Abstract
Neuroaxonal damage is the pathological substrate of permanent disability in various neurological disorders. Reliable quantification and longitudinal follow-up of such damage are important for assessing disease activity, monitoring treatment responses, facilitating treatment development and determining prognosis. The neurofilament proteins have promise in this context because their levels rise upon neuroaxonal damage not only in the cerebrospinal fluid (CSF) but also in blood, and they indicate neuroaxonal injury independent of causal pathways. First-generation (immunoblot) and second-generation (enzyme-linked immunosorbent assay) neurofilament assays had limited sensitivity. Third-generation (electrochemiluminescence) and particularly fourth-generation (single-molecule array) assays enable the reliable measurement of neurofilaments throughout the range of concentrations found in blood samples. This technological advancement has paved the way to investigate neurofilaments in a range of neurological disorders. Here, we review what is known about the structure and function of neurofilaments, discuss analytical aspects and knowledge of age-dependent normal ranges of neurofilaments and provide a comprehensive overview of studies on neurofilament light chain as a marker of axonal injury in different neurological disorders, including multiple sclerosis, neurodegenerative dementia, stroke, traumatic brain injury, amyotrophic lateral sclerosis and Parkinson disease. We also consider work needed to explore the value of this axonal damage marker in managing neurological diseases in daily practice.
Key points
-
Neuronal damage and loss are the pathological substrates of permanent disability in various acute and chronic neurological disorders.
-
Levels of neurofilament proteins increase in cerebrospinal fluid (CSF) and in the blood upon neuroaxonal damage.
-
First-generation (immunoblot) and second-generation (enzyme-linked immunosorbent assay) neurofilament assays captured only the tip of the iceberg in disease.
-
Third-generation (electrochemiluminescence) and fourth-generation (single-molecule array) assays permit highly sensitive, longitudinal detection of blood neurofilament levels even in mild disease and in healthy controls.
-
Multicentre studies are underway to consolidate neurofilaments as biomarkers that reflect brain tissue damage, enabling longitudinal monitoring of disease activity and drug effects in clinical trials in neurological diseases.
Similar content being viewed by others
Introduction
Neuroaxonal damage and loss are the pathological substrate of many acute and chronic neurological disorders that result in permanent disability. The ability to readily detect and follow such damage would be a great advantage in the assessment of disease activity, monitoring of treatment responses and prognosis. Therefore, a biomarker that accurately reflects neuroaxonal injury would be invaluable for reaching individual therapeutic decisions and measuring drug effects in clinical trials. Attempts to discover such a biomarker have involved investigation of several avenues, from cerebrospinal fluid (CSF) proteins to MRI, magnetic resonance spectroscopy and metabolic imaging, and have provided different insights with different limitations.
Neurofilaments are gaining increasing attention as candidate biomarkers of neuroaxonal injury because they are abundant structural scaffolding proteins that are exclusively expressed in neurons and that reach abnormal levels as a result of axonal damage in neurodegenerative, inflammatory, vascular and traumatic diseases not only in the CSF but also in serum. Neurofilaments are highly specific for neuronal cell damage and eventual neuronal cell death, offering a key advantage over other possible biomarkers.
Many, if not all, pathological processes that cause axonal damage release neurofilament proteins into the extracellular fluid, CSF and peripheral blood, depending on the extent of damage. High levels of neurofilaments, therefore, are general indicators of axonal damage irrespective of its cause and any clinical diagnosis that has been made, and blood levels of neurofilaments are useful for monitoring and predicting progression in various acute and chronic neurological diseases and for assessing the efficacy and/or toxicity of treatment.
Until recently, measurements of the neurofilament protein that is most promising as a biomarker, neurofilament light chain (NfL), in patients with neurological disorders could only be performed with CSF samples, mainly because assay sensitivity was insufficient for reliable quantification of NfL levels in the blood. Several studies of CSF have demonstrated that levels of neurofilament proteins are increased in a wide range of neurological diseases1. However, given that lumbar puncture is an invasive procedure, longitudinal analyses have been rare and not performed systematically. For the same reasons, neurofilaments have rarely been measured in diseases in which diagnostic lumbar punctures are infrequently indicated. Neurofilament levels in the blood can be quantified with enzyme-linked immunosorbent assay (ELISA)2,3 and more sensitive electrochemiluminescence (ECL) assay technology in many different diseases4,5, but neither technique can detect small, disease-related changes. Only the introduction of single-molecule array (SiMoA) assays has enabled reliable detection of NfL in blood samples across the whole range of concentrations, including those in healthy individuals6,7,8. Consequently, the past 2 years have witnessed a surge in the number of publications on neurofilament blood levels in a broad range of neurological disorders.
In this Review, we provide background on the structure and function of neurofilaments, consider the analytical aspects of neurofilament measurements and discuss current knowledge on age-dependent normal ranges of neurofilament concentrations. We also review the main neurological disorders in which neurofilament measurements could play a role in research or clinical settings, and highlight aspects that need to be addressed in future studies.
Neurofilaments — structure and function
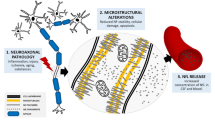
Neurofilaments are classified as intermediate filaments according to their diameter (~10 nm), which is between that of actin filaments (6 nm) and myosin filaments (15 nm). Human neurofilament heavy chain (NfH, 112.5 kDa on the basis of the DNA sequence and 200–220 kDa on the basis of polyacrylamide gel electrophoresis), neurofilament medium chain (NfM, 102.5 kDa and 145–160 kDa), NfL (61.5 kDa and 70–86 kDa) and α-internexin (55.4 kDa and 58–66 kDa) are class IV intermediate filaments, and peripherin (53.7 kDa and 57–59 kDa) is a class III intermediate filament (Fig. 1). The molecular masses are higher in polyacrylamide gel electrophoresis and in vivo owing to an abundance of negatively charged amino acids (glutamic acids) in their sequences and to post-translational modifications9.
a | Domain structure and post-translational modifications of neurofilament subunits9,11. Neurofilament light chain (NfL), neurofilament medium chain (NfM), neurofilament heavy chain (NfH), α-internexin and peripherin are the subunits of neurofilaments in the mature nervous system. All neurofilament subunits include a conserved α-helical rod domain that comprises several coiled coils, and variable amino-terminal globular head regions and carboxy-terminal tail domains. NfM and NfH subunits are unique among the intermediate filament proteins in that they have long carboxy-terminal domains with multiple Lys–Ser–Pro repeats that are heavily phosphorylated. Phosphorylation and O-linked glycosylation sites on neurofilament subunits are shown. b | Neurofilament assembly. Neurofilament protein monomers form parallel coiled-coil heterodimers9. Two dimers form staggered antiparallel tetramers through interactions between coil domains 1a, 1b and 2a12. The lateral association of eight tetramers results in formation of cylindrical structures known as unit-length filaments that have a diameter of ~16 nm and a length of ~60 nm. Gradual end-to-end annealing of these unit-length filaments results in filament elongation, which is followed by radial compaction to form the mature, long neurofilament polymer with a diameter of ~10 nm. The tail domains of NfM and NfH radiate outwards from the filament core because of the extensive negative charges from large numbers of glutamic acid and phosphorylated serine and threonine residues. E segment, glutamic-acid-rich segment; E1, glutamic-acid-rich segment 1; E2, glutamic-acid-rich segment 2. Adapted with permission from Yuan, A., Rao, M. V., Veeranna & Nixon, R. A. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb. Perspect. Biol. 9 (2017), © Cold Spring Harbor Laboratory Press.
