Abstract
RORγt is a lineage-specifying transcription factor that is expressed by immune cells that are enriched in the gastrointestinal tract and promote immunity, inflammation and tissue homeostasis1,2,3,4,5,6,7,8,9,10,11,12,13,14,15. However, fundamental questions remain with regard to the cellular heterogeneity among these cell types, the mechanisms that control protective versus inflammatory properties and their functional redundancy. Here we define all RORγt+ immune cells in the intestine at single-cell resolution and identify a subset of group 3 innate lymphoid cells (ILC3s) that expresses ZBTB46, a transcription factor specifying conventional dendritic cells16,17,18,19,20. ZBTB46 is robustly expressed by CCR6+ lymphoid-tissue-inducer-like ILC3s that are developmentally and phenotypically distinct from conventional dendritic cells, and its expression is imprinted by RORγt, fine-tuned by microbiota-derived signals and increased by pro-inflammatory cytokines. ZBTB46 restrains the inflammatory properties of ILC3s, including the OX40L-dependent expansion of T helper 17 cells and the exacerbated intestinal inflammation that occurs after enteric infection. Finally, ZBTB46+ ILC3s are a major source of IL-22, and selective depletion of this population renders mice susceptible to enteric infection and associated intestinal inflammation. These results show that ZBTB46 is a transcription factor that is shared between conventional dendritic cells and ILC3s, and identify a cell-intrinsic function for ZBTB46 in restraining the pro-inflammatory properties of ILC3s and a non-redundant role for ZBTB46+ ILC3s in orchestrating intestinal health.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data necessary to understand and evaluate the conclusions of this paper are provided. Bulk RNA-seq data have been deposited in the Gene Expression Omnibus database under the accession number GSE181865 and scRNA-seq data have been deposited under the accession number GSE181864. Source data are provided with this paper.
References
Eberl, G. et al. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Eberl, G. & Littman, D. R. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science 305, 248–251 (2004).
Ivanov, I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Ivanov, I. I., Zhou, L. & Littman, D. R. Transcriptional regulation of Th17 cell differentiation. Semin. Immunol. 19, 409–417 (2007).
Luci, C. et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 10, 75–82 (2009).
Zhou, L. & Littman, D. R. Transcriptional regulatory networks in Th17 cell differentiation. Curr. Opin. Immunol. 21, 146–152 (2009).
Sanos, S. L. et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 10, 83–91 (2009).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Martin, B., Hirota, K., Cua, D. J., Stockinger, B. & Veldhoen, M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity 31, 321–330 (2009).
Sutton, C. E. et al. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 (2009).
Buonocore, S. et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375 (2010).
Sawa, S. et al. Lineage relationship analysis of RORγt+ innate lymphoid cells. Science 330, 665–669 (2010).
Ohnmacht, C. et al. The microbiota regulates type 2 immunity through RORγt+ T cells. Science 349, 989–993 (2015).
Sefik, E. et al. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science 349, 993–997 (2015).
Eberl, G. RORγt, a multitask nuclear receptor at mucosal surfaces. Mucosal Immunol. 10, 27–34 (2017).
Satpathy, A. T. et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J. Exp. Med. 209, 1135–1152 (2012).
Meredith, M. M. et al. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J. Exp. Med. 209, 1153–1165 (2012).
Satpathy, A. T., Wu, X., Albring, J. C. & Murphy, K. M. Re(de)fining the dendritic cell lineage. Nat. Immunol. 13, 1145–1154 (2012).
Merad, M., Sathe, P., Helft, J., Miller, J. & Mortha, A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 31, 563–604 (2013).
Cabeza-Cabrerizo, M. et al. Dendritic cells revisited. Annu. Rev. Immunol. 39, 131–166 (2021).
Hepworth, M. R. et al. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science 348, 1031–1035 (2015).
Zhou, L. et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature 568, 405–409 (2019).
Teng, F. et al. A circadian clock is essential for homeostasis of group 3 innate lymphoid cells in the gut. Sci. Immunol. 4, eaax1215 (2019).
Fujino, S. et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52, 65–70 (2003).
Brown, C. C. et al. Transcriptional basis of mouse and human dendritic cell heterogeneity. Cell 179, 846–863 (2019).
