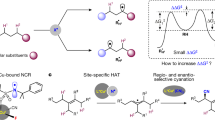
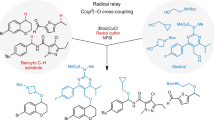
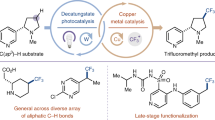
Abstract
Cytochrome P450 enzymes are known to catalyse bimodal oxidation of aliphatic acids via radical intermediates, which partition between pathways of hydroxylation and desaturation1,2. Developing analogous catalytic systems for remote C–H functionalization remains a significant challenge3,4,5. Here, we report the development of Cu(I)-catalysed bimodal dehydrogenation/lactonization reactions of synthetically common N-methoxyamides through radical abstractions of the γ-aliphatic C–H bonds. The feasibility of switching from dehydrogenation to lactonization is also demonstrated by altering reaction conditions. The use of a readily available amide as both radical precursor and internal oxidant allows for the development of redox-neutral C–H functionalization reactions with methanol as the sole side product. These C–H functionalization reactions using a Cu(I) catalyst with loading as low as 0.5 mol.% is applied to the diversification of a wide range of aliphatic acids including drug molecules and natural products. The exceptional compatibility of this catalytic system with a wide range of oxidatively sensitive functionality demonstrates the unique advantage of using a simple amide substrate as a mild internal oxidant.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Crystallographic data for compounds B63, B64, C27, C86, C101 and C102, as well as for derivatives of B62 (labelled as B62-ketone) and B65 (labelled as B65-Ac) are available in the Supplementary Information files and from the Cambridge Crystallographic Data Center under reference numbers CCDC 2279927, CCDC 2271734, CCDC 2271733, CCDC 2271730, CCDC 2271732, CCDC 2271731, CCDC 2296322and CCDC 2296327, respectively. All other data supporting the findings of this study are available in the Article and its Supplementary Information.
References
Rettie, A. E., Rettenmeier, A. W., Howald, W. N. & Baillie, T. A. Cytochrome P-450-catalyzed formation of Δ4-VPA, a toxic metabolite of valproic acid. Science 235, 890–893 (1987).
Zhou, J. et al. Spectroscopic studies of substrate interactions with clavaminate synthase 2, a multifunctional a-KG-dependent non-heme iron enzyme: correlation with mechanisms and reactivities. J. Am. Chem. Soc. 123, 7388–7398 (2001).
Kim, C., Dong, Y. & Que, L. Jr. Modeling nonheme diiron enzymes: hydrocarbon hydroxylation and desaturation by a high-valent Fe2O2 diamond core. J. Am. Chem. Soc. 119, 3635–3636 (1997).
Hull, J. F. et al. Manganese catalysts for C–H activation: an experimental/ theoretical study identifies the stereoelectronic factor that controls the switch between hydroxylation and desaturation pathways. J. Am. Chem. Soc. 132, 7605–7616 (2010).
Bigi, M. A., Reed, S. A. & White, M. C. Diverting non-haem iron catalyzed aliphatic C–H hydroxylations towards desaturations. Nat. Chem. 3, 216–222 (2011).
Larock, R. C. Comprehensive Organic Transformation (Wiley, 2018).
Ishihara, Y. & Baran, P. S. Two-phase terpene total synthesis: historical perspective and application to the Taxol® problem. Synlett 12, 1733–1745 (2010).
Qiu, Y. & Gao, S. Trends in applying C–H oxidation to the total synthesis of natural products. Nat. Prod. Rep. 33, 562–581 (2016).
Buist, P. H. Fatty acid desaturases: selecting the dehydrogenation channel. Nat. Prod. Rep. 21, 249–262 (2004).
Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, P. & Krska, S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Hong, B., Luo, T. & Lei, X. Late-stage diversification of natural products. ACS Cent. Sci. 6, 622–635 (2020).
Breslow, R. & Baldwin, S. W. Conversion of cholestanol to 12-oxocholestanol and to cholest-14-enol and-8(14)-enol by remote oxidation. J. Am. Chem. Soc. 92, 732–734 (1970).
Breslow, R. Biomimetic chemistry and artificial enzymes: catalysis by design. Acc. Chem. Res. 28, 146–153 (1995).
