Abstract
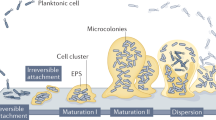
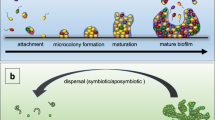
The biofilm matrix can be considered to be a shared space for the encased microbial cells, comprising a wide variety of extracellular polymeric substances (EPS), such as polysaccharides, proteins, amyloids, lipids and extracellular DNA (eDNA), as well as membrane vesicles and humic-like microbially derived refractory substances. EPS are dynamic in space and time and their components interact in complex ways, fulfilling various functions: to stabilize the matrix, acquire nutrients, retain and protect eDNA or exoenzymes, or offer sorption sites for ions and hydrophobic substances. The retention of exoenzymes effectively renders the biofilm matrix an external digestion system influencing the global turnover of biopolymers, considering the ubiquitous relevance of biofilms. Physico-chemical and biological interactions and environmental conditions enable biofilm systems to morph into films, microcolonies and macrocolonies, films, ridges, ripples, columns, pellicles, bubbles, mushrooms and suspended aggregates — in response to the very diverse conditions confronting a particular biofilm community. Assembly and dynamics of the matrix are mostly coordinated by secondary messengers, signalling molecules or small RNAs, in both medically relevant and environmental biofilms. Fully deciphering how bacteria provide structure to the matrix, and thus facilitate and benefit from extracellular reactions, remains the challenge for future biofilm research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wingender, J., Neu, T. R. & Flemming, H.-C. in Microbial Extracellular Polymeric Substances (eds. Wingender, J., Neu, T. R. & Flemming, H.-C.) 1–19 (Springer, 1999).
Steinberg, N. & Kolodkin-Gal, I. The matrix reloaded: how sensing the extracellular matrix synchronizes bacterial communities. J. Bacteriol. 197, 2092–2103 (2015).
Frantz, C., Stewart, K. M. & Weaver, V. M. The extracellular matrix at a glance. J. Cell Sci. 123, 4195–4200 (2010).
Karygianni, I., Ren, Z. & Thurnheer, T. Biofilm matrixome: extracellular components in structured microbial communities. Trends Microbiol. 28, 668–681 (2020).
Serra D. O. & Hengge, R. in Extracellular Sugar-Based Biopolymers Matrices. Biologically-Inspired Systems Vol. 12 (eds Cohen, E. & Merzendorfer, H.) 355–392 (Springer, 2019).
Dueholm, M. S. & Nielsen, P. H. in The Perfect Slime. Microbial Extracellular Polymeric Substances (EPS) (eds Flemming, H.-C., Neu, T. R. & Wingender, J.) 113–133 (IWA, 2017).
Erskine, E. et al. Formation of functional, non-amyloidogenic fibres by recombinant Bacillus subtilis TasA. Mol. Microbiol. 110, 897–913 (2018).
Neu, T. R. & Lawrence, J. R. in The Perfect Slime. Microbial Extracellular Polymeric Substances (EPS) (eds Flemming, H.-C., Neu, T. R. & Wingender, J.) 25–60 (IWA, 2017.
Toyofuku, M., Nomura, N. & Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 17, 13–24 (2019). This work presents a comprehensive and systematic overview of OMVs, outer–inner membrane vesicles and cytoplasmatic membrane vesicles and their formation and functions.
Choi, S. Y. et al. Chromobacterium violaceum delivers violacein, a hydrophobic antibiotic, to other microbes in membrane vesicles. Environ. Microbiol. 22, 705–713 (2020).
Wurl, O. & Cunliffe, M. in The Perfect Slime. Microbial Extracellular Polymeric Substances (EPS) (eds Flemming, H.-C., Neu, T. R. & Wingender, J.) 249–268 (IWA, 2017).
Flemming, H.-C. et al. Biofilms: an emergent form of microbial life. Nat. Rev. Microbiol. 14, 563–575 (2016).
Jennings, L. K. et al. Pseudomonas aeruginosa aggregates in cystic fibrosis sputum produce exopolysaccharides that likely impede current therapies. Cell Rep. 34, 108782 (2021).
Costerton, J. W. et al. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41, 435–464 (1987).
Hengge, R. Linking bacterial growth, survival and multicellularity — small signaling molecules as triggers and drivers. Curr. Opin. Microbiol. 55, 57–66 (2020). This paper shows clearly how the transition to multicellularity is achieved by a regulatory signalling network, promoting either growth or stress resistance, and how the matrix represents a self-constructed homeostatic ‘niche’.
Penesyan, A., Paulsen, I. T., Kjelleberg, S. & Gillings, M. R. Three faces of biofilms: a microbial lifestyle, a nascent multicellular organism, and an incubator for diversity. NPJ Biofilms Microbiomes 7, 80 (2021).
Dragoš, A. et al. Division of labour during biofilm matrix production. Curr. Biol. 28, 1903–1913 (2018).
Decho, A. W. & Guiterrez, T. Microbial extracellular substances (EPSs) in ocean systems. Front. Microbiol. 8, 922 (2017).
Oppenheimer-Shaanan, Y. et al. Spatio-temporal assembly of functional mineral scaffolds within microbial biofilms. NPJ Biofilms Microbiomes 2, 15031 (2016).
Staudt, C., Horn, H., Hempel, D. C. & Neu, T. R. in Biofilms in Medicine, Industry and Environmental Technology (eds Lens, P. et al.) 308–327 (IWA, 2003).
Bennke, C. M., Neu, T. R., Fuchs, B. M. & Amann, R. Mapping glycoconjugate-mediated interactions of marine Bacteroidetes with diatoms. Syst. Appl. Microbiol. 36, 417–425 (2013).
Maqbool, T., Cho, J., Shin, K. H. & Hur, J. Using stable isotope labeling approach and two dimensional correlation spectroscopy to explore the turnover cycles of different carbon structures in extracellular polymeric substances. Water Res. 170, 115355 (2020).
Choong, F. X. et al. Real-time optotracing of curli and cellulose in live Salmonella biofilms using luminescent oligothiophenes. NPJ Biofilms Microbiomes 2, 16024 (2016).
Seviour, T. W. et al. Extracellular polymeric substances of biofilms: suffering from an identity crisis. Water Res. 151, 1–7 (2019). This work presents a wide spectrum of EPS components, and an inspiring multidisciplinary road map for addressing the nature, function and control of these components is proposed.