Schraml, B. U. et al. Genetic tracing via DNGR-1 expression history defines dendritic cells as a hematopoietic lineage. Cell 154, 843–858 (2013).
Pokrovskii, M. et al. Characterization of transcriptional regulatory networks that promote and restrict identities and functions of intestinal innate lymphoid cells. Immunity 51, 185–197 (2019).
Britanova, L. & Diefenbach, A. Interplay of innate lymphoid cells and the microbiota. Immunol. Rev. 279, 36–51 (2017).
Chen, W. Y. et al. Inhibition of the androgen receptor induces a novel tumor promoter, ZBTB46, for prostate cancer metastasis. Oncogene 36, 6213–6224 (2017).
Kugathasan, S. et al. Loci on 20q13 and 21q22 are associated with pediatric-onset inflammatory bowel disease. Nat. Genet. 40, 1211–1215 (2008).
Meredith, M. M. et al. Zinc finger transcription factor zDC is a negative regulator required to prevent activation of classical dendritic cells in the steady state. J. Exp. Med. 209, 1583–1593 (2012).
Castellanos, J. G. et al. Microbiota-induced TNF-like ligand 1A drives group 3 innate lymphoid cell-mediated barrier protection and intestinal T cell activation during colitis. Immunity 49, 1077–1089 (2018).
Gajdasik, D. W. et al. Th1 responses in vivo require cell-specific provision of OX40L dictated by environmental cues. Nat. Commun. 11, 3421 (2020).
Yao, C. et al. Prostaglandin E2–EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat. Med. 15, 633–640 (2009).
Napolitani, G., Acosta-Rodriguez, E. V., Lanzavecchia, A. & Sallusto, F. Prostaglandin E2 enhances Th17 responses via modulation of IL-17 and IFN-γ production by memory CD4+ T cells. Eur. J. Immunol. 39, 1301–1312 (2009).
Vély, F. et al. Evidence of innate lymphoid cell redundancy in humans. Nat. Immunol. 17, 1291–1299 (2016).
Rankin, L. C. et al. Complementarity and redundancy of IL-22-producing innate lymphoid cells. Nat. Immunol. 17, 179–186 (2016).
Song, C. et al. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J. Exp. Med. 212, 1869–1882 (2015).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 (2008).
Sonnenberg, G., Monticelli, L., Elloso, M., Fouser, L. & Artis, D. CD4+ lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34, 122–134 (2011).
Ota, N. et al. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat. Immunol. 12, 941–948 (2011).
Dudakov, J. A., Hanash, A. M. & van den Brink, M. R. Interleukin-22: immunobiology and pathology. Annu. Rev. Immunol. 33, 747–785 (2015).
Basu, R. et al. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37, 1061–1075 (2012).
Leppkes, M. et al. RORγ-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology 136, 257–267 (2009).
Bernink, J. H. et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 14, 221–229 (2013).
Wang, Y. et al. ZBTB46 is a shear-sensitive transcription factor inhibiting endothelial cell proliferation via gene expression regulation of cell cycle proteins. Lab. Invest. 99, 305–318 (2019).
Loschko, J. et al. Inducible targeting of cDCs and their subsets in vivo. J. Immunol. Methods 434, 32–38 (2016).
Caton, M. L., Smith-Raska, M. R. & Reizis, B. Notch–RBP-J signaling controls the homeostasis of CD8− dendritic cells in the spleen. J. Exp. Med. 204, 1653–1664 (2007).
Ahlfors, H. et al. IL-22 fate reporter reveals origin and control of IL-22 production in homeostasis and infection. J. Immunol. 193, 4602–4613 (2014).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Choi, G. B. et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 (2016).
Finotto, S. et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science 295, 336–338 (2002).
Kühn, R., Löhler, J., Rennick, D., Rajewsky, K. & Müller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 (1993).
Lee, P. P. et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15, 763–774 (2001).
Loschko, J. et al. Absence of MHC class II on cDCs results in microbial-dependent intestinal inflammation. J. Exp. Med. 213, 517–534 (2016).
Schlenner, S. M. et al. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity 32, 426–436 (2010).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Hadley, W. ggplot2: Elegant Graphics for Data Analysis (Springer, 2016).