Groves, J. T., Nemo, T. E. & Myers, R. S. Hydroxylation and epoxidation catalyzed by iron-porphine complexes. Oxygen transfer from iodosylbenzene. J. Am. Chem. Soc. 101, 1032–1033 (1979).
Huang, X. & Groves, J. T. Beyond ferryl-mediated hydroxylation: 40 years of the rebound mechanism and C–H activation. J. Biol. Inorg. Chem. 22, 185–207 (2017).
Zhu, R.-Y., Farmer, M. E., Chen, Y.-Q. & Yu, J.-Q. A simple and versatile amide directing group for C–H functionalizations. Angew. Chem. Int. Ed. 55, 10578–10599 (2016).
Wang, D.-H., Wasa, M., Giri, R. & Yu, J.-Q. Pd(II)-catalyzed cross-coupling of sp3 C–H bonds with sp2 and sp3 boronic acids using air as the oxidant. J. Am. Chem. Soc. 130, 7190–7191 (2008).
Zard, S. Z. Recent progress in the generation and use of nitrogen-centred radicals. Chem. Soc. Rev. 37, 1603 (2008).
Fazekas, T. J. et al. Diversification of aliphatic C–H bonds in small molecules and polyolefins through radical chain transfer. Science 375, 545–550 (2022).
Davies, J., Svejstrup, T. D., Fernandez Reina, D. F., Sheikh, N. S. & Leonori, D. Visible-light-mediated synthesis of amidyl radicals: transition-metal-free hydroamination and N-arylation reactions. J. Am. Chem. Soc. 138, 8092–8095 (2016).
Davies, J., Morcillo, S. P., Douglas, J. J. & Leonori, D. Hydroxylamine derivatives as nitrogen-radical precursors in visible-light photochemistry. Chemistry 24, 12154–12163 (2018).
Jin, W. & Yu, S. Photoinduced and palladium-catalyzed remote desaturation of amide derivatives. Org. Lett. 23, 6931–6935 (2021).
Kwon, K., Simons, R. T., Nandakumar, M. & Roizen, J. L. Strategies to generate nitrogen-centered radicals that may rely on photoredox catalysis: development in reaction methodology and applications in organic synthesis. Chem. Rev. 122, 2353–2428 (2022).
Bach, R. D. & Schlegel, H. B. The bond dissociation energy of the N–O bond. J. Phys. Chem. A 125, 5014–5021 (2021).
Stateman, L. M., Dare, R. M., Paneque, A. N. & Nagib, D. A. Aza-heterocycles via copper-catalyzed, remote C–H desaturation of amines. Chem 8, 210–224 (2022).
Faulkner, A., Race, N. J., Scottb, J. S. & Bower, J. F. Copper catalyzed Heck-like cyclizations of oxime esters. Chem. Sci. 5, 2416–2421 (2014).
Kochi, J. K. The decomposition of peroxides catalyzed by copper compounds and the oxidation of alkyl radicals by cupric salts. J. Am. Chem. Soc. 85, 1958–1968 (1963).
Kochi, J. K. Mechanisms of organic oxidation and reduction by metal complexes: electron and ligand transfer processes form the basis for redox reactions of radicals and metal species. Science 155, 415–424 (1967).
Kochi, J. K., Bemis, A. & Jenkins, C. L. Mechanism of electron transfer oxidation of alkyl radicals by copper(II) complexes. J. Am. Chem. Soc. 90, 4616–4625 (1968).
Barton, D. H. R., Beckwith, A. L. J. & Goosen, A. Photochemical transformations. Part XVI. A novel synthesis of lactones. J. Chem. Soc. 181–190 (1965).
Neale, R. S., Marcus, N. L. & Schepers, R. G. The chemistry of nitrogen radicals. IV. The rearrangement of N-halamides and the synthesis of iminolactones. J. Am. Chem. Soc. 88, 3051–3058 (1966).
Chen, K., Richter, J. M. & Baran, P. S. 1,3-diol synthesis via controlled, radical-mediated C–H functionalization. J. Am. Chem. Soc. 130, 7247–7249 (2008).
Chen, K. & Baran, P. S. Total synthesis of eudesmane terpenes by site-selective C–H oxidations. Nature 459, 824–828 (2009).