Flemming, H.-C. & Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633 (2010).
Bruckner, C. G., Rehm, C., Grossart, H. P. & Kroth, P. G. Growth and release of extracellular organic compounds by benthic diatoms depend on interactions with bacteria. Environ. Microbiol. 13, 1052–1063 (2011).
Pierce, C. et al. The Candida albicans biofilm matrix: composition, structure and function. J. Fungi 3, 14 (2017).
Tomer, A. et al. in Mycoremediation and Environmental Sustainability. Fungal Biology (eds Prasad, R., Nayak, S. C., Kharwar, R. N. & Dubey, N. K.) 187–200 (Springer, 2021).
Turnheer, T., Gmür, R., Shapiro, S. & Guggenheim, B. Mass transport of macromolecules within an in vitro model of supragingival plaque. Appl. Environ. Microbiol. 60, 1702–1709 (2003).
Boles, B. R. & Horswill, A. R. Swimming cells promote a dynamic environment within biofilms. Proc. Natl Acad. Sci. USA 109, 32 (2012).
Piard, J. C. et al. in The Perfect Slime. Microbial Extracellular Polymeric Substances (EPS) (eds Flemming, H.-C., Neu, T. R. & Wingender, J.) 179–191 (IWA, 2017).
Costerton, J. W., Geesey, G. G. & Cheng, K.-J. How bacteria stick. Sci. Am. 238, 86–95 (1978).
Turnbull, J. E. & Field, R. A. Emerging glycomics technologies. Nat. Chem. Biol. 3, 74–77 (2007).
Sutherland, I. W. in The Perfect Slime. Microbial Extracellular Polymeric Substances (EPS) (eds Flemming, H.-C., Neu, T. R. & Wingender, J.) 15–24 (IWA, 2017).
Limoli, D. H. et al. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.MB-0011-2014 (2015). This work presents a good overview of the aggregative, structural and protective properties of polysaccharides in the matrix which provide the successful adaptation of bacteria to nearly every niche.
Pestrak, M. J., Eggleston, H. C. & Wozniak, D. J. in The Perfect Slime. Microbial Extracellular Polymeric Substances (EPS) (eds Flemming, H.-C., Neu, T. R. & Wingender, J.) 79–112 (IWA, 2017). This work is a good overview of P. aeruginosa polysaccharides, and their composition, structure, biosynthesis, functions and regulation.
Tseng, B. S. et al. A biofilm matrix-associated protease inhibitor protects Pseudomonas aeruginosa from proteolytic attack. mBio 9, e00543-18 (2018).
Whitfield, C., Wear, S. S. & Sande, C. Assembly of bacterial capsular polysaccharides and exopolysaccharides. Annu. Rev. Microbiol. 74, 521–543 (2020).
Abdian, P. L. & Zorreguieta, A. in The Perfect Slime. Microbial Extracellular Polymeric Substances (EPS) (eds Flemming, H.-C., Neu, T. R., Wingender, J.) 227–247 (IWA, 2017).
Caudan, C., Filali, A., Spérandio, M. & Girbal-Neuhauser, E. Multiple EPS interactions involved in the cohesion and structure of aerobic granules. Chemosphere 117, 262–270 (2014).
Felz, S., Neu, T. R., van Loosdrecht, M. C. M. & Lin, Y. Aerobic granular sludge contains hyaluronic acid-like and sulfated glycosaminoglycan-like polymers. Water Res. 169, 115291 (2020).
de Graaff, D. R. et al. Sialic acids in the extracellular polymeric substances of seawater-adapted aerobic granular sludge. Wat. Res. 155, 343–351 (2019).
Bonnans, C., Chou, J. & Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 (2014).
Brown, A. J. XLIII.— On an acetic ferment which forms cellulose. J. Chem. Soc. Trans. 49, 432–439 (1886).
Römling, U. & Galperin, M. Y. Bacterial cellulose biosynthesis: diversity of operons, subunits, products, and functions. Trends Microbiol. 23, 545–557 (2015).
Yamananka, S. et al. The structure and mechanical properties of sheets prepared from bacterial cellulose. J. Mat. Sci. 24, 3141–3145 (1989).
Ziemba, C., Shabtai, Y., Piatkovsky, M. & Herzberg, M. Cellulose effects on morphology and elasticity of Vibrio fischeri biofilms. NPJ Biofilms Microbiomes 2, 1 (2016).
Thongsomboon, W. et al. Phosphoethanolamine cellulose: a naturally produced chemically modified cellulose. Science 359, 6373 (2018).
Grossart, H.-P., Tang, K. M., Kiørboe, T. & Ploug, H. Comparison of cell-specific activity between free-living and attached bacteria using isolates and natural assemblages. FEMS Microbiol. Lett. 266, 194–200 (2007).
Wingender, J. & Jaeger, K. E. in Encyclopedia of Environmental Microbiology (ed. Bitton, G.). 1207–1223 (Wiley, 2002).
Zhang, P. et al. Identification and function of extracellular protein in wastewater treatment using proteomic approaches: a minireview. J. Environ. Manag. 233, 24–29 (2019).
Tielen, P. et al. Interaction between extracellular lipase LipA and the polysaccharide alginate of Pseudomonas aeruginosa. BMC Microbiol. 13, 159 (2013).
Li, Q. & Sand, W. Mechanical and chemical studies on EPS from Sulfobacillus thermosulfidooxidans: from planktonic to biofilm cells. Coll. Surf. B Biointerfaces 153, 34–40 (2017).
Lindsay, S., Oates, A. & Bourdillon, K. The detrimental impact of extracellular proteases on wound healing. Int. Wound J. 14, 1237–1247 (2017).
McDougald, D., Rice, S. A., Barraud, N., Steinberg, P. D. & Kjelleberg, S. A. Should we stay or should we go: mechanics and ecological consequences of biofilm dispersal. Nat. Rev. Microbiol. 10, 39–50 (2012). This classic work on the reasons for and mechanisms of biofilm dispersal shows that the disassembly of biofilms is as equally regulated as their formation.
Rumbaugh, K. P. & Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 18, 571–581 (2020). This work highlights the role of differentiated dispersal cells and develops a broad conceptual framework for the diversity of mechanisms leading to biofilm disassembly, portraying dispersal as an active event in which biofilm cells convert to the planktonic mode of growth.
Flemming, H.-C. & Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 17, 247–260 (2019).
