatry 7, 142 (2016).
Demyer, W., Zeman, W. & Palmer, C. G. The face predicts the brain: diagnostic significance of median facial anomalies for holoprosencephaly (arhinencephaly). Pediatrics 34, 256–263 (1964).
Pachano, T., Haro, E. & Rada-Iglesias, A. Enhancer-gene specificity in development and disease. Development 149, dev186536 (2022).
Milunsky, J. M. et al. TFAP2A mutations result in branchio-oculo-facial syndrome. Am. J. Hum. Genet. 82, 1171–1177 (2008).
Zhang, J. et al. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature 381, 238–241 (1996).
Laugsch, M. et al. Modeling the pathological long-range regulatory effects of human structural variation with patient-specific hiPSCs. Cell Stem Cell 24, 736–752 (2019).
Robin, P. A fall of the base of the tongue considered as a new cause of nasopharyngeal respiratory impairment: Pierre Robin sequence, a translation. 1923. Plast. Reconstr. Surg. 93, 1301–1303 (1994).
Amarillo, I. E., Dipple, K. M. & Quintero-Rivera, F. Familial microdeletion of 17q24.3 upstream of SOX9 is associated with isolated Pierre Robin sequence due to position effect. Am. J. Med. Genet. A 161A, 1167–1172 (2013).
Benko, S. et al. Highly conserved non-coding elements on either side of SOX9 associated with Pierre Robin sequence. Nat. Genet. 41, 359–364 (2009).
Gordon, C. T. et al. Long-range regulation at the SOX9 locus in development and disease. J. Med. Genet. 46, 649–656 (2009).
Gordon, C. T. et al. Identification of novel craniofacial regulatory domains located far upstream of SOX9 and disrupted in Pierre Robin sequence. Hum. Mutat. 35, 1011–1020 (2014).
Long, H. K. et al. Loss of extreme long-range enhancers in human neural crest drives a craniofacial disorder. Cell Stem Cell 27, 765–783 (2020).
Funato, N., Kokubo, H., Nakamura, M., Yanagisawa, H. & Saga, Y. Specification of jaw identity by the Hand2 transcription factor. Sci. Rep. 6, 28405 (2016).
Chen, Q., Dai, J. & Bian, Q. Integration of 3D genome topology and local chromatin features uncovers enhancers underlying craniofacial-specific cartilage defects. Sci. Adv. 8, eabo3648 (2022).
Lettice, L. A. et al. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum. Mol. Genet. 12, 1725–1735 (2003).
Herranz, D. et al. A NOTCH1-driven MYC enhancer promotes T cell development, transformation and acute lymphoblastic leukemia. Nat. Med. 20, 1130–1137 (2014).
Bahr, C. et al. A Myc enhancer cluster regulates normal and leukaemic haematopoietic stem cell hierarchies. Nature 553, 515–520 (2018).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Wang, H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/cas-mediated genome engineering. Cell 153, 910–918 (2013).
Clark, J. F., Dinsmore, C. J. & Soriano, P. A most formidable arsenal: genetic technologies for building a better mouse. Genes. Dev. 34, 1256–1286 (2020).
Stern, C. D. Reflections on the past, present and future of developmental biology. Dev. Biol. 488, 30–34 (2022).
Prescott, S. L. et al. Enhancer divergence and cis-regulatory evolution in the human and chimp neural crest. Cell 163, 68–83 (2015). This study carried out epigenomic profiling of human and chimpanzee CNCCs and identified divergence of craniofacial cis-regulatory landscapes, potentially underlying morphological face variation in higher primates.
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Dong, X. et al. Efficient engineering of human auricular cartilage through mesenchymal stem cell chaperoning. J. Tissue Eng. Regen. Med. 16, 825–835 (2022).
Russell, J. J. et al. Non-model model organisms. BMC Biol. 15, 55 (2017).
Depew, M. J. & Bertocchini, F. Avenues for investigating the neural crest and its derivatives in non-model (unconventional) vertebrates: a craniofacial skeleton perspective. Methods Mol. Biol. 1976, 207–221 (2019).
Hockman, D. et al. A genome-wide assessment of the ancestral neural crest gene regulatory network. Nat. Commun. 10, 4689 (2019).
Horie, R. et al. Shared evolutionary origin of vertebrate neural crest and cranial placodes. Nature 560, 228–232 (2018).
Abzhanov, A., Protas, M., Grant, B. R., Grant, P. R. & Tabin, C. J. Bmp4 and morphological variation of beaks in Darwin’s finches. Science 305, 1462–1465 (2004).
Brink, K. S. et al. Tooth removal in the leopard gecko and the de novo formation of replacement teeth. Front. Physiol. 12, 576816 (2021).