The neurofilament proteins contain intrinsically unstructured regions. One key feature of these unstructured regions is that a high proportion of residues are lysine10,11; lysine and serine are the dominant amino acids in the neurofilament tail domain10. A relatively conserved, central α-helical rod region, a short variable head domain at the amino-terminal end and a tail of highly variable length at the carboxy-terminal end are highly characteristic of the neurofilament protein subunits9 (Fig. 1). The head domain contains serine and threonine residues and O-linked glycosylation and phosphorylation sites. The tail domain contains abundant glutamic and lysine-rich stretches of variable length with multiple serine phosphorylation sites. The central rod domain contains hydrophobic repeats that facilitate the formation of coil-to-coil dimers.
Formation of neurofilament protein dimers is the first step in heteropolymer assembly. Antiparallel aggregation of these dimers leads to the formation of tetramers, and eight laterally associated tetramers form the cylindrical unit-length filament (UFL) structure9,12. Annealing of UFLs leads to longitudinal elongation of neurofilaments, which is followed by radial compaction to form the final long neurofilaments with diameters of 10 nm (ref.12) (Fig. 1).
Post-translational modifications of neurofilaments include addition of O-linked N-acetylglucosamine (O-GlcNAc) to individual serine and threonine residues, nitration, oxidation, ubiquitylation and, most importantly, phosphorylation9,13. All subunits are phosphorylated on their head domain, but only NfM and NfH are extensively phosphorylated on their carboxy-terminal domains, and this phosphorylation increases the resistance of these subunits to proteases14. Under normal conditions, neurofilaments are highly stable within axons, and their turnover is low. The filaments form a liquid crystal gel network in diseases such as amyotrophic lateral sclerosis (ALS), dementia with Lewy bodies (DLB) or Parkinson disease (PD); neurofilament accumulation is related to subunit stoichiometry and the degree of phosphorylation14.
The precise functions of neurofilaments remain unknown, but they are thought to be critical for radial growth and stability of axons, thereby enabling effective, high-velocity nerve conduction15,16. Several reports indicate that neurofilaments interact with other proteins and organelles, including mitochondria and microtubules9, suggesting that they have important functions beyond preserving axonal stability.
Several mutations identified in the genes that encode neurofilament proteins can lead to abnormal neurofilament aggregation and accumulation, with the consequence of axonal dysfunction and neurodegeneration. For example, mutations in the NEFL gene, which encodes NfL, lead to Charcot–Marie–Tooth disease (CMT) type 2E (CMT2E) or type 1F (CMT1F). Mutations of the genes that encode peripherin (PRPH), NfH (NEFH) and NfM (NEFM) have been associated with increased susceptibility to ALS and familial PD. Mutations in genes other than those that encode neurofilament proteins can have secondary effects on neurofilament aggregation; such mutations include those in heat-shock 27-kDa protein 1 in CMT2F, gigaxonin in giant axonal neuropathy and superoxide dismutase 1 (SOD1) in ALS9,17.
Assays to detect soluble neurofilaments
In the past three decades, impressive advances have been made in the development of sensitive immunoassay technologies. With these advances, the detection of neurofilaments has improved (Fig. 2), moving towards ever more clinically useful capabilities.
When an axon is damaged, cytoskeletal proteins, including neurofilaments, are released into the extracellular space and subsequently into the cerebrospinal fluid (CSF) and, at lower concentrations, into the blood. First-generation (immunoblots) and second-generation (enzyme-linked immunosorbent assay (ELISA)) immunoassays can typically detect neurofilaments in the CSF but have limited sensitivity for detection in the blood. Third-generation (electrochemiluminescence) and, in particular, fourth-generation (single-molecule array) immunoassays can reliably measure blood levels of neurofilament light and detect subtle longitudianl changes in disease and in healthy controls.
First-generation immunoassays were semi-quantitative at best. Immunoblots based on electrophoretic protein separation or dot blots were, however, consistent in that they reliably demonstrated the presence of neurofilament isoforms in the CSF and blood of patients with a range of diseases11.
Second-generation sandwich ELISA technology produced the first reliable quantitative data that enabled assessment of the prognostic and diagnostic value of NfH and NfL in the CSF in human disease2,18,19,20. Human body fluid compartments that were analysed with this technique extended to the interstitial and extracellular fluid21, serum and plasma, amniotic fluid and the vitreous body22. Meta-analyses and international validation studies demonstrated that high precision could be achieved in expert laboratories, but they also highlighted the need for improved assay standardization23,24.
Third-generation ECL technology led to a substantial improvement in analytical sensitivity4,5,25,26,27. ECL-based assays are known to be highly sensitive, exhibit a broad dynamic range and require a small sample volume. However, fourth-generation SiMoA technology is 126-fold and 25-fold more sensitive than ELISA and ECL assays, respectively, for quantification of NfL7. This technology improved the analytical sensitivity to an extent that reliable quantification of NfL levels in blood became possible across the range of concentrations that are observed in disease and in physiological conditions6,7,8,28. This cutting-edge method is based on single-molecule arrays and the simultaneous counting of singulated capture microscopic beads (2.7 µm diameter) that carry sandwich antibody complexes (two antibodies and one antigen). The analytical sensitivity is manifold higher than that obtained with use of the same antibodies in the ELISA format designed for CSF measurements19 and enables reliable measurement of the low NfL concentrations that are present in blood samples from young healthy individuals6,8, so that minor changes in levels of this protein that occur in normal ageing or after mild injury can be monitored. A close correlation between NfL levels in the serum or plasma and levels in the CSF, which has been demonstrated in numerous studies and various neurological diseases, allows conclusions about the degree of ongoing neuroaxonal injury to be drawn from blood levels without the need to obtain CSF by lumbar puncture4,8,29,30,31,32,33,34,35,36. Investigations of NfM have been sparse22,37, but commercial SiMoA kits for the detection of NfL and phosphorylated NfH are available.
Neurofilaments in ageing
Normal ageing is associated with neurodegenerative processes that can be detected with assessment of various markers, such as volumetric loss of brain tissue and levels of fluid biomarkers including neurofilaments. The advantage of an easy-to-access body fluid biomarker, such as neurofilament in the blood, is that it provides real-time information about neuroaxonal damage in the CNS at low cost and with the ability to repeat measurements in a relatively non-invasive manner38. In CSF, the normal upper reference value for NfL levels increases 2.5-fold between the ages of 20 years and 50 years and doubles further by the age of 70 years39. This age-related increase in levels in the lumbar CSF could be due to the physiological phenomenon of reduced CSF turnover40 with age but could also indicate slow, ongoing axonal injury. The latter possibility is supported by the finding that CSF levels of NfL in cognitively healthy elderly individuals correlate with hippocampal atrophy independently of age and Alzheimer disease (AD) biomarkers41. However, a detailed understanding of the mechanisms that underlie age-related increases in neurofilament levels is lacking. In addition to structural damage and loss of neurons, metabolic alterations in the turnover of neurofilament proteins might play a role: experimental evidence demonstrates complex changes in the expression of mRNA, post-translational mRNA modification and neurofilament protein turnover42.