Richers, J., Heilmann, M., Drees, M. & Tiefenbacher, K. Synthesis of lactones via C–H functionalization of nonactivated C(sp3)–H bonds. Org. Lett. 18, 6472–6475 (2016).
Hoste, J. On a new copper specific group. Anal. Chim. Acta 4, 23–27 (1950).
Ribas, X. et al. Aryl C–H activation by CuII to form an organometallic aryl-CuIII species: a novel twist on copper disproportionation. Angew. Chem. Int. Ed. 41, 2991–2994 (2002).
Xu, J. et al. Copper-catalyzed trifluoromethylation of terminal alkenes through allylic C–H bond activation. J. Am. Chem. Soc. 133, 15300–15303 (2011).
Wu, X., Riedel, J. & Dong, V. M. Transforming olefins into γ,δ-unsaturated nitriles through copper catalysis. Angew. Chem. Int. Ed. 56, 11589–11593 (2017).
Beckwith, A. L. J. & Zavitsas, A. A. Allylic oxidations by peroxy esters catalyzed by copper salts. The potential for stereoselective syntheses. J. Am. Chem. Soc. 108, 8230–8234 (1986).
Acknowledgements
We thank the Scripps Research Institute, National Institutes of Health (NIGMS, 2R01GM084019) for financial support. The content is solely our responsibility and does not necessarily represent the official views of the National Institutes of Health. We thank D. Strassfeld for proofreading and providing helpful suggestions in preparing the manuscript; Z. Li and Y.-K. Lin for proofreading supplementary information and repeating reactions; M. Gembicky and J. Bailey of the UCSD Crystallography Facility for X-ray crystallographic analysis; D.-H. Huang, L. Pasternack and G. Kroon of the Nuclear Magnetic Resonance Facility of the Scripps Researcher Services for their assistance with NMR analysis; and B. Webb and E. Billings of the Scripps Center for Metabolomics and Mass Spectrometry and Q. N. Wong of the Scripps Automated Synthesis Facility for assistance with mass spectrometry. This paper is dedicated to the memory of the late Dong-Hui Wang.
Author information
Authors and Affiliations
Contributions
J.-Q.Y. conceived the concept. S.Z. and Z.-J.Z. discovered and developed the dehydrogenation/lactonization reaction. S.Z. and Z.-J.Z. conducted the mechanistic studies. S.Z., Z.-J.Z. and J.-Q.Y. wrote the manuscript. J.-Q.Y. directed the project.
Corresponding author
Ethics declarations
Competing interests
J.-Q.Y., S.Z. and Z.-J.Z. are inventors on a patent application related to this work (US Patent application 63/605,065) filed by The Scripps Research Institute. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Continued Substrate scope for the dehydrogenation reaction.
Reaction conditions: A (0.1 mmol), CuF2 (10 mol%), AcOH (8 eq.) or CSA (0.5 eq.), dioxane (0.50 mL), at 100-125 °C for 2-20 h (see the SI for details). Isolated yields are reported. aL (20 mol%) was added. bThe solvent is DCE. rr is the ratio of γ,δ-alkene/β,γ-alkene.
Extended Data Fig. 2 Continued substrate scope for the lactonization reaction.
Reaction conditions: A (0.1 mmol), CuF2 (10 mol%) or [(CH3CN)4Cu]BF4 (10 mol%), TFA (5 eq.), dioxane (0.50 mL), at 125 °C for 1-20 h (see the SI for details). Isolated yields are reported. aThe solvent is dioxane/MeNO2 (0.25 mL/0.25 mL). aThe acid is TsOH•H2O (1 eq.). b0.5 eq. CSA was used. c2.5 eq. TFA was used. d.r. = diastereomer ratio, see the SI for details.
Extended Data Fig. 3 Mechanistic studies.
(a) Investigation of Cu(I) generated in-situ. (b) Radical clock experiment. (c) Inverted regioselectivity of elimination from a cationic intermediate. See the SI for details.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19, Tables 1–8 and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, S., Zhang, ZJ. & Yu, JQ. Copper-catalysed dehydrogenation or lactonization of C(sp3)–H bonds. Nature 629, 363–369 (2024). https://doi.org/10.1038/s41586-024-07341-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07341-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.