Compagnucci, C. et al. Pattern and polarity in the development and evolution of the gnathostome jaw: both conservation and heterotopy in the branchial arches of the shark, Scyliorhinus canicula. Dev. Biol. 377, 428–448 (2013).
Kyllar, M. et al. Radiography, computed tomography and magnetic resonance imaging of craniofacial structures in pig. Anat. Histol. Embryol. 43, 435–452 (2014).
Fons, J. M. et al. Getting out of an egg: merging of tooth germs to create an egg tooth in the snake. Dev. Dyn. 249, 199–208 (2020).
Martik, M. L. et al. Evolution of the new head by gradual acquisition of neural crest regulatory circuits. Nature 574, 675–678 (2019).
Rodríguez-Seguí, S., Akerman, I. & Ferrer, J. GATA believe it: new essential regulators of pancreas development. J. Clin. Invest. 122, 3469–3471 (2012).
Pollen, A. A. et al. Establishing cerebral organoids as models of human-specific brain evolution. Cell 176, 743–756 (2019).
Carroll, S. B. Evo-Devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 134, 25–36 (2008).
Gokhman, D. et al. Human–chimpanzee fused cells reveal cis-regulatory divergence underlying skeletal evolution. Nat. Genet. 53, 467–476 (2021).
Thomas, D. C., Moorthy, J. D., Prabhakar, V., Ajayakumar, A. & Pitchumani, P. K. Role of primary cilia and Hedgehog signaling in craniofacial features of Ellis–van Creveld syndrome. Am. J. Med. Genet. C. Semin. Med. Genet. 190, 36–46 (2022).
Pilot, M. et al. Diversifying selection between pure-breed and free-breeding dogs inferred from genome-wide SNP analysis. G3 6, 2285–2298 (2016).
Hu, Y. & Albertson, R. C. Hedgehog signaling mediates adaptive variation in a dynamic functional system in the cichlid feeding apparatus. Proc. Natl Acad. Sci. USA 111, 8530–8534 (2014).
Burga, A. et al. A genetic signature of the evolution of loss of flight in the Galapagos cormorant. Science 356, eaal3345 (2017).
Young, N. M., Chong, H. J., Hu, D., Hallgrímsson, B. & Marcucio, R. S. Quantitative analyses link modulation of sonic hedgehog signaling to continuous variation in facial growth and shape. Development 137, 3405–3409 (2010).
Wilkins, A. S., Wrangham, R. W. & Tecumseh Fitch, W. The ‘domestication syndrome’ in mammals: a unified explanation based on neural crest cell behavior and genetics. Genetics 197, 795–808 (2014).
Wilkins, A. S., Wrangham, R. & Fitch, W. T. The neural crest/domestication syndrome hypothesis, explained: reply to Johnsson, Henriksen, and Wright. Genetics 219, iyab098 (2021).
Wilson, L. A. B., Balcarcel, A., Geiger, M., Heck, L. & Sánchez-Villagra, M. R. Modularity patterns in mammalian domestication: assessing developmental hypotheses for diversification. Evol. Lett. 5, 385–396 (2021).
Johnsson, M., Henriksen, R. & Wright, D. The neural crest cell hypothesis: no unified explanation for domestication. Genetics 219, iyab097 (2021).
Anastasiadi, D., Piferrer, F. & Wittkopp, P. Epimutations in developmental genes underlie the onset of domestication in farmed European sea bass. Mol. Biol. Evol. 36, 2252–2264 (2019).
Zanella, M. et al. Dosage analysis of the 7q11.23 Williams region identifies BAZ1B as a major human gene patterning the modern human face and underlying self-domestication. Sci. Adv. 5, eaaw7908 (2019).
Theofanopoulou, C. et al. Self-domestication in Homo sapiens: insights from comparative genomics. PLoS ONE 12, e0185306 (2017).
Acknowledgements
The authors thank R. Aho for the original preparation of all artwork, N. Tian for generating the library of references, P. Martin for editing the manuscript, G. Panagiotakos for discussions and input, and E. Nora, D. Wagner and S. Weinberg for comments on select sections of the Review. The authors received funding from the NIH (R01 DE024745, R01 DE028324, R01 DE028753, U01 DE024430 FaceBase2) and the March of Dimes and Birth Defects Foundation to L.S.; and from the Swiss National Science Foundation (CRSII5_173868), the European Research Council under the European Union’s Horizon 2020 research and innovation programme (810111-EpiCrest2Reg) and the Novartis Research Foundation to F.M.R.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Genetics thanks P. A. Trainor and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Branchial arches
-
Also known as pharyngeal arches. Segmented structures arising as a series of endodermal outpockets on the sides of the developing pharynx that are filled with ectomesenchymal cells derived from cranial neural crest and mesodermal cells. They give rise to multiple facial and visceral structures, including skeletal, muscular and neural elements.