A highly significant correlation between age and NfL blood levels is also observed: the use of SiMoA technology has shown that NfL levels in the blood of healthy controls increase by 2.2% per year between the ages of 18 years and 70 years8,43. The strong correlation between CSF and blood levels of NfL at the group level suggests that the two measures reflect similar physiological processes4,8,29,30,31,32,33,34,35,36. Nevertheless, the possibility that degenerative processes in the PNS contribute to neurofilament levels in peripheral blood must be acknowledged4,44,45.
Establishing universal reference values for healthy controls by analysis of samples under standardized and controlled conditions in reference laboratories is important for further development of neurofilaments as a biomarker. These reference values would enable correct interpretation of levels seen in various pathological conditions, thereby maximizing the potential of neurofilaments in the management of diseases that involve neuroaxonal injury.
Neurofilaments in neurological disease
CSF and blood levels of neurofilament proteins have been measured in various neurological diseases (Box 1), and evidence has accumulated that they can be clinically useful biomarkers in many of these diseases. Below, we discuss the evidence in each of the studied diseases.
Multiple sclerosis
Multiple sclerosis (MS) is a chronic disease of presumed autoimmune origin that is, at least initially, characterized by episodes of focal inflammation in the brain and spinal cord that predominantly involve the white matter but can also involve the grey matter46. The formation of new lesions can be visualized with MRI, the only established biomarker of disease activity used in routine clinical practice today. However, MRI primarily detects lesions in the white matter, and grey matter damage is largely missed with standard imaging techniques47,48. In addition, MRI does not allow selective detection of neuroaxonal degeneration, which seems to be the most important determinant of long-term disability49,50,51. Several MRI-based volumetric measures, including analysis of cortical thickness, have been used to assess neuronal degeneration, but the specificity and sensitivity of these measures at the individual level are limited52.
Use of second-generation immunoassays to measure NfL, pioneered by Rosengren et al.18,53, revealed three key aspects of disease associated with CSF levels of NfL: the degree of disability, disease activity and the time since the last relapse in patients with relapsing–remitting MS (RRMS)53. These initial findings were replicated and extended by subsequent, larger studies of clinical aspects associated with CSF levels of NfL54,55,56,57 and NfH26,55,58,59. Further, CSF levels of NfL reduce as a consequence of disease-modifying therapy (DMT). For example, initiation of the high-efficacy DMT natalizumab resulted in normalization of CSF NfL levels to those seen in healthy controls within 6–12 months60, suggesting that NfL can be used to monitor therapeutic efficacy. Similar observations were made in placebo-controlled61 and observational62,63 studies of fingolimod in patients with RRMS and in studies of mitoxantrone and rituximab64 and of natalizumab65 in progressive MS.
Despite the promising results in MS, a major barrier to widespread adoption of NfL analysis in MS research and clinical practice has been the requirement of CSF sampling; however, this problem has finally been overcome by use of third-generation29 and especially fourth-generation immunoassays8. Of particular interest is the demonstration that serum levels of NfL can be used to separate not only patients with MS from healthy controls but also patients with MS who have enhancing MRI lesions from patients without such lesions8. Furthermore, serum NfL levels in patients with MS have been independently associated with disability and relapse status8,38, and the risk of future relapses and disability worsening is higher among patients with high serum levels of NfL than among those with lower levels8,38. Finally, patients with ongoing DMT had lower serum NfL concentrations than did untreated patients8. Yet another study found that patients who switched from injectable therapies to fingolimod had significantly lower serum NfL levels than when they were receiving injectables over a 2-year period30. Associations with disease activity and treatment-related reductions in serum NfL levels were confirmed by another observational study in which a fourth-generation immunoassay was used in a large, independent cohort of patients with RRMS36. A longitudinal observational study published in 2018 demonstrated that patients with increased serum levels of NfL at baseline, independent of other clinical and MRI variables, experience significantly more brain and spinal cord volume loss over 2 years and 5 years of follow-up38.
Collectively, the findings described (Table 1) make a strong case for bringing fourth-generation serum NfL assays from the bench to the clinics in the management of MS. Further studies are required to show how these assays can be used for monitoring disease activity and for therapeutic decision-making.
Dementias
Dementia — defined in this context as cognitive disturbances that are severe enough to interfere with activities of daily living — can be caused by several different neurodegenerative disorders, of which AD is the most prevalent, frontotemporal dementia (FTD) is the second-most prevalent among people aged <60 years, and DLB is the second-most prevalent among patients aged >60 years. Currently, the clinical diagnosis of the different types of dementia relies largely on documenting cognitive decline or on post-mortem evaluation. However, it is becoming clear that early brain damage occurs decades before the onset of clinical symptoms. This observation opens a window of opportunity for secondary prevention and suggests the value of a shift from using clinical hallmarks for diagnosis to monitoring of biological measures that reflect ongoing pathological processes. Several studies have addressed the question of whether neurofilaments can provide such a biological measure (Table 2).
An early study in 1999 demonstrated a mild increase in CSF levels of NfL in AD and substantially higher levels in FTD67. These findings were confirmed in a subsequent study that also showed that the increase in CSF levels of NfL in AD is seen only in patients with late-onset disease, whereas NfL levels did not significantly differ between healthy controls and patients with early-onset AD68.
Subsequent meta-analyses of findings obtained with second-generation immunoassays consistently demonstrated that CSF levels of NfL are increased in the mild cognitive impairment and dementia stages of AD23,69 and are independent of amyloid-β (Aβ) load69,70. The diagnostic specificity of NfL levels was lower than that of the hallmark AD biomarkers, namely Aβ1–42 levels, Aβ1–42:Aβ1–40 ratio and phosphorylated tau levels69,71. Nevertheless, evidence indicates that NfL levels correlate with and are predictive of brain atrophy and worsening of cognition independently of Aβ pathology70,72. Moreover, NfL levels in the blood have some predictive value for progression to AD dementia in patients with subjective memory complaints43, so the potential for use of NfL levels in combination with clinical evaluation and other biomarkers to detect the earliest stage of the disease should be assessed. Furthermore, the greatest value of NfL in AD dementia could be in monitoring responses to treatment, as seen in MS, as reductions in plasma levels of NfL were observed in animal models of AD when treated with a β-secretase (BACE) inhibitor31.
Measurement of NfL levels is also likely to be of value in the diagnosis of FTD, in which CSF23,68 and serum35,73 levels of NfL are high and approach those observed in ALS (see Amyotrophic lateral sclerosis section below). Indeed, among the neurodegenerative dementias, the highest CSF NfL concentrations are observed in FTD and vascular dementia, followed by AD23,74. Among patients with FTD, CSF levels of NfL are higher in those with TAR DNA-binding protein 43 (TDP43) inclusions than in those with tau pathology (confirmed by genetic testing or post-mortem evaluation)75. Moreover, CSF levels of NfL increased when symptoms developed in patients with genetic FTD, and these levels were inversely correlated with survival35. Several studies have confirmed a strong relationship between CSF and serum levels of NfL and time to death in patients with FTD35,75.