Falkowski, P. G., Fenchel, T. & Delong, E. F. The microbial engines that drive Earth’s biogeochemical cycles. Science 320, 1034 (2008).
Battin, T. J., Besemer, K., Bengtsson, M. M., Romani, A. M. & Packmann, A. I. The ecology and biogeochemistry of stream biofilms. Nat. Rev. Microbiol. 14, 251–263 (2016). This work demonstrates the extent to which biofilms dominate microbial life in streams and rivers, drive crucial ecosystem processes and contribute to global biogeochemical fluxes.
Álvarez-Mena, A., Camara-Almirón, J., de Vicente, A. & Romero, D. Multifunctional amyloids in the biology of Gram-positive bacteria. Microorganisms 8, 2020 (2020).
Schubeis, T. et al. Untangling a repetitive amyloid sequence: correlating biofilm-derived and segmentally labeled curli fimbriae by solid-state NMR spectroscopy. Angew. Chem. Int. Ed. 54, 14669–14672 (2015).
Deshmukh, M., Evans, M. L. & Chapman, M. R. Amyloid by design: intrinsic regulation of microbial amyloid assembly. J. Mol. Biol. 12, 3631–3641 (2018).
Cámara-Almirón, J., Caro-Astorga, J., de Vincente, A. & Romero, D. Beyond the expected: the structural and functional diversity of bacterial amyloids. Crit. Rev. Microbiol. 44, 653–666 (2018).
Van Gerven, N., van der Verren, S. E., Reiter, D. M. & Remaut, H. The role of functional amyloids in bacterial virulence. J. Mol. Biol. 430, 3657–3684 (2018).
Levkovich, S. A., Gazit, E. & Bar-Yosev, D. L. Two decades of studying functional amyloids in microorganisms. Trends Microbiol. 29, 251–265 (2020).
Jain, N. & Chapman, M. R. Bacterial functional amyloids: order from disorder. BBA Prot. Proteom. 1867, 954–960 (2019).
Otto, S. et al. Privatization of biofilm matrix in structurally heterogeneous biofilms. mSystems 5, e00425-20 (2020).
Steinberg, N. et al. The extracellular matrix protein TasA is a developmental cue that maintains a motile subpopulation within Bacillus subtilis biofilms. Sci. Signal. 13, eaaw8905 (2020).
Taglialegna, A. et al. Staphylococcal bap proteins build amyloid scaffold biofilm matrices in response to environmental signals. PLoS Pathog. 12, e1005711 (2016).
Salinas, N., Povolotsky, T. L., Landau, M. & Kolodkin-Gal, H. Emerging roles of functional bacterial amyloids in gene regulation, toxicity, and immunomodulation. Microbiol. Mol. Biol. Rev. 85, e00062-20 (2021).
Christensen, L. F. B. et al. The sheats of Methanospirillum are made of a new type of amyloid protein. Front. Microbiol. 9, 2729 (2018).
Morris, R. J. et al. Natural variations in the biofilm-associated protein BslA from the genus. Bacillus. Sci. Rep. 7, 6730 (2017).
Arnouteli, S., Bamford, N. C., Stanley-Wall, N. R. & Kovács, Á. T. Bacillus subtilis biofilm formation and social interactions. Nat. Rev. Microbiol. 19, 600–614 (2021).
Kobayashi, K. & Iwano, M. BslA (YuaB) forms a hydrophobic layer on the surface of Bacillus subtilis biofilms. Mol. Microbiol. 85, 51–66 (2012).
Hobley, L. A. et al. BslA is a self-assembling bacterial hydrophobin that coats the Bacillus subtilis biofilm. Proc. Natl Acad. Sci. USA 110, 33 (2013).
Werb, M. et al. Surface topology affects wetting behavior of Bacillus subtilis biofilms. NPJ Biofilms Microbiomes 3, 11 (2017).
Yu, H. Q. Molecular insights into extracellular polymeric substances in activated sludge. Environ. Sci. Microbiol. 54, 7742–7750 (2020).
Spaeth, R., Flemming, H.-C. & Wuertz, S. Sorption properties of biofilms. Water Sci. Technol. 37, 207–210 (1998).
Schwartz, K., Ganesan, M., Payne, D. E., Solomon, M. J. & Boles, B. R. Extracellular DNA facilitates the formation of functional amyloids in Staphylococcus aureus biofilms. Mol. Microbiol. 99, 123–134 (2016).
Okshevsky, M. & Meyer, R. L. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit. Rev. Microbiol. 41, 341–352 (2015).
Kästner, M. & Miltner, A. in The Future of Soil Carbon (eds Garcia, C., Nannipieri, P. & Hernandez, T.) 125–163 (Elsevier, 2018).
Chrzanowski, L., Lawniczak, L. & Czaczyk, K. Why do microorganisms produce rhamnolipids? World J. Microbiol. Biotechnol. 28, 401–419 (2012).
Aldeek, F. et al. Patterned hydrophobic domains in the exopolymer matrix of Shewanella oneidensis MR-1 biofilms. Appl. Environ. Microbiol. 79, 1400–1402 (2013).
Campoccia, D., Montanaro, L. & Arciola, C. R. Extracellular DNA (eDNA). A major ubiquitous element of the bacterial biofilm architecture. Int. J. Mol. Sci. 22, 9100 (2021). This work presents a comprehensive overview of the fundamental structural role of eDNA and the contribution it offers to the complex architecture of the biofilm matrix by interaction with various other EPS components.
de Aldecoa, A. L. I., Zafra, O. & González-Pastor, J. E. Mechanism and regulation of extracellular DNA release and its biological role in microbial communities. Front. Microbiol. 8, 1390 (2017).
Panlilio, H. & Rice, C. V. The role of extracellular DNA in the formation, architecture, stability, and treatment of bacterial biofilms. Biotechnol. Bioeng. 118, 2129–2141 (2021). This work addresses, in particular, the role of eDNA for structure and stability of the EPS matrix and develops approaches to disrupt infectious biofilms.
McDonough, E. K., Kamp, H. & Camili, A. Vibrio cholerae phosphatases required for the utilization of nucleotides and extracellular DNA as phosphate sources. Mol. Microbiol. 99, 453–469 (2016).
Seviour, T. W. et al. The biofilm matrix scaffold of Pseudomonas aeruginosa contains G-quadruplex extracellular DNA structures. NPJ Biofilms Microbiomes 7, 27 (2021).