Dodt, M., Roehr, J. T., Ahmed, R. & Dieterich, C. FLEXBAR—flexible barcode and adapter processing for next-generation sequencing platforms. Biology 1, 895–905 (2012).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 47, e47 (2019).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Robinson, J. T., Thorvaldsdóttir, H., Wenger, A. M., Zehir, A. & Mesirov, J. P. Variant review with the Integrative Genomics Viewer. Cancer Res. 77, e31–e34 (2017).
Acknowledgements
We thank members of the G.F.S. laboratory for discussions and critical reading of the manuscript. Research in the G.F.S. laboratory is supported by the National Institutes of Health (R01AI143842, R01AI123368, R01AI145989, U01AI095608, R21CA249274, R01AI162936 and R01CA274534); an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund; the Meyer Cancer Center Collaborative Research Initiative; The Dalton Family Foundation; and Linda and Glenn Greenberg. W.Z. is supported by a fellowship from the Crohn’s and Colitis Foundation (831404). L.Z. is supported by a fellowship from the Crohn’s and Colitis Foundation (608975). G.F.S. is a CRI Lloyd J. Old STAR. We thank the Epigenomics Cores of Weill Cornell Medicine and G. Putzel for bioinformatics assistance; S. Mozumder for technical assistance; and J. Conrad for administrative assistance. The JRI IBD Live Cell Bank is supported by the JRI, Jill Roberts Center for IBD, Cure for IBD, the Rosanne H. Silbermann Foundation, the Sanders Family and Weill Cornell Medicine Division of Pediatric Gastroenterology and Nutrition.
Author information
Authors and Affiliations
Consortia
Contributions
W.Z. and G.F.S. conceived the project. W.Z. performed most experiments and analysed the data. L.Z., J.Z. and C.C. helped with experiments and data analyses. R.E.S. and JRI Live Cell Bank contributed to the acquisition and processing of clinical samples. C.Z. performed bioinformatic analyses. G.E. provided essential mouse models, scientific advice and expertise. W.Z. and G.F.S. wrote the manuscript, with input from all of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Bart Lambrecht and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
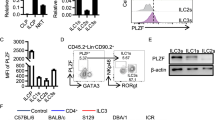
Extended Data Fig. 1 Gating of immune cells from the large intestine of mice.
a. Cells were isolated from the large intestine lamina propria of RORγtEGFP reporter mice. Two populations, TCRβ+ cells and TCRβ− cells, of RORγt+ immune cells were sorted separately and equally mixed at a 1:1 ratio, permitting enrichment of non-T lymphocytes populations. b. Gating strategy for different immune cells with surface markers. Lineage markers included: CD3, CD5, NK1.1, TCRγδ, Ly6G. B cells were gated as CD45+CD19+; Macrophages (Mac) were gated as CD45+CD64+MHCII+; cDCs were gated as CD45+Lin−CD64−CD11hiMHCIIhi. CD4+ T cells were gated as CD45+Lin+CD4+; ILC2s were gated as CD45+Lin−CD64−CD11c−CD127+CD90+KLRG1+; ILC3s were gated as CD45dimLin−CD64−CD11c−CD127+CD90hiKLRG1−; ILC3s were further gated as CCR6+, NKp46+ or DN subset. c. Gating strategy for different immune cells with transcription factors. Lineage 1: CD3, CD5, NK1.1, TCRγδ, Ly6G. Lineage 2: F4/80 and B220. cDCs were gated as CD45+Lin−CD11hiMHCIIhi; TH17 cells were gated as CD45+Lin+CD4+FoxP3−RORγt+; ILC3s were gated as CD45+Lin−CD11c−CD127+CD90+RORγt+, and further gated as CCR6+, T-bet+, or DN; ILC2s were gated as CD45+Lin−CD11c−CD127+CD90+RORγt−T-bet−.
Extended Data Fig. 2 RORγt expression among ILCs and cDCs.
a. Representative flow cytometry plots and quantifications of RORγt+ cells in cDCs and ILCs in the large intestine (LI) (n = 3 mice) or spleen of C57BL/6 mice by protein staining (n = 4 mice). ILCs were gated as CD45+Lin−CD11c−CD127+CD90.2+. b. Representative flow cytometry plots and quantifications of RORγt-EGFP+ cells in cDCs and ILCs in RORγtEGFP reporter mice (n = 3 mice). c. Representative flow cytometry plots and quantifications of YFP+ cells in cDCs and ILCs in RorcCreROSA26lsl-EYFP fate-mapping mice (n = 3 mice). d. Representative flow cytometry plots of RORγt-EGFP+ cells in endothelial cells in RORγtEGFP reporter mice. Data are representative of two or three independent experiments and shown as the means ± S.E.M in a-c.