The results of fourth-generation immunoassays76 for the detection of neurofilaments in blood similarly reflect neuroaxonal damage in neurodegenerative dementias, including FTD73, familial and sporadic AD43,77 and atypical parkinsonian disorders78. In sporadic AD, plasma NfL concentrations are already increased in the mild cognitive impairment stage and correlate with cognitive, biochemical and imaging hallmarks of the disease43. In familial AD, blood NfL concentrations start to increase ~10 years before the expected onset77.
Elevated serum levels of NfL have also been described in patients with primary progressive aphasia79; higher levels were identified in patients with the non-fluent or agrammatic and semantic variants than in those with the logopenic variant. NfL levels correlated with clinical progression and brain volume loss in all patients with primary progressive aphasias79.
Very high CSF and blood levels of NfL have been observed in patients with sporadic and familial Creutzfeldt–Jakob disease. In this acute and severe condition, CSF levels of NfL were increased before symptom onset, and the sensitivity and specificity of serum NfL concentration for diagnosis of Creutzfeldt–Jakob disease were 100% and 85.5%, respectively80.
Stroke
Most existing data on neurofilaments in stroke are CSF measurements in subarachnoid haemorrhage (SAH). Studies have shown that NfH and NfL levels are higher among patients with aneurysmal SAH than among healthy controls or patients without neurological disease81,82,83. The exact causes of neurofilament elevation in SAH in the absence of associated focal lesions (parenchymal haematoma or ischaemia owing to vasospasm) are not entirely clear, but they are presumably attributable to diffuse neuroaxonal injury or are iatrogenic after, for example, placement of an external ventricular drain. Regardless, evidence suggests that neurofilament levels consistently correlate with the clinical severity and extent of morphological brain damage81,82.
The ability to analyse NfL levels in blood samples with fourth-generation immunoassays has facilitated the study of this marker in stroke, in which there is usually no indication for a lumbar puncture. This approach has been used to show that serum levels of NfL are higher among patients with spontaneous cervical artery dissection who had an ischaemic stroke than among those with transient ischaemic attacks or isolated local symptoms84. Similarly, serum NfL concentration was found to be increased in patients with a single, recent, small subcortical infarct compared with the concentration in age-matched and sex-matched healthy controls85. In the same study, assessment of the temporal dynamics of NfL at 3 months and 15 months after stroke revealed especially high levels in patients with new, clinically silent brain lesions related to small vessel disease detected with MRI during follow-up, suggesting that NfL levels indicate active small vessel disease. Interestingly, serum NfL levels increased during the first few days after stroke onset and remained elevated in a follow-up assessment at 3 months. Comparable findings of neurofilament dynamics have been reported in other studies5,84,86. Prolonged release of NfL into the blood after acute neuronal injury might be caused by persistent blood–brain barrier breakdown, but ongoing post-ischaemic immunological or inflammatory processes could also explain these findings.
Traumatic brain injury
Mild traumatic brain injury (TBI), also called concussion, is caused by non-penetrating head trauma and is increasingly recognized as a major health problem87. Most patients with mild TBI recover within hours to days, but a percentage have symptoms for weeks to months after the head impact, a condition called post-concussive syndrome. Furthermore, an unknown proportion of people who are exposed to repeated concussions, primarily contact sports athletes such as boxers and American football players and soldiers who are exposed to explosive blasts, develop a chronic neurodegenerative disease called chronic traumatic encephalopathy (CTE)88, previously known as dementia pugilistica89.
Mild TBI and post-concussive syndrome are vaguely defined clinical entities and their diagnosis is based only on the presence of one or several nonspecific symptoms (such as loss of consciousness, dizziness, headache and poor concentration), causing a major issue in research, clinical management and drug development in this field87. Consequently, sensitive biomarkers are needed to identify and grade neuronal injury in individuals with mild TBI and post-concussive syndrome. Furthermore, biomarkers that enable grading of the severity of neuronal injury after a mild TBI might be important as objective tools for guiding sports physicians with return-to-play decisions for their athletes.
Studies of contact sports athletes with mild TBI show that CSF levels of NfL increase more than levels of tau, suggesting that minor head injuries affect long myelinated white matter axons more than they affect shorter cortical axons90,91. In severe TBI, fourth-generation NfL immunoassays6 have demonstrated a marked increase in blood NfL levels that also predicted clinical outcome92, thereby confirming earlier findings from second-generation immunoassay studies of CSF and blood samples93. Interestingly, marked increases in blood NfL levels have been detected in amateur boxers after a bout; higher NfL levels were observed in boxers who had received more head impacts, and levels approached normalization after 3 months of rest from boxing94. Similarly, blood levels of NfL were found to increase during the course of a season in American football players95. Taken together, these results (Table 3) support the idea that the blood level of NfL is a sensitive indicator of axonal injury after mild TBI and is a promising candidate for clinical application and contact sports medicine.
Amyotrophic lateral sclerosis
Motor neuron diseases are neurodegenerative disorders characterized by degeneration of the upper and lower motor neurons, and the most common form is ALS96. Given that axonal impairment can be seen early in the disease, measurement of neurofilaments in the CSF of patients with ALS was an obvious and straightforward experiment and led to the observation that NfL levels are increased in this condition18,97,98.
Several independent studies have confirmed that neurofilament levels are significantly elevated in patients with ALS compared with several other disorders (Table 4); the largest prospective study included 455 patients34,99,100,101,102,103,104. Diagnostic sensitivities and specificities were up to ~80%. Higher levels were also associated with faster disease progression. Increases in NfL and NfH levels were also observed in the early clinical phase of patients with genetic ALS and in patients with sporadic ALS33,105,106,107,108. The first clinical sign of the disease seems to be associated with a massive increase in neurofilament levels in the CSF33, and a corresponding increase in NfL levels has been observed in the blood33. Furthermore, increased levels of blood NfH have been seen in patients with sporadic ALS101,103,109. The difference in dynamics of NfL levels compared with those of NfH levels in ALS and controls might partly be explained by earlier issues with assay sensitivity. However, a new hypothesis — adaptive protein stoichiometry — suggests that the neurodegenerative process itself alters the quantitative relationship of neurofilament subunits, leading to a relative overexpression of NfL compared with NfM and NfH that minimizes ATP requirements for subunit translation in the motor neuron22. This hypothesis might be correct in ALS, as a clear differentiation between NfL levels in patients with ALS and those in healthy controls was seen with fourth-generation NfL assays108. However, large prospective studies have not been performed.