Sørensen, S. J., Bailey, M., Hansen, L. H., Kroer, N. & Wuertz, S. Studying plasmid horizontal gene transfer in situ: a critical review. Nat. Rev. Microbiol. 3, 701–710 (2005).
Devaraj, A. et al. The extracellular DNA lattice of bacterial biofilms is structurally related to Holliday junction recombination intermediates. Proc. Natl Acad. Sci. USA 116, 50 (2019). This work is the first paper to demonstrate that specific proteins normally associated with intracellular functions such as transcription and translation interact with eDNA outside the cell to provide biofilm structural stability.
Buzzo, J. R. et al. Z-form extracellular DNA is a structural component of the bacterial biofilm matrix. Cell 184, 1–19 (2021).
Randrianjatovo-Gbalou, I., Rouquette, P., Levebvre, D., Girbal-Neuhauser, E. & Marcato-Romain, C.-E. In situ analysis of Bacillus licheniformis biofilms: amyloid-like polymers and eDNA are involved in the adherence and aggregation of the extracellular matrix. Appl. Microbiol. 122, 1262–1274 (2017).
Saxena, P., Joshi, Y., Rawat, K. & Bisht, R. Biofilms: architecture, resistance, quorum sensing and control mechanisms. Indian J. Microbiol. 59, 3–12 (2019).
Dawson, L. F. et al. Extracellular DNA, cell surface protein and c-di-GMP promote biofilm formation in Clostidioides difficile. Sci. Rep. 11, 3244 (2021).
Kavanaugh, J. S. et al. Identification of extracellular DNA-binding proteins in the biofilm matrix. mBio 10, e01137-19 (2019). This work discovers a series of lipoproteins with DNA-binding activity and develops an electrostatic net model in which membrane-attached lipoproteins function as anchor points between eDNA in the matrix and the bacterial cell surface.
Jacubovics, N. S., Goodman, S. D., Mashburn-Warren, L., Stafford, G. P. & Cieplik, F. The dental plaque biofilm matrix. Periodontol 2000 86, 32–56 (2021).
Chiba, A. et al. Staphylococcus aureus utilizes environmental RNA as a building material in specific polysaccharide-dependent biofilms. NPJ Biofilms Microbiomes 8, 17 (2022).
Deatherage, B. L. & Cookson, B. T. Membrane vesicle release in bacteria, eukaryotes and archaea: a conserved yet underappreciated aspect of microbial life. Infect. Immun. 80, 1948–1957 (2012).
Kikuchi, Y. et al. Diversity of physical properties of bacterial extracellular membrane vesicles revealed through atomic force microscopy imaging. Nanoscale 12, 7950–7959 (2020).
Nagakubo, T., Nomura, N. & Toyofuku, M. Cracking open bacterial membrane vesicles. Front. Microbiol. 10, 3026 (2020).
Schooling, S. R. & Beveridge, T. J. Membrane vesicles: an overlooked component in the matrices of biofilms. J. Bacteriol. 188, 5945–5957 (2006).
Elhenawy, W., Debelyy, M. O. & Feldman, M. F. Preferential packing of acidic glycosidases and proteases into Bacteroides outer membrane vesicles. mBio 5, e00909-14 (2014).
Brown, L., Wolf, J. M., Prados-Rosales, R. & Casadevall, A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 13, 620–630 (2015).
Liu, Y., Defourny, K. A. Y., Smid, E. J. & Abee, L. T. Gram-positive bacterial extracellular vesicles and their impact on health and disease. Front. Microbiol. 9, 1502 (2018).
Toyofuku, M. Bacterial communication through membrane vesicles. Biosci. Biotechnol. Biochem. 83, 1599–1605 (2019).
Morinaga, K., Yoshida, K., Takahashi, K., Nomura, N. & Toyofuku, M. Pecularities of biofilm formation by Paracoccus denitrificans. Appl. Microbiol. Biotechnol. 104, 2427–2433 (2020).
Schwechheimer, C. & Kuehn, M. J. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 13, 605–619 (2015).
Baeza, N. & Mercade, E. Relationship between membrane vesicles, extracellular ATP and biofilm formation in Antarctic Gram-negative bacteria. Microb. Ecol. 81, 645–656 (2020).
Park, M., Sun, Q., Liu, F., DeLisa, M. P. & Chen, W. Positional assembly of enzymes on bacterial outer membrane vesicles for cascade reactions. PLoS ONE 9, e97103 (2014).
He, X. et al. Membrane vesicles are the dominant structural components of ceftazidime-induced biofilm formation in an oxacillin-sensitive MRSA. Front. Microbiol. 10, 571 (2019).
Seike, S. et al. Outer membrane vesicles released from Aeromonas strains are involved in biofilm formation. Front. Microbiol. 11, 613650 (2021).
Toyofuku, M. et al. Prophage-triggered membrane vesicle formation through peptoglycan damage in Bacillus subtilis. Nat. Comm. 8, 481 (2017).
Piccolo, A. et al. in The Future of Soil Carbon (eds Garcia, C., Nainpieri, P. & Hernandez, T.) 87–124 (Elsevier, 2018).
Jiao, N. et al. Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean. Nat. Rev. Microbiol. 8, 593–599 (2010).
Zheng, T., Miltner, A., Liang, C., Nowak, K. M. & Kästner, M. Turnover of Gram-negative bacterial biomass-derived carbon through the microbial food web of an agricultural soil. Soil Biol. Biochem. 152, 108070 (2021).
Liang, C., Amelung, W., Lehmann, J. & Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Change Biol. 25, 3578–3590 (2019).
Yang, J., Toyofuku, M., Sakai, R. & Nomura, N. Influence of the alginate production on cell-to-cell communication in Pseudomonas aeruginosa PAO1. Environ. Microbiol. Rep. 9, 239–249 (2017).
Spitzer, J. From water and ions to crowded biomacromolecules: in vivo structuring of a prokaryotic cell. Microbiol. Mol. Biol. Rev. 75, 491–506 (2011). This work develops a theoretical model of molecular crowding in confined spaces as the base for enhanced multiple interactions among biomacromolecules, enabling multiple biochemical and physiological functions.
Mittal, S., Chowhan, R. K. & Singh, L. R. Macromolecular crowding: friend or foe. Biochem. Biophys. Acta 1850, 1822–1831 (2015).
Berk, V. et al. Molecular architecture and assembly principles of Vibrio cholerae biofilms. Science 337, 236–239 (2012).