Extended Data Fig. 3 ZBTB46 is significantly enriched in CCR6+ LTi-like ILC3s from the intestine.
a. Quantifications of frequencies of ZBTB46–GFP+ cells in indicated ILC3 subsets in the large intestine (LI) or small intestine (SI) of Zbtb46GFP/+ mice (n = 4 mice for both LI and SI). b-d. Unbiased gating for ZBTB46–GFP+ cells from the large intestine of Zbtb46GFP/+ mice. c. Quantification of the frequency of ZBTB46–GFP+ cDCs or ILC3s in total CD45+ immune cells from the large intestine of Zbtb46GFP/+ mice (n = 4 mice). d. Quantification of the frequency of ZBTB46–GFP+ ILC3 subsets from the large intestine of Zbtb46GFP/+ mice (n = 4 mice). e. Representative flow plots and quantifications of frequencies of Zbtb46Cre fate-mapped cells in indicated ILC3 subsets in the large intestine (LI) or small intestine (SI) of Zbtb46CreROSA26lsl-EYFP mice (n = 4 for both LI and SI). f. Representative flow histograms and quantifications of ZBTB46 MFI in indicated ILC3 subsets from the large intestine (LI) or small intestine (SI) of C57BL/6 or Zbtb46GFP/GFP mice (n = 3 for both LI and SI). The dash line indicates ZBTB46 MFI in CCR6+ ILC3s from Zbtb46GFP/GFP. Data are representative of two or three independent experiments and shown as the means ± S.E.M. Statistics are calculated by One-way ANOVA with Dunnett’s multiple comparisons in a, d, e, and f, two-tailed unpaired Student’s t-test in c.
Extended Data Fig. 4 ZBTB46 is expressed by ILC3s in mucosal and lymphoid tissues.
a. Representative flow plots of ZBTB46–GFP+ cells in γδT cells in the large intestine of C57BL/6 or Zbtb46GFP/+ mice (n = 4 mice). b. Representative flow cytometry histogram and quantification of ZBTB46 MFI in indicated T cell subsets from the large intestine of C57BL/6 or Zbtb46GFP/GFP mice (n = 3 mice). The dash line indicates ZBTB46 MFI in CD4+ T cells from Zbtb46GFP/GFP. c. Quantification of ZBTB46–GFP+ cell frequencies of indicated cells in the small intestine (SI), mesenteric lymph nodes (mLN), periphery lymph nodes (pLN), Spleen, Lung, Fat, Skin, and salivary gland of Zbtb46GFP/+ mice (n = 4 mice for all tissues). d. Quantification of ZBTB46–GFP+ cell frequencies of ILC3 subsets in the large intestine of Zbtb46GFP/+ female or male mice (n = 8 or 5 mice per group for all subsets). Data are representative of two independent experiments a-c, and data are pooled from 2 individual experiments in d. Data are shown as the means ± S.E.M.in b-d, means ± S.D. in a. Statistics are calculated by One-way ANOVA with Dunnett’s multiple comparisons in c and by two-tailed unpaired Student’s t-test in d.
Extended Data Fig. 5 Gating strategy for different immune cells in human intestine.
a. Lineage markers included: CD19, CD94, CD14, CD123, FcεR1a, CD34. B cells were gated as CD45+CD19+CD3−; Macrophages (Mac) were gated as CD45+CD19−CD3−CD64+MHCII+; cDCs were gated as CD45+CD19−CD3−CD64−CD11hiMHCIIhi. CD4+ T cells were gated as CD45+CD3+CD4+; ILC3s were gated as CD45+Lin−CD3−CD127+CD117+. b,c. Representative flow cytometry histogram of ZBTB46 staining and quantification of ZBTB46 MFI in indicated cells from human tonsils (n = 4 individual donors per group). FMO: full staining minus one; Isotype: Isotype control. Data are representative of two independent experiments in b,c, and shown as the means ± S.E.M. Statistics are calculated by One-way ANOVA with Dunnett’s multiple comparisons.