Although helpful for diagnostic purposes, the reason for the high CSF levels of neurofilaments in ALS is still not entirely clear, even under the assumption that neurofilament levels reflect neuroaxonal damage. One small study showed a correlation of NfL levels with axonal impairment assessed with diffusion tensor imaging (DTI)110, but this correlation was not seen in a larger cohort of patients with ALS (n = 75)102. NfL concentrations in the blood seem to be stable at a very high level during follow-up of patients with ALS, whereas DTI values increase34,79; only a slight increase in blood levels of NfH has been described100. One mechanistic hypothesis is based on evidence that TDP43, the major neuropathological hallmark of ALS, directly interacts with neurofilament production and causes the sudden and massive release of neurofilaments in ALS111. More prospective studies of neurofilament levels in ALS, especially in the blood, are needed to define stable cut-off levels for diagnosis and prognosis and to explore whether neurofilament levels in blood can be used to stratify patient groups in clinical studies.
Parkinson disease
Although PD is one of the most common neurodegenerative disorders, no validated neurochemical biomarkers are currently available to aid clinical diagnosis. In PD and other synucleinopathies, α-synuclein is the main component of neuronal inclusions. Many studies have been performed to assess whether α-synuclein in the CSF could be an effective biomarker of PD; ELISA has been used in most of these studies, which have produced contradictory results112,113.
In 1998, NfL was first investigated in the CSF of 49 patients undergoing differential diagnosis for a parkinsonian syndrome, including patients with atypical parkinsonian syndromes such as progressive supranuclear palsy (PSP) and multiple system atrophy (MSA). These investigations demonstrated increased CSF NfL levels in patients with PSP and MSA compared with patients with PD114. This increase in PSP and MSA but not in PD was also seen for NfH115. In a larger study that included >450 patients with PSP, MSA or PD, almost no overlap was observed between CSF levels of NfL in patients with atypical parkinsonian disease and those with PD; NfL levels were increased mainly in the atypical disorders116. The finding was validated in an independent cohort117.
In a study published in 2016, high levels of NfL were observed in the blood of patients with PSP, and these high levels were still present 1 year later. In patients with higher NfL levels, neurological, functional and neuropsychological deterioration over the year was more severe. Higher baseline levels of NfL predicted greater whole-brain and superior cerebellar peduncle volume loss118. On the basis of these findings, the investigators concluded that NfL could be used not only to aid diagnosis but also to monitor pharmacodynamic effects, especially in clinical trials. The findings of this study were also validated in three independent cohorts, leading to the suggestion that NfL can be used in both primary care and specialized clinics78.
Huntington disease
Huntington disease (HD) is a progressive neurodegenerative disorder caused by CAG repeat expansions in the HTT gene, leading to the formation of mutant huntingtin (mHTT). No proven disease-modifying treatments yet exist119. The slow and insidious progression of neurodegeneration in HD has made it challenging to detect disease-related changes in the levels of neurofilament proteins in the blood120. However, increased CSF levels of NfL have been demonstrated in patients with HD121,122, and fourth-generation technology has revealed a strong relationship between plasma levels of NfL, HD onset and subsequent progression of neurodegeneration119. If confirmed, blood NfL levels could be included as a secondary outcome measure in future clinical trials in HD.
Bipolar disorder
Some evidence suggests that neurodegeneration and neuroaxonal injury can be associated with some subtypes of bipolar disorder123. Although these aspects are not prominent features of the condition, CSF levels of NfL were slightly increased in a subset of patients in one study124, particularly those who were treated with atypical antipsychotics, presumably reflecting a disease-associated or treatment-associated effect that is not yet understood. However, no clear relationship was seen between NfL levels and clinical outcomes, such as manic or hypomanic and depressive episodes (cross-sectional data), suicide attempts, psychotic symptoms or inpatient care125. Although the current evidence that neuroaxonal injury can be detected in bipolar disorder by measuring neurofilaments is limited, the available results warrant longitudinal studies of well-characterized patients to examine how neurofilament concentrations change over time in relation to disease activity and phase (depression and mania) and whether neurofilaments can indicate adverse effects of treatments. Fourth-generation measurement technology will facilitate such studies by enabling measurements to be taken from blood samples.
Conclusions and future aspects
In summary, highly sensitive neurofilament measurements have the potential to fill a gap in the assessment of neuroaxonal damage in various neurological disorders. For the first time, this approach provides a sensitive assessment of the consequences of brain tissue damage with only a blood sample, an important advance to aid research and towards use of the assays in clinical practice. In relation to clinical trials, the reviewed characteristics of neurofilaments, especially of NfL, make these proteins optimal candidates as markers of outcome in phase II trials in neurological disorders. Definitive phase III trials must use clinical end points (clinical events with a clear effect on the duration or quality of life) to confirm a clinical benefit, but the aim of phase II trials is to identify drugs with sufficient activity to continue to phase III trials, so earlier end points are preferable.
To validate neurofilament measurements as phase II trial end points, two additional properties must be verified: a correlation with the clinical end points used in phase III trials, and an ability to detect a treatment effect. To verify these properties, a promising approach is retrospective analysis of data from randomized clinical trials in which blood samples suitable for measurement of neurofilaments have been collected. Comparison of neurofilament levels between subgroups of patients enrolled in the trials would determine whether the drug tested had an effect on the neurofilament biomarker. Moreover, neurofilament levels could be correlated with all the other relevant clinical and paraclinical measures collected in the trial.
The main factors limiting the application of neurofilament measurements to disease monitoring individuals are the lack of normal values of neurofilament across all age groups, a detailed understanding of how comorbidities affect blood neurofilament measurements, and the need for thorough multicentre analytical assay validation to achieve standardized and reliable measurements across different sites. In light of the effect of ageing on neurofilament levels, generation of normative data in large collections of controls is a priority. Coordinated multicentre research activities are already ongoing to tackle these obstacles.
References
Deisenhammer, F. et al. EFNS guidelines on disease-specific CSF investigations. Eur. J. Neurol. 16, 760–770 (2009).
Petzold, A., Keir, G., Green, A. J., Giovannoni, G. & Thompson, E. J. A specific ELISA for measuring neurofilament heavy chain phosphoforms. J. Immunol. Methods 278, 179–190 (2003).
Petzold, A., Rejdak, K. & Plant, G. T. Axonal degeneration and inflammation in acute optic neuritis. J. Neurol. Neurosurg. Psychiatry 75, 1178–1180 (2004).
Gaiottino, J. et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS ONE 8, e75091 (2013).
Kuhle, J. et al. Serum neurofilament light chain is a biomarker of human spinal cord injury severity and outcome. J. Neurol. Neurosurg. Psychiatry 86, 273–279 (2015).
Gisslen, M. et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine 3, 135–140 (2016). This study was the first time that NfL was measured with a single-molecule array assay and showed that CSF and plasma concentrations were highly correlated, indicating that plasma NfL concentration is a promising marker in HIV-associated brain damage.
Kuhle, J. et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin. Chem. Lab. Med. 54, 1655–1661 (2016).
Disanto, G. et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 81, 857–870 (2017). This study was the first in which serum NfL levels in MS were measured with a Simoa assay; higher CSF and serum levels and clinical and MRI disease activity were associated with higher risk of relapses and disability worsening.
Yuan, A., Rao, M. V., Veeranna & Nixon, R. A. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb. Perspect. Biol. 9, a018309 (2017). This paper provides a comprehensive and detailed overview of the structure and biological roles of the neurofilament proteins.