Fong, J. C. N. et al. Structural dynamics of RbmA governs plasticity of Vibrio cholerae biofilms. eLife 6, e26163 (2017).
Peng, N. et al. The exopolysaccharide–eDNA interaction modulates 3D architecture of Bacillus subtilis biofilm. BMC Microbiol. 20, 111 (2020).
Kanampalliwar, A. & Singh, D. V. Extracellular DNA builds and interacts with Vibrio polysaccharide in the biofilm matrix formed by Vibrio cholerae. Environ. Microbiol. Rep. 12, 594–606 (2020).
Jennings, L. K. et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm. Proc. Natl Acad. Sci. USA 112, 11353–11358 (2015).
Irie, Y. et al. Self-produced exopolysaccharide is a signal that stimulates biofilm formation in Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA 109, 20632–20636 (2012).
Yu, H. Y. et al. Elastase LasB of Pseudomonas aeruginosa promotes biofilm formation partly through rhamnolipid-mediated regulation. Can. J. Microbiol. 60, 227–235 (2014).
Seviour, T. W. et al. Functional amyloids keep quorum sensing molecules in check. J. Biol. Chem. 290, 6457–6469 (2015). This paper shows how functional amyloids contain hydrophobic domains which bind signalling molecules with transient affinity, providing a pool of hydrophobic quorum sensing molecules.
Grande, R. et al. Detection and physicochemical characterization of membrane vesicles (MVs) of Lactobacillus reuteri DSM 17938. Front. Microbiol. 8, 1040 (2017).
Das, D. et al. Phenazine virulence factor binding to extracellular DNA is important for Pseudomonas aeruginosa biofilm formation. Sci. Rep. 5, 8398 (2015).
Schiessl, K. et al. Phenazine production promotes antibiotic tolerance and metabolic heterogeneity in Pseudomonas aeruginosa biofilms. Nat. Commun. 10, 762 (2019).
Saunders, S. H. et al. Extracellular DNA promotes efficient electron transfer by pyocyanin in Pseudomonas aeruginosa biofilms. Cell 182, 191–932 (2020).
Zhang, Z. et al. Organic loading rate (OLR) regulation for enhancement of aerobic sludge granulation: role of key microorganism and their function. Sci. Tot. Environ. 653, 630–637 (2019).
van Loosdrecht, M. C. M., Heijnen, J. J., Eberl, H., Kreft, J. & Picioreanu, C. Mathematical modelling of biofilm structures. Ant. Leeuwenhoek 81, 245–256 (2002).
Fazli, M. et al. Regulation of biofilm formation in Pseudomonas and Burkholderia species. Env. Microbiol. 16, 1961–1981 (2014).
Wolska, K., Grudniak, A. M., Rudnicka, Z. & Markowska, K. Genetic control of bacterial biofilms. J. Appl. Gen. 57, 225–238 (2016). This work gives a clear and comprehensive overview on quorum sensing molecules, c-di-GMP and small RNAs as regulators in the life cycle of some Gram-negative species.
Poulin, M. B. & Kuperman, L. L. Regulation of biofilm exopolysaccharide production by cyclic di-guanosine monophosphate. Front. Microbiol. 12, 730980 (2021).
Teschler, J. K. et al. Living in the matrix: assembly and control of Vibrio cholerae biofilms. Nat. Rev. Microbiol. 13, 255–268 (2015).
Moormeier, D. E. & Bayles, K. W. Staphylococcus aureus biofilm: a complex developmental organism. Mol. Microbiol. 104, 365–367 (2017).
Schilcher, K. & Horswill, A. R. Staphylococcal biofilm development: structure, regulation and treatment strategies. Microbiol. Mol. Biol. Rev. 84, e00026-19 (2020).
Neu, T. R. & Lawrence, J. R. in Productive Biofilms (eds Muffler, K. & Ulber, R.) 1–51 (Springer International, 2014).
Karampatzakis, A. et al. Measurement of oxygen concentrations in bacterial biofilms using transient state monitoring by single plane illumination microscopy. Biomed. Phys. Engin. Expr. 3, 035020 (2017).
Wagner, M. & Horn, H. Optical coherence tomography in biofilm research: a comprehensive review. Biotechnol. Bioeng. 114, 1386–1402 (2017).
Lawrence, J. R., Korber, D. R., Hoyle, B. D., Costerton, J. W. & Caldwell, D. E. Optical sectioning of microbial biofilms. J. Bacteriol. 173, 6558–6567 (1991).
Lawrence, J. R., Swerhone, G. D. W., Kuhlicke, U. & Neu, T. R. In situ evidence for metabolic and chemical microdomains in the structured polymer matrix of bacterial microcolonies. FEMS Microbiol. Ecol. 92, fiw183 (2016).
Lawrence, J. R., Swerhone, G. D. W. & Neu, T. R. Visualization of the sorption of nickel within exopolymer microdomains of bacterial microcolonies using confocal and scanning electron microscopy. Microbes Eniron 34, 76–82 (2019).
Lawrence, J. R., Winkler, M. & Neu, T. R. Multi-parameter laser imaging reveals complex microscale biofilm matrix in a thick (4000 µm) aerobic methanol oxidizing community. Front. Microbiol. 9, 2186 (2018).
Karwautz, C., Kus, G., Stöckl, M., Neu, T. R. & Lueders, T. Microbial megacities fueled by methane oxidation in a mineral spring cave. ISME J. 12, 87–100 (2018).
Neu, T. R. & Kuhlicke, U. Fluorescence lectin bar-coding of glycoconjugates in the extracellular matrix of biofilm and bioaggregate forming microorganisms. Microorganisms 5, 5 (2017).
Neu, T. R. & Lawrence, J. R. in Aquatic Biofilms: Ecology, Water Quality and Wastewater Treatment (eds Romani, A. M., Guasch, H. & Balaguer, M. D.) 29–45 (Caister Acad. Press, 2016).
Raman, R., Raguraman, S., Venkataraman, G., Paulson, J. C. & Sasisekharan, R. Glycomics: an integrated system approach to structure–function relationships of glycans. Nat. Meth. 2, 817–824 (2005).
Kellman, B. P. & Lewis, N. E. Big-data glycomics: tools to connect glycan biosynthesis to extracellular communication. Trends Biochem. Sci. 46, 284–300 (2021).