Extended Data Fig. 6 ZBTB46+ ILC3s and cDCs are distinct cell lineages.
a. Representative flow cytometry plots showing the YFP+ cells in cDCs and CCR6+ ILC3s from the large intestine of indicated fate-mapping mice. b. Schematic of progenitor cell transfer. pre-DC or CHILP were sort-purified from the bone marrow of Zbtb46GFP/+ and transferred into Rag2−/−Il2rg−/− recipient mice. Six weeks later, ILC3s and ZBTB46–GFP expression were analysed in the intestine of recipient mice. PBS injection was used as a negative control. pre-DCs were gated as: (Ter119, CD19, B220, CD3, CD5, NK1.1, Ly6G, CD11b)−CD127−MHCII−CD117−CD16/32−CD11c+Flt3+GFP+; CHILP were gated as (Ter119, B220, CD19, NK1.1, CD3, CD5, CD11b, CD11c, Ly6G)−CD45+CD127+α4β7+Flt3−CD25−CD117+. c. Representative flow plots of recipient mice injected with indicated progenitor cells. γc staining was used to differentiate the host-derived and recipient-derived ILCs. d. Gating strategies for progenitors and LTi cells from the fetal tissue. e. RORγt (RORE) motif analysis of the Zbtb46 locus in ATAC-seq data from CCR6+ ILC3s. f. Zbtb46 expression relative to Hprt in sort-purified CCR6+ ILC3s with or without exposure to the RORγt inhibitor GSK805 (n = 11 mice). g. Quantification of ZBTB46 MFI in CCR6+ or CCR6− ILC3s from the large intestine of indicated mice (n = 6 or 5 mice per group for both subsets). h. Quantification of cell numbers of ZBTB46–GFP+ cells in indicated ILC3 subsets from the large intestine of water or ABX-treated Zbtb46GFP/+ mice (n = 5 mice for all subsets). i. Correlation analysis of ZBTB46 expression and disease scores in patients with ulcerative colitis. Data are pooled from three or two individual experiments in f and g, data are representative of two independent experiments in h. Data are shown as means ± S.E.M in g and h. Statistics are calculated by two-tailed paired Student’s t-test in f, two-tailed unpaired Student’s t-test in g and h, and the correlation is determined by simple linear regression in i.
Extended Data Fig. 7 Comparison of naive RorcCreZbtb46f/f mice relative to littermates.
a. Representative flow cytometry histograms and quantification of ZBTB46 MFI in double-negative (DN) or T-bet+ ILC3s from the large intestine of indicated mice (n = 4 mice per group for both subsets). b. Representative flow cytometry histograms of ZBTB46 in cDCs and different T cell subsets in the large intestine of indicated mice. c. Quantification of geometric MFI of ZBTB46 in cDCs and CD4+ T cells (n = 8 or 7 mice per group for both cell types). d. The frequency of cDCs in the large intestine of indicated mice (n = 8 or 7 mice per group). e. Quantification of CCR6+ ILC3 cell numbers in the large intestine of indicated mice (n = 5 mice per group). f. Quantification of the frequencies of IL-22+ ILC3s in indicated mice (n = 8 or 7 mice per group). g. Quantification of the frequencies of IL-17A+ ILC3s in indicated mice (n = 4 or 5 mice per group). h. Quantification of the cell numbers of IL-22+ ILC3s in indicated mice (n = 4 mice per group). i. Quantification of the cell numbers of IL-17A+ ILC3s (n = 4 or 5 mice per group) in indicated mice. j,k. Representative flow plots and quantification of the frequencies of MHCII+ CCR6+ ILC3s in the large intestine of indicated mice (n = 5 mice per group). l. Quantification of MHCII MFI in CCR6+ ILC3s in indicated mice (n = 5 mice per group). m. Quantification of mLN weight and the numbers of Peyer’s patches in indicated mice (n = 9 mice per group). n. Go pathway analysis of the upregulated genes in the RorcCreZbtb46f/f mice compared to Zbtb46f/f mice. o. Quantification of the frequencies of COX-2+ (n = 4 or 5 mice per group) or IL-6 MFI (n = 4 or 5 mice per group) in ILC3s in the large intestine of indicated mice. p. Zbtb46 motif analysis of the Tnfsf4 and Ptgs2 loci in ATAC-seq data from CCR6+ ILC3s. Data are representative of three independent experiments in a, b, e, g-l, o. Data are pooled from two independent experiments in c, d, f, m. Data are shown as the means ± S.E.M. Statistics are calculated by two-tailed unpaired Student’s t-test.