Dyson, H. J. & Wright, P. E. Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 6, 197–208 (2005).
Petzold, A. Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J. Neurol. Sci. 233, 183–198 (2005).
Herrmann, H. & Aebi, U. Intermediate filaments: structure and assembly. Cold Spring Harb. Perspect. Biol. 8, a018242 (2016).
Nixon, R. A. & Sihag, R. K. Neurofilament phosphorylation: a new look at regulation and function. Trends Neurosci. 14, 501–506 (1991).
Beck, R., Deek, J. & Safinya, C. R. Structures and interactions in ‘bottlebrush’ neurofilaments: the role of charged disordered proteins in forming hydrogel networks. Biochem. Soc. Trans. 40, 1027–1031 (2012).
Barry, D. M. et al. Expansion of neurofilament medium C terminus increases axonal diameter independent of increases in conduction velocity or myelin thickness. J. Neurosci. 32, 6209–6219 (2012).
Rao, M. V. et al. The neurofilament middle molecular mass subunit carboxyl-terminal tail domains is essential for the radial growth and cytoskeletal architecture of axons but not for regulating neurofilament transport rate. J. Cell Biol. 163, 1021–1031 (2003).
Brown, R. H. Jr & Al-Chalabi, A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 377, 1602 (2017).
Rosengren, L. E., Karlsson, J. E., Karlsson, J. O., Persson, L. I. & Wikkelso, C. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J. Neurochem. 67, 2013–2018 (1996).
Norgren, N., Karlsson, J. E., Rosengren, L. & Stigbrand, T. Monoclonal antibodies selective for low molecular weight neurofilaments. Hybrid. Hybridomics 21, 53–59 (2002). This paper provides a seminal description of NfL‑specific monoclonal antibodies that are now used in most studies.
Norgren, N., Rosengren, L. & Stigbrand, T. Elevated neurofilament levels in neurological diseases. Brain Res. 987, 25–31 (2003).
Petzold, A. et al. In vivo monitoring of neuronal loss in traumatic brain injury: a microdialysis study. Brain 134, 464–483 (2011).
Zucchi, E. et al. A motor neuron strategy to save time and energy in neurodegeneration: adaptive protein stoichiometry. J. Neurochem. https://doi.org/10.1111/jnc.14542 (2018).
Petzold, A., Keir, G., Warren, J., Fox, N. & Rossor, M. N. A systematic review and meta-analysis of CSF neurofilament protein levels as biomarkers in dementia. Neurodegener. Dis. 4, 185–194 (2007).
Petzold, A. et al. Neurofilament ELISA validation. J. Immunol. Methods 352, 23–31 (2010). This study was an evaluation of the most widely used NfL ELISA kit across 35 different laboratories, and identified that a lack of accurate and consistent protein standards is the main reason for a poor inter-laboratory coefficient of variation.
Kuhle, J. et al. A highly sensitive electrochemiluminescence immunoassay for the neurofilament heavy chain protein. J. Neuroimmunol. 220, 114–119 (2010).
Kuhle, J. et al. Neurofilament heavy chain in CSF correlates with relapses and disability in multiple sclerosis. Neurology 76, 1206–1213 (2011).
Kuhle, J. et al. Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology 88, 826–831 (2017).
Rissin, D. M. et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 28, 595–599 (2010). This study provided the initial description of the high-sensitivity, fourth-generation Simoa assay technology that enables reliable quantification of NfL in serum and plasma samples.
Kuhle, J. et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult. Scler. 22, 1550–1559 (2016).
Piehl, F. et al. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult. Scler. 24, 1046–1054 (2018).
Bacioglu, M. et al. Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron 91, 56–66 (2016).
Wilke, C. et al. Neurofilament light chain in FTD is elevated not only in cerebrospinal fluid, but also in serum. J. Neurol. Neurosurg. Psychiatry 87, 1270–1272 (2016).
Weydt, P. et al. Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann. Neurol. 79, 152–158 (2016). This study of asymptomatic and symptomatic ALS mutation carriers and family controls showed that blood NfL levels are normal in the asymptomatic stage and increase at the time of early symptom onset, thereby linking NfL to the symptomatic phase of ALS.
Lu, C. H. et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 84, 2247–2257 (2015).
Meeter, L. H. et al. Neurofilament light chain: a biomarker for genetic frontotemporal dementia. Ann. Clin. Transl Neurol. 3, 623–636 (2016).
Novakova, L. et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 89, 2230–2237 (2017).
Martinez-Morillo, E. et al. Neurofilament medium polypeptide (NFM) protein concentration is increased in CSF and serum samples from patients with brain injury. Clin. Chem. Lab. Med. 53, 1575–1584 (2015).
Barro, C. et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 141, 2382–2391 (2018).
Yilmaz, A. et al. Neurofilament light chain protein as a marker of neuronal injury: review of its use in HIV-1 infection and reference values for HIV-negative controls. Expert. Rev. Mol. Diagn. 17, 761–770 (2017).
Reiber, H. Flow rate of cerebrospinal fluid (CSF) — a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J. Neurol. Sci. 122, 189–203 (1994).
Idland, A. V. et al. CSF neurofilament light levels predict hippocampal atrophy in cognitively healthy older adults. Neurobiol. Aging 49, 138–144 (2017).
Szaro, B. G. & Strong, M. J. Post-transcriptional control of neurofilaments: new roles in development, regeneration and neurodegenerative disease. Trends Neurosci. 33, 27–37 (2010).
Mattsson, N., Andreasson, U., Zetterberg, H. & Blennow, K. Alzheimer’s Disease Neuroimaging Initiative. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 74, 557–566 (2017). In this paper, plasma NfL was associated with Alzheimer disease diagnosis and cognitive performance, suggesting that NfL is a noninvasive biomarker for this disease.
Bischof, A. et al. Serum neurofilament light chain: a biomarker of neuronal injury in vasculitic neuropathy. Ann. Rheum. Dis. 77, 1093–1094 (2017).
Sandelius, A. et al. Plasma neurofilament light chain concentration in the inherited peripheral neuropathies. Neurology 90, e518–e524 (2018).
Brownlee, W. J., Hardy, T. A., Fazekas, F. & Miller, D. H. Diagnosis of multiple sclerosis: progress and challenges. Lancet 389, 1336–1346 (2017).
Lucchinetti, C. F. et al. Inflammatory cortical demyelination in early multiple sclerosis. N. Engl. J. Med. 365, 2188–2197 (2011).
Huizinga, R., Gerritsen, W., Heijmans, N. & Amor, S. Axonal loss and gray matter pathology as a direct result of autoimmunity to neurofilaments. Neurobiol. Dis. 32, 461–470 (2008).
Trapp, B. D. et al. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 338, 278–285 (1998).
Kuhlmann, T., Lingfeld, G., Bitsch, A., Schuchardt, J. & Bruck, W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain 125, 2202–2212 (2002).
Tallantyre, E. C. et al. Clinico-pathological evidence that axonal loss underlies disability in progressive multiple sclerosis. Mult. Scler. 16, 406–411 (2010).
Rocca, M. A. et al. Brain MRI atrophy quantification in MS: From methods to clinical application. Neurology 88, 403–413 (2017).