- Branchio-oto-renal syndrome
-
This syndrome is characterized by neck and external ear abnormalities, including hearing loss, and kidney defects. Symptom severity varies greatly from person to person.
- Collinear
-
Refers to the physical gene order within each Hox cluster on the chromosome (telomeric to centromeric), which correlates with the serial activation of these genes along the anterior–posterior embryonic body axis.
- Epithelial-to-mesenchymal transition
-
(EMT). Process by which epithelial cells lose their cell polarity and cell–cell adhesion, and gain migratory and invasive properties to become mesenchymal cells.
- Frontonasal prominence
-
(FNP). Midline, unpaired embryonic structure that develops between the telencephalon, the forming oral cavity and the nasal pits, into the forehead.
- Homeodomain
-
The DNA-binding homeobox domain (homeodomain) is encoded by a 180-bp homeobox DNA sequence, found within genes encoding transcription factors that are involved in pattern formation during development in animals, fungi, plants and numerous single-cell eukaryotes.
- Homeotic transformation
-
Morphological variation in body plan in which one structure is changed into the likeness of another structure, arising from loss-of-function or gain-of-function mutations of the developmentally crucial homeotic genes.
- Hox genes
-
Also known as homeotic genes. A subset of homeodomain genes that specify the morphology of the distinct structures of the body plan of an embryo along the anterior–posterior (head-to-tail) body axis. Mammals have 39 Hox genes, organized into four clusters of 9–11 paralogous genes (some clusters lack select paralogues), resulting from successive evolutionary duplications.
- Hyoid bone
-
Horseshoe-shaped bone situated in the anterior midline of the neck between the base of the lower jaw and the thyroid cartilage that provides an attachment structure for the tongue. The greater horns of the hyoid bone arise from branchial arch 3, whereas the lesser horns originate from branchial arch 2.
- Induced pluripotent stem cells
-
(iPSCs). Pluripotent stem cells that can be generated directly from a somatic cell by the introduction of specific transcription factor genes (MYC, OCT3, OCT4, SOX2 and KLF4).
- Lateral nasal prominence
-
(LNP). Ectoderm-covered swelling filled with mesenchymal cells of cranial neural crest origin that separates the embryonic olfactory pit from the developing eye. The wings of the nose (alae nasi) develop from the LNP.
- Mandibular prominences
-
(MdPs). Caudal prominences formed by bifurcation of embryonic branchial arch 1. Each MdP fuses antero-ventrally with the MdP on the other side of the embryonic face to form the lower jaw.
- Maxillary prominence
-
(MxP). Rostral prominence formed by bifurcation of embryonic branchial arch 1, which joins with the ipsilateral medial nasal prominence to form the upper jaw.
- Meckel’s cartilage
-
Bilaterally paired, rod-like, cartilaginous ventral component of the lower jaw, within the branchial arch 1-derived mandibular prominences of vertebrate embryos.
- Medial nasal prominence
-
(MNP). Ectoderm-covered swelling filled with mesenchymal cells of cranial neural crest origin that lies medial to the olfactory pit in the embryo. The nasal tip and philtrum (midline groove) of the lip (in humans) develop from the MNP.
- Middle ear ossicles
-
The incus, malleus and stapes, which transfer vibrations from the eardrum to the inner ear. The incus and malleus are derived from branchial arch 1, whereas the stapes is derived from branchial arch 2.
- Multi-omics
-
Branch of biological science comprising various experimental approaches, such as genomics, transcriptomics, proteomics, metabolomics and phenomics. The goal of multi-omics is the combined characterization and quantification of large data sets that translate into the structure and function of an organism.
- Neurocristopathies
-
A class of human disorders that result from abnormal expression, migration, differentiation or death of neural crest cells during embryonic development.
- Paralogous
-
Genes related to each other through a gene duplication event. A paralogous gene in the same organism gains novel regulation and function, but also often keeps redundant functions with its paralogues. An example of paralogous genes is provided by genes in similar linear positions in the distinct Hox clusters.
- Rhombomere 4
-
The rhombomeres (up to eight in total) are transient compartments of neuroepithelial precursor cells formed in the developing hindbrain of vertebrate embryos. They appear as a series of swellings with meristic organization in the early developing neural tube.
- Stomodeum
-
The primitive oral cavity, which forms between the frontonasal process and branchial arch 1.
- Topologically associating domain
-
(TAD). Self-interacting genomic region of approximately 1 Mb. DNA sequences within a TAD are likely to interact physically with each other more frequently than with sequences outside the TAD.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Selleri, L., Rijli, F.M. Shaping faces: genetic and epigenetic control of craniofacial morphogenesis. Nat Rev Genet 24, 610–626 (2023). https://doi.org/10.1038/s41576-023-00594-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41576-023-00594-w