Extended Data Fig. 8 ZBTB46 regulates ILC3s in a cell-intrinsic manner.
a. Schematic of mixed bone marrow (BM) chimera. The BM of CD45.2 Zbtb46GFP/GFP and CD45.1 C57BL/6 mice was equally mixed and transferred into Rag2−/−Il2rg−/− recipient mice. Mice were analysed 6–8 weeks after transplantation. b,c. Representative flow plots of the composition of ILC3s or ILC2s, and quantification of the ratio of CD45.2 to CD45.1 cells in ILC3s or ILC2s in the large intestine of recipient mice (n = 4 mice). d. Representative flow plots of the composition of CD4+ T cells in non-transplanted (control) or BM chimera mice. e. Quantification of the frequencies of indicated CD4+ T cells in the large intestine of BM chimera mice (n = 5 mice). f. Quantification of the frequencies of indicated RORγt+ CD4+ T cells in the large intestine of BM chimera mice (n = 5 mice). g. Quantification of the frequencies of IL-17A+ CD4+ T cells in indicated congenic T cells of BM chimera mice (n = 4 mice). h. Representative flow cytometry histograms of ZBTB46 in CD4+ T cells in the large intestine of indicated mice. i. Quantification of the frequencies of TH17 cells in indicated mice (n = 6 or 3 mice per group). j. Quantification of the frequencies of IL-17A+ CD4+ T cells in indicated mice (n = 6 or 3 mice per group). k-s, Mice were orally infected with C. rodentium for two weeks prior to analysis. k. Representative flow cytometry histograms of ZBTB46 in cDCs and different T cell subsets. l. Quantification of the frequencies of ILC3 subsets in the large intestine of indicated mice (n = 5 or 4 mice per group for both subsets). m,n. Quantification of the frequencies of Ki67+ ILC3 (n = 4 or 5 mice per group) and Ki67+ CCR6+ ILC3s (n = 4 or 5 mice per group) in the large intestine of indicated mice. o-r, Quantification of the frequencies of IL-22+ (n = 5 mice per group), GMCSF+ (n = 4 or 5 mice per group), IL-17A+ (n = 4 or 5 mice per group), and IL-17F+ (n = 4 or 5 mice per group) ILC3s in the large intestine of indicated mice. s. Quantification of the frequencies of IFNγ+ CD4+ T cells in the large intestine of indicated mice (n = 6 or 5 mice per group). Data are representative of two or three independent experiments in b-s. Data are shown as the means ± S.E.M. Statistics are calculated by two-tailed unpaired Student’s t-test.
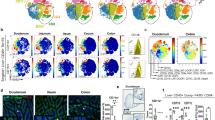
Extended Data Fig. 9 ZBTB46+ ILC3s are a dominant source of tissue-protective IL-22.