Lycke, J. N., Karlsson, J. E., Andersen, O. & Rosengren, L. E. Neurofilament protein in cerebrospinal fluid: a potential marker of activity in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 64, 402–404 (1998). This work identified for the first time that neurofilament levels in CSF could be a biomarker in MS.
Norgren, N. et al. Neurofilament and glial fibrillary acidic protein in multiple sclerosis. Neurology 63, 1586–1590 (2004).
Teunissen, C. E. et al. Combination of CSF N-acetylaspartate and neurofilaments in multiple sclerosis. Neurology 72, 1322–1329 (2009).
Khalil, M. et al. CSF neurofilament and N-acetylaspartate related brain changes in clinically isolated syndrome. Mult. Scler. 19, 436–442 (2013).
Arrambide, G. et al. Neurofilament light chain level is a weak risk factor for the development of MS. Neurology 87, 1076–1084 (2016).
Petzold, A. et al. CSF neurofilament levels: a potential prognostic marker in Guillain-Barre syndrome. Neurology 67, 1071–1073 (2006).
Petzold, A., Steenwijk, M. D., Eikelenboom, J. M., Wattjes, M. P. & Uitdehaag, B. M. Elevated CSF neurofilament proteins predict brain atrophy: a 15-year follow-up study. Mult. Scler. 22, 1154–1162 (2016).
Gunnarsson, M. et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann. Neurol. 69, 83–89 (2011). This longitudinal study showed a treatment effect of natalizumab on CSF levels of NfL in relapsing–remitting MS, regardless of previous treatment and whether patients experienced a relapse in the 3 months before natalizumab treatment.
Kuhle, J. et al. Fingolimod and CSF neurofilament light chain levels in relapsing-remitting multiple sclerosis. Neurology 84, 1639–1643 (2015).
Novakova, L. et al. Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of fingolimod efficacy in multiple sclerosis. Mult. Scler. 23, 62–71 (2017).
Novakova, L. et al. Cerebrospinal fluid biomarkers as a measure of disease activity and treatment efficacy in relapsing-remitting multiple sclerosis. J. Neurochem. 141, 296–304 (2017).
Axelsson, M. et al. Immunosuppressive therapy reduces axonal damage in progressive multiple sclerosis. Mult. Scler. 20, 43–50 (2014).
Romme Christensen, J. et al. Natalizumab in progressive MS: results of an open-label, phase 2A, proof-of-concept trial. Neurology 82, 1499–1507 (2014).
Malmestrom, C., Haghighi, S., Rosengren, L., Andersen, O. & Lycke, J. Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology 61, 1720–1725 (2003).
Rosengren, L. E., Karlsson, J. E., Sjogren, M., Blennow, K. & Wallin, A. Neurofilament protein levels in CSF are increased in dementia. Neurology 52, 1090–1093 (1999).
Sjogren, M. et al. Cytoskeleton proteins in CSF distinguish frontotemporal dementia from AD. Neurology 54, 1960–1964 (2000).
Olsson, B. et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 15, 673–684 (2016).
Mattsson, N. et al. Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer’s disease. EMBO Mol. Med. 8, 1184–1196 (2016).
Zetterberg, H. Neurofilament light: a dynamic cross-disease fluid biomarker for neurodegeneration. Neuron 91, 1–3 (2016).
Zetterberg, H. et al. Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol. 73, 60–67 (2015).
Rohrer, J. D. et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology 87, 1329–1336 (2016).
Skillback, T. et al. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology 83, 1945–1953 (2014).
Pijnenburg, Y. A., Verwey, N. A., van der Flier, W. M., Scheltens, P. & Teunissen, C. E. Discriminative and prognostic potential of cerebrospinal fluid phosphoTau/tau ratio and neurofilaments for frontotemporal dementia subtypes. Alzheimers Dement. (Amst.) 1, 505–512 (2015).
Andreasson, U., Blennow, K. & Zetterberg, H. Update on ultrasensitive technologies to facilitate research on blood biomarkers for central nervous system disorders. Alzheimers Dement. (Amst.) 3, 98–102 (2016).
Weston, P. S. J. et al. Serum neurofilament light in familial Alzheimer disease: a marker of early neurodegeneration. Neurology 89, 2167–2175 (2017).
Hansson, O. et al. Blood-based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology 88, 930–937 (2017).
Steinacker, P. et al. Neurofilament as a blood marker for diagnosis and monitoring of primary progressive aphasias. Neurology 88, 961–969 (2017).
Steinacker, P. et al. Neurofilaments in blood and CSF for diagnosis and prediction of onset in Creutzfeldt-Jakob disease. Sci. Rep. 6, 38737 (2016).
Nylen, K. et al. CSF -neurofilament correlates with outcome after aneurysmal subarachnoid hemorrhage. Neurosci. Lett. 404, 132–136 (2006).
Lewis, S. B., Wolper, R. A., Miralia, L., Yang, C. & Shaw, G. Detection of phosphorylated NF-H in the cerebrospinal fluid and blood of aneurysmal subarachnoid hemorrhage patients. J. Cereb. Blood Flow Metab. 28, 1261–1271 (2008).
Zanier, E. R. et al. Neurofilament light chain levels in ventricular cerebrospinal fluid after acute aneurysmal subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry 82, 157–159 (2011).
Traenka, C. et al. Serum neurofilament light chain levels are associated with clinical characteristics and outcome in patients with cervical artery dissection. Cerebrovasc. Dis. 40, 222–227 (2015).
Gattringer, T. et al. Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology 89, 2108–2114 (2017). This study provided evidence of a possible role for serum NfL as a marker of ongoing cerebral small vessel disease and for the occurrence of new vascular MRI lesions, even when these lesions are clinically silent.
Bergman, J. et al. Neurofilament light in CSF and serum is a sensitive marker for axonal white matter injury in MS. Neurol. Neuroimmunol. Neuroinflamm. 3, e271 (2016).
Blennow, K. et al. Traumatic brain injuries. Nat. Rev. Dis. Primers 2, 16084 (2016).
McKee, A. C. et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 131, 75–86 (2016).
Dekosky, S. T., Blennow, K., Ikonomovic, M. D. & Gandy, S. Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat. Rev. Neurol. 9, 192–200 (2013).
Zetterberg, H. et al. Neurochemical aftermath of amateur boxing. Arch. Neurol. 63, 1277–1280 (2006).
Neselius, S. et al. CSF-biomarkers in Olympic boxing: diagnosis and effects of repetitive head trauma. PLoS ONE 7, e33606 (2012).
Shahim, P. et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci. Rep. 6, 36791 (2016).
Al Nimer, F. et al. Comparative assessment of the prognostic value of biomarkers in traumatic brain injury reveals an independent role for serum levels of neurofilament light. PLoS ONE 10, e0132177 (2015).
Shahim, P., Zetterberg, H., Tegner, Y. & Blennow, K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 88, 1788–1794 (2017).
Oliver, J. M. et al. Serum neurofilament light in american football athletes over the course of a season. J. Neurotrauma 33, 1784–1789 (2016).