Laughlin, S. T. & Bertozzi, C. R. Imaging the glycome. Proc. Natl Acad. Sci. USA 106, 12–17 (2009).
Siegrist, M. S., Swarts, B. M., Fox, D. M., Lim, S. A. & Bertozzi, C. R. Illumination of growth, division and secretion by metabolic labeling of the bacterial cell surface. FEMS Microbiol. Rev. 39, 184–202 (2015).
Geta-Zatorsky, N. et al. In vivo imaging and tracking of host–microbiota interactions via metabolic lableing of gut anaerobic bacteria. Nat. Med. 21, 1091–1100 (2015).
Gregor, I. & Enderlein, J. Image scanning microscopy. Curr. Opin. Chem. l Biol. 51, 74–83 (2019).
Kumar, A. et al. Dual-view plane illumination microscopy for rapid and spatially isotropic imaging. Nat. Protoc. 9, 2555–2573 (2014).
Rooney, L. M., Amos, W. B., Hoskisson, P. A. & McConnell, G. Intra-colony channels in E. coli function as a nutrient uptake system. ISME J. 14, 2461–2473 (2020).
Peterson, B. W. et al. Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol. Rev. 39, 234–245 (2015).
Stewart, E., Ganesan, M., Younger, J. G. & Solomon, M. J. Artificial biofilms establish the role of matrix interactions in staphylococcal biofilm assembly and disassembly. Sci. Rep. 5, 13081 (2015).
Gloag, E. S., Fabbri, S., Wozniak, D. J. & Stoodley, P. Biofilm mechanics: implications in infection and survival. Biofilm 2, 100017 (2020). This work describes very well how biofilm viscoelasticity due to the cohesion of multiple EPS components contributes to biofilm resilience to medical treatment and contributes to the virulence of chronic biofilm infections.
Billings, N. et al. Material properties of biofilms — a review of methods for understanding permeability and mechanics. Rep. Progr. Phys. 78, 036601 (2015).
Seviour, T. et al. The biofilm matrix scaffold of Pseudomonas aeruginosa contains G-quadruplex extracellular structures. NPJ Biofilms Microbiomes 7, 27 (2021).
Díaz-Pascual, F. et al. Breakdown of Vibrio cholerae biofilm architecture induced by antibiotics disrupts community barrier function. Nat. Microbiol. 4, 2136–2145 (2019).
Bergstrom, J. S. & Boyce, M. C. Mechanical behavior of particle filled elastomers. Rubber Chem. Technol. 72, 633–656 (1999).
Qi, L. & Christopher, G. F. Rheological variability of Pseudomonas aeruginosa biofilms. Rheol. Acta 60, 219–230 (2021).
Fabbri, S. et al. Fluid-driven interfacial instabilities and turbulence in bacterial biofilms. Environ. Microbiol. 19, 4417–4431 (2017).
Risse-Buhl, U. et al. The role of hydrodynamics in shaping the composition and architecture of epilithic biofilms in fluvial ecosystems. Water Res. 127, 211–222 (2017).
Picioreanu, C., Blauert, F., Horn, H. & Wagner, M. Determination of mechanical properties of biofilms by modelling the deformation measured using optical coherence tomography. Water Res. 145, 588–598 (2018).
Prades, L. et al. Computational and experimental investigation of biofilm disruption dynamics induced by high-velocity gas jet impingement. mBio 11, 1 (2020).
Fabbri, S. & Stoodley, P. in The Perfect Slime. Microbial Extracellular Polymeric Substances (EPS) (eds Flemming, H.-C., Neu, T. R. & Wingender, J.) 153–177 (IWA, 2017).
Wilking, J. N., Angelini, T. E., Seminara, A., Brenner, M. P. & Weitz, D. A. Biofilms as complex fluids. MRS Bull. 36, 385–391 (2011).
Cao, H., Habimana, O., Safari, A., Heffernan, R. & Casey, E. Revealing region-specific viscoelastic properties by means of a micro-rheological approach. NPJ Biofilms Microbiomes 2, 5 (2016).
Saikat, J. et al. Nonlinear rheological characteristics of single species bacterial biofilms. NPJ Biofilms Microbiomes 6, 19 (2020).
Charlton, S. G. V. et al. Regulating, measuring and modeling the viscoelasticity of bacterial biofilms. J. Bacteriol. 201, e00101-19 (2019).
Pattem, J. et al. A multi-scale biophysical approach to develop structure–property relationships in oral biofilms. Sci. Rep. 8, 5691 (2018).
Lieleg, O., Caldara, M., Baumgärtel, R. & Ribbeck, K. Mechanical robustness of Pseudomonas aeruginosa biofilms. Soft Matter 7, 3307–3314 (2011).
Lei, W., Bruchmann, J., Rüping, J. L., Levkin, P. A. & Schwartz, T. Biofilm bridges forming structural networks on patterned lubricant-infused surfaces. Adv. Sci. 6, 1900519 (2019).
Rozenbaum, R. T. et al. Role of viscoelasticity in bacterial killing by antimicrobials in differently grown Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 63, e01972 (2019).
Rahman, M. U. et al. Microrheology of Pseudomonas aeruginosa biofilms grown in wound beds. NPJ Biofilms Microbiomes 8, 49 (2022).
Flemming, H.-C. et al. Who put the film in biofilm? The migration of a term from wastewater engineering to medicine and beyond. NPJ Biofilms Microbiomes 7, 10 (2021).
Quan, K. et al. Water in bacterial biofilms: pores, channels, storage and transport functions. Crit. Rev. Microbiol. 48, 283–302 (2021).
Katharios-Lanwermeyer, S. & O´Toole, G. A. Biofilm maintenance as an active process: evidence that biofilms work hard to stay put. J. Bacteriol. 204, e00587-21 (2022).
Fritts, R. K., McCully, A. L. & McKinlay, J. B. Extracellular metabolism sets the table for microbial cross-feeding. Microbiol. Mol. Biol. Rev. 85, e00135-20 (2021).
Arshad, Z. et al. Using stable isotope probing and fluorescence spectroscopy to examine the roles of substrate and soluble microbial products in extracellular polymeric substance formation in activated sludge processes. Sci. Tot. Environ. 178, 147875 (2021).
Costa, O. Y. A., Pijl, A. & Kuramae, E. E. Dynamics of active potential bacterial and fungal interactions of acidobacterial EPS in soil. Soil Biol. Biochem. 148, 107916 (2020).