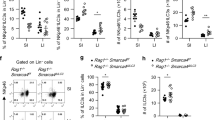
a. UMAPs show the expression of Il22 or Zbtb46 in all clusters of RORγt+ immune cells from the large intestine of RORγtEGFP mice. The dash circle indicates IL-22+ ILC3s. b. Representative flow plot of IL-22 and ZBTB46 expression in the large intestine of Zbtb46GFP/+ mice. c. Quantification of CCR6+ ILC3s in total Il22-YFP+ cells from the large intestine of Il22CreROSA26lsl-EYFP mice (n = 4 mice). d. Representative flow plots presenting unbiased gating for Il22-YFP+ cells in the large intestine of Il22CreROSA26lsl-EYFP fate-mapping mice. Lineage 1: CD3, CD5, NK1.1, TCRγδ, Ly6G. Lineage 2: F4/80 and B220. e. Representative flow plots and quantification of CCR6+ cells in the large intestine of indicated mice. Cells were gated from Lineage−CD90.2+CD127+ cells (n = 4 or 5 mice per group). f. Quantification of the frequencies of cDCs in the large intestine of indicated mice (n = 5 mice per group). g. Quantification of the frequencies of RORγt+ CD4+ T Cells in the large intestine of indicated mice (n = 9 or 8 mice per group). h, i. Representative flow plots and quantification of TH17 or IL-17A+ CD4+ T cells in the large intestine of indicated mice (n = 5 mice per group). j,k. Representative flow plots and quantification of the frequencies of RORγt+ γδ T cells in the large intestine of indicated mice (n = 9 or 8 mice per group). l. Representative flow plots and quantification of the frequencies of IL-22+ cells in the large intestine of indicated mice (n = 4 or 5 mice per group). Cells were gated from Lineage−CD90.2+CD127+ cells. m. Quantification of the frequencies IL-22+ ILC3s in the large intestine of indicated mice (n = 4 or 5 mice per group). Cells were gated from Lineage−CD90.2+CD127+RORγt+ ILC3s. n. Quantification of the frequencies IL-22+ CD4+ T cells in the large intestine of indicated mice (n = 4 or 5 mice per group). o-q. Mice were orally infected with C. rodentium for two weeks prior to analysis. o. Quantification of the frequencies of CCR6+ ILCs in the large intestine of indicated mice (n = 5 or 3 mice per group). Cells were gated from Lineage−CD90.2+CD127+ cells. p. Quantification of survival rate of indicated mice following C. rodentium infection (n = 21 or 23 mice per group). q. Quantification of the frequencies of RORγt+ γδ T cells out of total CD45+ cells in the large intestine of indicated mice (n = 10 or 6 mice per group). Data are representative of two independent experiments in b-d, and three independent experiments in e,f, h, i, l-o. Data are pooled from two independent experiments in g, k, and q. Data are pooled from four independent experiments in p. Data are shown as the means ± S.E.M. in c, e, f, g, i, k-o, q, and means ± S.D. in h. Statistics are calculated by two-tailed unpaired Student’s t-test in e-g, i, k-o, q, and by Log-rank (Mantel–Cox) test in p.
Extended Data Fig. 10 Regulation and function of ZBTB46+ ILC3s.
a. Representative flow cytometry histogram of RORγt expression in CD4+ ILCs from the large intestine of indicated mice. Cells were gated from Lineage−CD90.2+CD127+CD4+ cells. b,c. Representative flow cytometry histogram of ZBTB46 expression and quantification of ZBTB46 MFI in CD4+ ILCs from the large intestine of indicated mice (n = 4 or 5 mice per group). Cells were gated from Lineage−CD90.2+CD127+CD4+ cells. d. Representative flow cytometry histogram of RORγt expression in CCR6+ ILC3s from the large intestine of indicated mice. Cells were gated from Lineage−CD90.2+CD127+RORγt+CCR6+ cells. e,f. Representative flow cytometry histogram of ZBTB46 expression and quantification of ZBTB46 MFI in CCR6+ ILC3s from the large intestine of indicated mice (n = 4 or 5 mice per group). Cells were gated from Lineage−CD90.2+CD127+RORγt+CCR6+ cells. Data are representative of two independent experiments in a-f. Data are shown as the means ± S.E.M. Statistics are calculated by two-tailed unpaired Student’s t-test. g. ZBTB46 defines and regulates ILC3s that protect the intestine.
Supplementary information
Supplementary Table 1
Clinical metadata on paediatric patients with IBD and matched controls.
Supplementary Table 2
Differentially expressed genes among Zbtb46-deficient CCR6+ ILC3s and controls.
Source data
Rights and permissions
About this article
Cite this article
Zhou, W., Zhou, L., Zhou, J. et al. ZBTB46 defines and regulates ILC3s that protect the intestine. Nature 609, 159–165 (2022). https://doi.org/10.1038/s41586-022-04934-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04934-4
This article is cited by
-
Group 3 innate lymphoid cells in intestinal health and disease
Nature Reviews Gastroenterology & Hepatology (2024)
-
The emerging family of RORγt+ antigen-presenting cells
Nature Reviews Immunology (2024)
-
Metabolic control of innate lymphoid cells in health and disease
Nature Metabolism (2022)
-
ZBTB46+ ILC3s promote intestinal health
Nature Reviews Gastroenterology & Hepatology (2022)
-
Novel antigen-presenting cell imparts Treg-dependent tolerance to gut microbiota
Nature (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.