Al-Chalabi, A. et al. Amyotrophic lateral sclerosis: moving towards a new classification system. Lancet Neurol. 15, 1182–1194 (2016).
Brettschneider, J., Petzold, A., Sussmuth, S. D., Ludolph, A. C. & Tumani, H. Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology 66, 852–856 (2006).
Zetterberg, H., Jacobsson, J., Rosengren, L., Blennow, K. & Andersen, P. M. Cerebrospinal fluid neurofilament light levels in amyotrophic lateral sclerosis: impact of SOD1 genotype. Eur. J. Neurol. 14, 1329–1333 (2007).
Mendonca, D. M. et al. Neurofilament heavy subunit in cerebrospinal fluid: a biomarker of amyotrophic lateral sclerosis? Amyotroph. Lateral Scler. 12, 144–147 (2011).
McCombe, P. A. et al. Serial measurements of phosphorylated neurofilament-heavy in the serum of subjects with amyotrophic lateral sclerosis. J. Neurol. Sci. 353, 122–129 (2015).
Li, S. et al. Phosphorylated neurofilament heavy chain levels in paired plasma and CSF of amyotrophic lateral sclerosis. J. Neurol. Sci. 367, 269–274 (2016).
Steinacker, P. et al. Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. J. Neurol. Neurosurg. Psychiatry 87, 12–20 (2016).
De, S. M. et al. Comparison of elevated phosphorylated neurofilament heavy chains in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 89, 367–373 (2017).
Poesen, K. et al. Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology 88, 2302–2309 (2017).
Oeckl, P. P. et al. Multicenter validation of CSF neurofilaments as diagnostic biomarkers for ALS. Amyotroph. Lateral. Scler. Frontotemporal Degener. 17, 404–413 (2016).
Gendron, T. F. et al. Phosphorylated neurofilament heavy chain: a biomarker of survival for C9ORF72-associated amyotrophic lateral sclerosis. Ann. Neurol. 82, 139–146 (2017).
Lehmer, C. et al. Poly-GP in cerebrospinal fluid links C9orf72-associated dipeptide repeat expression to the asymptomatic phase of ALS/FTD. EMBO Mol. Med. 9, 859–868 (2017).
Feneberg, E. et al. Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology 90, e22–e30 (2018).
Boylan, K. B. et al. Phosphorylated neurofilament heavy subunit (pNF-H) in peripheral blood and CSF as a potential prognostic biomarker in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 84, 467–472 (2013).
Menke, R. A. et al. CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann. Clin. Transl Neurol. 2, 748–755 (2015).
Volkening, K., Leystra-Lantz, C., Yang, W., Jaffee, H. & Strong, M. J. Tar DNA binding protein of 43 kDa (TDP-43), 14-3-3 proteins and copper/zinc superoxide dismutase (SOD1) interact to modulate NFL mRNA stability. Implications for altered RNA processing in amyotrophic lateral sclerosis (ALS). Brain Res. 1305, 168–182 (2009).
Halbgebauer, S., Ockl, P., Wirth, K., Steinacker, P. & Otto, M. Protein biomarkers in Parkinson’s disease: focus on cerebrospinal fluid markers and synaptic proteins. Mov. Disord. 31, 848–860 (2016).
Eusebi, P. et al. Diagnostic utility of cerebrospinal fluid alpha-synuclein in Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 32, 1389–1400 (2017).
Holmberg, B., Rosengren, L., Karlsson, J. E. & Johnels, B. Increased cerebrospinal fluid levels of neurofilament protein in progressive supranuclear palsy and multiple-system atrophy compared with Parkinson’s disease. Mov. Disord. 13, 70–77 (1998).
Brettschneider, J. et al. Neurofilament heavy-chain NfH(SMI35) in cerebrospinal fluid supports the differential diagnosis of Parkinsonian syndromes. Mov. Disord. 21, 2224–2227 (2006).
Hall, S. et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch. Neurol. 69, 1445–1452 (2012).
Magdalinou, N. K. et al. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 86, 1240–1247 (2015).
Rojas, J. C. et al. Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Ann. Clin. Transl Neurol. 3, 216–225 (2016).
Byrne, L. M. et al. Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: a retrospective cohort analysis. Lancet Neurol. 16, 601–609 (2017).
Wild, E. J., Petzold, A., Keir, G. & Tabrizi, S. J. Plasma neurofilament heavy chain levels in Huntington’s disease. Neurosci. Lett. 417, 231–233 (2007).
Constantinescu, R., Romer, M., Oakes, D., Rosengren, L. & Kieburtz, K. Levels of the light subunit of neurofilament triplet protein in cerebrospinal fluid in Huntington’s disease. Parkinsonism Relat. Disord. 15, 245–248 (2009).
Niemela, V., Landtblom, A. M., Blennow, K. & Sundblom, J. Tau or neurofilament light -which is the more suitable biomarker for Huntington’s disease? PLoS ONE 12, e0172762 (2017).
Cousins, D. A. & Grunze, H. Interpreting magnetic resonance imaging findings in bipolar disorder. CNS Neurosci. Ther. 18, 201–207 (2012).
Jakobsson, J. et al. Elevated concentrations of neurofilament light chain in the cerebrospinal fluid of bipolar disorder patients. Neuropsychopharmacology 39, 2349–2356 (2014).
Isgren, A. et al. Markers of neuroinflammation and neuronal injury in bipolar disorder: relation to prospective clinical outcomes. Brain Behav. Immun. 65, 195–201 (2017).
Steinacker, P. et al. Diagnostic and prognostic significance of neurofilament light chain NF-L, but not progranulin and S100B, in the course of amyotrophic lateral sclerosis: Data from the German MND-net. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 18, 112–119 (2017).
Acknowledgements
M.O. is supported by the German Federal Ministry for Education and Research (German FTLD consortium), the Thierry Latran Foundation and the ALS association. H.Z. is supported by grants from the Swedish Research Council, the European Research Council and the Knut and Alice Wallenberg Foundation. K.B. is supported by grants from the Swedish Research Council, the Swedish Alzheimer Association, the Swedish Brain Foundation and the Torsten Söderberg Foundation. J.K. is supported by grants from the Swiss National Science Foundation (320030_160221). F.P. is supported by grants from the Swedish Research Council.
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khalil, M., Teunissen, C.E., Otto, M. et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 14, 577–589 (2018). https://doi.org/10.1038/s41582-018-0058-z
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-018-0058-z
This article is cited by
-
Cluster analysis dissecting cognitive deficits in older adults with major depressive disorder and the association with neurofilament light chain
BMC Geriatrics (2024)
-
Associations of plasma neurofilament light chain with cognition and neuroimaging measures in community-dwelling early old age men
Alzheimer's Research & Therapy (2024)
-
Associations of sleep disorders with serum neurofilament light chain levels in Parkinson’s disease
BMC Neurology (2024)
-
Correlation analysis between smoke exposure and serum neurofilament light chain in adults: a cross-sectional study
BMC Public Health (2024)
-
Study protocol: fish oil supplement in prevention of oxaliplatin-induced peripheral neuropathy in adjuvant colorectal cancer patients – a randomized controlled trial. (OxaNeuro)
BMC Cancer (2024)