Boutrif, M., Garel, M., Cortell, T. & Tamburini, C. Assimilation of marine extracellular polymeric substances by deep-sea prokaryotes in the NW Mediterranean Sea. Environ. Microbiol. Rep. 3, 705–709 (2011).
Bharti, S. et al. Rv1717 is a cell wall-associated β-galactosidase of Mycobacterium tuberculosis that Is involved in biofilm dispersion. Front. Microbiol. 11, 611122 (2021).
Wettstatt, S. Breaking free from home: biofilm dispersal by a glycosidase from Desulfovibrio vulgaris. Environ. Microbiol. 22, 557–558 (2020).
Cherny, K. & Sauer, K. Untethering and degradation of the polysaccharide matrix are essential steps in the dispersion response of Pseudomonas aeruginosa biofilms. J. Bacteriol. 202, e00575-19 (2020).
Pires, D. P., Oliveira, H., Melo, L. D. R., Sillancorva, S. & Azeredo, J. Bacteriophage-encoded depolymerases: their diversity and biotechnological applications. Appl. Microbiol. Biochechnol. 100, 2141–2151 (2016).
Lapébie, P., Lombard, V., Drula, E., Terrapon, N. & Henrissat, B. Bacteroidetes use thousands of enzyme combinations to break down glucans. Nat. Commun. 10, 2043 (2019).
Glowacki, R. W. P. & Martens, E. C. If you eat it or secrete it, they will grow: the expanding list of nutrients utilized by the human gut bacteria. J. Bacteriol. 203, e00481-20 (2021).
Larsbrink, J. & McKee, L. Bacteroidetes bacteria in the soil: glycan acquisition, enzyme secretion, and gliding motility. Adv. Appl. Microbiol. 110, 63–98 (2020).
Brethauer, S., Shahab, R. & Studer, M. Impact of biofilms on the conversion of cellulose. Appl. Microbiol. Biotechnol. 104, 5201–5212 (2020).
López-Mondéjar, R., Algora, C. & Baldrian, P. Lignocellulytic systems of soil bacteria: a vast and diverse toolbox for biotechnological conversion processes. Biotechnol. Adv. 37, 107374 (2019).
Mitrofanova, O., Mardanova, A., Evtugyn, V., Bogomolnaya, L. & Sharipova, M. Effects of Bacillus serine proteases on the bacterial biofilms. Biomed. Res. Int. 2017, 8525912 (2017).
Esoda, C. N. & Kuehn, M. J. Pseudomonas aeruginosa leucine aminopeptidase influences early biofilm composition and structure via vesicle-associated antibiofilm activity. mBio 10, e02548-19 (2019).
Ningthoujam, S. et al. In vitro degradation of β-amyloid fibrils by microbial keratinase. Alzheimers Dement. (NY) 5, 154–163 (2019).
Pressler, K. et al. Characterization of Vibrio cholerae’s extracellular nuclease Xds. Front. Microbiol. 10, 2057 (2019).
Wasmund, K. et al. Genomic insights into diverse bacterial taxa that degrade extracellular DNA in marine sediments. Nat. Microbiol. 6, 885–898 (2021).
Meylan, S., Andrews, I. W. & Collins, J. J. Targeting antibiotic tolerance, pathogen by pathogen. Cell 172, 1228–1238 (2018).
Brauner, A., Fridman, O., Gefen, O. & Balaban, N. O. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 14, 320–330 (2016).
Stewart, P. S. et al. Conceptual model of biofilm antibiotic tolerance that integrates phenomena of diffusion, metabolism, gene expression, and physiology. J. Bacteriol. 201, e00307-19 (2019).
Doroshenko, N. et al. Extracellular DNA impedes the transport of vancomycin in Staphylococcus epidermidis biofilms preexposed to subinhibitory concentrations of vancomycin. Antimicrob. Agents Chemother. 58, 7273–7282 (2014).
Tseng, B. S. et al. The extracellular matrix protects Pseudomonas aeruginosa biofilms by limiting the penetration of tobramycin. Environ. Microbiol. 15, 2865–2878 (2013).
Colvin, K. M. et al. The Pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomona aeruginosa. PLoS Pathog. 7, e1001264 (2011).
Cerca, N., Jefferson, K. K., Oliveira, R., Pier, G. B. & Azeredo, J. Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infect. Immun. 74, 4849–4855 (2006).
Jones, E. A., McGillivary, G. & Bakaletz, L. O. Extracellular DNA within a nontypeable Haemophilus influenzae-induced biofilm binds human β-defensin-3 and reduces its antimicrobial activity. J. Innate Immun. 5, 24–38 (2013).
Wingender, J., Grobe, S., Fiedler, S. & Flemming, H.-C. in Biofilms in Aquatic Systems Vol. 242 (eds Keevil, C. W., Godfree, A. F., Holt, D. & Dow, C.) 93–100 (Royal Society of Chemistry, 1999).
Hahn, M. M., González, J. F. & Gunn, J. S. Salmonella biofilms tolerate hydrogen peroxide by a combination of extracellular polymeric substance barrier function and catalase enzymes. Front. Cell. Infect. Microbiol. 11, 683081 (2021).
Powell, L. et al. Quantifying the effects of antibiotic treatment on the extracellular polymer network of antimicrobial resistant and sensitive biofilms using multiple particle tracking. NPJ Biofilms Microbiomes 7, 1–11 (2021).
Rosman, C. W., van der Mei, H. C. & Sjollema, J. Influence of sub-inhibitory concentrations of antimicrobials on micrococcal nuclease and biofilm formation in Staphylococcus aureus. Sci. Rep. 11, 13241 (2021).
Ranieri, M. R. M., Whitchurch, C. M. & Burrows, L. L. Mechanisms of biofilm stimulation by subinhibitory concentrations of antimicrobials. Curr. Opin. Microbiol. 45, 164–169 (2018).
Lin, J., Wang, Z., Zang, Y., Zhang, D. & Xin, Q. Detection of respiration changes inside biofilms with microelectrodes during exposure to antibiotics. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 54, 202–207 (2019).
Narten, M., Rosin, N., Schobert, M. & Tielen, P. Susceptibility of Pseudomonas aeruginosa urinary tract isolates and influence of urinary tract conditions on antibiotic tolerance. Curr. Microbiol. 64, 7–16 (2012).
von Ohle, C. et al. Real-time microsensor measurement of local metabolic activities in ex vivo dental biofilms exposed to sucrose and treated with chlorhexidine. Appl. Environ. Microbiol. 76, 2326–2334 (2010).
Bui, L. M. G., Conlon, B. P. & Kidd, S. P. Antibiotic tolerance and the alternative lifestyles of Staphylococcus aureus. Essays Biochem. 61, 71–79 (2017).
Ciofu, O., Moser, C., Jensen, P. Ø. & Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. https://doi.org/10.1038/s41579-022-00682-4 (2022).
Dworkin, J. & Shaw, I. M. Exit from dormancy in microbial organisms. Nat. Rev. Microbiol. 8, 890–896 (2010).
Yan, J. & Bassler, B. L. Surviving as a community: antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe 26, 15–21 (2019).
Sindeldecker, D. et al. Novel aminoglycoside-tolerant phoenix colony variants of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 64, e00623-20 (2020).
Abe, K., Nomura, N. & Suzuki, S. Biofilms: hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol. Ecol. 96, fiaa031 (2020).
Acknowledgements
The authors are grateful to I. C. H. Tan and C. Mayer for help with designing the drafts for Figs. 2 and 5a, respectively.
Author information
Authors and Affiliations
Contributions
H.-C.F., T.R.N., P.H.N., T.S. and P.S. researched data for the article. H.-C.F., E.D.v.H., T.R.N., P.H.N., T.S., P.S., J.W. and S.W. contributed substantially to discussion of the content. H.-C.F., T.R.N., P.H.N. and P.S. wrote the article. H.-C.F., T.R.N., J.W. and P.S. reviewed and/or edited the manuscript before submission. H.-C.F. assembled the team and coordinated the writing process.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Kendra Rumbaugh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Extracellular polymeric substances
-
(EPS). Microbial biopolymers such as polysaccharides, proteins, extracellular DNA (eDNA) and others, forming the biofilm matrix.
- Extracellular matrix
-
The non-cellular component present within all tissues and organs, sometimes also used as an alternative to the term extracellular polymeric substances (EPS); frequently used in the medical context.
- Amyloids
-
Aggregates of proteins in fibrillar morphology. Pathogenic amyloids form by misfolding of previously normal structures. In biofilms, amyloids fulfil many functions, including, for example, matrix stabilization, nutrient storage, desiccation resistance and others.
- Humic-like microbially derived refractory substances
-
Remains of bacterial cells that are not readily degraded after cell death. As high molecular weight compounds they remain present within microbial communities, contributing to the polymeric matrix.
- Humic substances
-
The organic components of humus. Humic substances are hetero-polycondensates based on a motif of aromatic nuclei with phenolic and carboxylic substituents linked together. They can form aggregates, provide cation complexation sites and regulate the bioavailability of metal ions.
- Transparent exopolymer particles
-
Extracellular acidic polysaccharides produced by phytoplankton and bacteria in saltwater, freshwater and wastewater; they are extremely abundant and play a significant role in biogeochemical cycling of carbon and other elements in water.
- Collective biological systems
-
Systems such as forests, beehives, coral reefs or kelp fields that show emerging properties which exceed those of the sum of the single individuals. Also known as ‘extended organisms’.
- Psl
-
An extracellular polysaccharide of Pseudomonas aeruginosa and an important structural and functional feature of P. aeruginosa biofilms. Psl is rich in galactose and mannose.
- Pel
-
An extracellular polysaccharide of Pseudomonas aeruginosa and an important structural and functional feature of P. aeruginosa biofilms. The structure of Pel is not fully characterized but it is a cationic polysaccharide, differing from Psl and alginate.
- Ecotin
-
A protease inhibitor, formed in the periplasmic space of Gram-negative bacteria, inhibiting neutrophil elastase.
- Anammox
-
(Ammonium oxidation). The reaction of nitrite and ammonium ions leading directly to dinitrogen and water.
- Rhamnolipids
-
Amphiphilic glycolipids consisting of a monosaccharide or disaccharide connected by a glycosidic bond to a fatty acid; they act in various roles in the EPS matrix.
- Necromass
-
The mass of dead biological material, including microorganisms.
- Phenazine
-
The chemical description of the class of dibenzo annulated pyrazine; it embraces pyocyanine as a subclass in which one of the nitrogen atoms is substituted with a methyl group.
- Glycomics
-
The study of all glycan structures in biology and a subset of glycobiology. Glycomics focuses on the identification of structure and function of the total collection of glycans (the glycome) produced by biological systems under specified conditions of time, space and environment.
- Mesolens
-
A novel microscope objective lens that combines a high numerical aperture with a large field of view of up to 6 mm combined with high spatial resolution.
- Nanoscopy
-
A term describing light microscopy techniques at a resolution across the diffraction limit of light. The techniques include localization or blink microscopy, stimulated emission depletion microscopy and, more recently, MinFlux. By exploiting switchable fluorochromes, achieving a resolution of 20–10 nm down to a few nanometres becomes possible.
- Persisters
-
A subpopulation of transiently antibiotic-tolerant bacterial cells that are often slow growing or growth arrested, and are able to resume growth after a lethal stress.
- Phoenix phenotypes
-
Phoenix colonies that grow out of the zone of clearance of antibiotic-loaded beads from lawn biofilms while there are still very high concentrations of antibiotic present, suggesting an antibiotic-resistant phenotype.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Flemming, HC., van Hullebusch, E.D., Neu, T.R. et al. The biofilm matrix: multitasking in a shared space. Nat Rev Microbiol 21, 70–86 (2023). https://doi.org/10.1038/s41579-022-00791-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-022-00791-0
This article is cited by
-
In vitro and in silico studies of enterobactin-inspired Ciprofloxacin and Fosfomycin first generation conjugates on the antibiotic resistant E. coli OQ866153
BMC Microbiology (2024)
-
Deciphering antifungal and antibiofilm mechanisms of isobavachalcone against Cryptococcus neoformans through RNA-seq and functional analyses
Microbial Cell Factories (2024)
-
Cracking the code of seasonal seawater biofouling: enhanced biofouling control with quorum sensing inhibitor-functionalized membranes
npj Clean Water (2024)
-
Promastigote EPS secretion and haptomonad biofilm formation as evolutionary adaptations of trypanosomatid parasites for colonizing honeybee hosts
npj Biofilms and Microbiomes (2024)
-
Beneficial applications of biofilms
Nature Reviews Microbiology (2024)