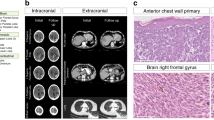
Abstract
Patients with high-risk neuroblastoma generally present with widely metastatic disease and often relapse despite intensive therapy. As most studies to date focused on diagnosis-relapse pairs, our understanding of the genetic and clonal dynamics of metastatic spread and disease progression remain limited. Here, using genomic profiling of 470 sequential and spatially separated samples from 283 patients, we characterize subtype-specific genetic evolutionary trajectories from diagnosis through progression and end-stage metastatic disease. Clonal tracing timed disease initiation to embryogenesis. Continuous acquisition of structural variants at disease-defining loci (MYCN, TERT, MDM2-CDK4) followed by convergent evolution of mutations targeting shared pathways emerged as the predominant feature of progression. At diagnosis metastatic clones were already established at distant sites where they could stay dormant, only to cause relapses years later and spread via metastasis-to-metastasis and polyclonal seeding after therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
For all the WGS samples (n = 247), somatic mutation calls for SNVs, Indels, SVs and CNAs are available under controlled access at https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs003111.v1.p1. Gene/transcript level read counts for all RNA-seq samples (n = 210) are available under controlled access at https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs003111.v1.p1. Somatic mutation calls and CNAs for all MSK-IMPACT samples (n = 223) are available in Supplementary Table 2 and at https://www.cbioportal.org/study/summary?id=nbl_msk_2023. Due to lack of patient consent to data sharing, raw data for 337 tumors are not available including 223 WGS samples, 89 RNA-seq samples and 223 MSK-IMPACT clinical sequencing samples. Raw data for the rest of the tumors (133 WGS and 121 RNA-seq) are available under controlled access in three different studies at dbGAP or EGA: 35 WGS samples at https://ega-archive.org/datasets/EGAD00001000135, 26 WGS tumors at https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs002620.v1.p1 and 72 WGS and 70 RNA-seq tumors at https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs003111.v1.p1. Treatment information is available for patients > =2 WGS tumor under controlled access at https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs003111.v1.p1. Detailed information about the availability of somatic/raw data for all tumors is provided in Supplementary Table 1. Additional databases/datasets used for the analysis are human reference genome (GRCh37d5), transcript from Ensembl v75, COSMIC (v92), OncoKB (v1), gnomAD (v3.1) and Clin_var (2022_03_20).
Code availability
Additional scripts and data used for generating the figures are available at https://doi.org/10.5281/zenodo.7783022.
References
Maris, J. M. Recent advances in neuroblastoma. N. Engl. J. Med. 362, 2202–2211 (2010).
London, W. B. et al. Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children’s Oncology Group early-phase trials.Cancer 123, 4914–4923 (2017).
Abbasi, M. R. et al. Impact of disseminated neuroblastoma cells on the identification of the relapse-seeding clone. Clin. Cancer Res. 23, 4224–4232 (2017).
Eleveld, T. F. et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat. Genet. 47, 864–871 (2015).
Schramm, A. et al. Mutational dynamics between primary and relapse neuroblastomas. Nat. Genet. 47, 872–877 (2015).
Chicard, M. et al. Genomic copy number profiling using circulating free tumor DNA highlights heterogeneity in neuroblastoma. Clin. Cancer Res. 22, 5564–5573 (2016).
Van Roy, N. et al. Shallow whole genome sequencing on circulating cell-free DNA allows reliable noninvasive copy-number profiling in neuroblastoma patients. Clin. Cancer Res. 23, 6305–6314 (2017).
Chicard, M. et al. Whole-exome sequencing of cell-free DNA reveals temporo-spatial heterogeneity and identifies treatment-resistant clones in neuroblastoma. Clin. Cancer Res. 24, 939–949 (2018).
Fransson, S. et al. Whole-genome sequencing of recurrent neuroblastoma reveals somatic mutations that affect key players in cancer progression and telomere maintenance. Sci. Rep. 10, 22432 (2020).
Karlsson, J. et al. Four evolutionary trajectories underlie genetic intratumoral variation in childhood cancer. Nat. Genet. 50, 944–950 (2018).
Andersson, N. et al. Extensive clonal branching shapes the evolutionary history of high-risk pediatric cancers. Cancer Res. 80, 1512–1523 (2020).
Schmelz, K. et al. Spatial and temporal intratumour heterogeneity has potential consequences for single biopsy-based neuroblastoma treatment decisions. Nat. Commun. 12, 6804 (2021).
Peifer, M. et al. Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature 526, 700–704 (2015).
Brady, S. W. et al. Pan-neuroblastoma analysis reveals age- and signature-associated driver alterations. Nat. Commun. 11, 5183 (2020).
Monclair, T. et al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J. Clin. Oncol. 27, 298–303 (2009).
Pugh, T. J. et al. The genetic landscape of high-risk neuroblastoma. Nat. Genet. 45, 279–284 (2013).
Valentijn, L. J. et al. TERT rearrangements are frequent in neuroblastoma and identify aggressive tumors. Nat. Genet. 47, 1411–1414 (2015).
Amoroso, L. et al. Genomic coamplification of CDK4/MDM2/FRS2 is associated with very poor prognosis and atypical clinical features in neuroblastoma patients. Genes Chromosomes Cancer 59, 277–285 (2020).
Alexandrov, L. B. et al. The repertoire of mutational signatures in human cancer. Nature 578, 94–101 (2020).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Kucab, J. E. et al. A compendium of mutational signatures of environmental agents. Cell 177, 821–836.e16 (2019).
Wang, T. et al. MYCN drives glutaminolysis in neuroblastoma and confers sensitivity to an ROS augmenting agent. Cell Death Dis. 9, 220 (2018).
Gröbner, S. N. et al. The landscape of genomic alterations across childhood cancers. Nature 555, 321–327 (2018).
Ma, X. et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature 555, 371–376 (2018).
Wei, J. S. et al. Clinically relevant cytotoxic immune cell signatures and clonal expansion of T-cell receptors in high-risk MYCN-not-amplified human neuroblastoma. Clin. Cancer Res. 24, 5673–5684 (2018).
Layer, J. P. et al. Amplification of N-Myc is associated with a T-cell-poor microenvironment in metastatic neuroblastoma restraining interferon pathway activity and chemokine expression. Oncoimmunology 6, e1320626 (2017).
Geoerger, B. et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1–2 trial.Lancet 399, 1718–1729 (2022).
Geoerger, B. et al. Atezolizumab for children and young adults with previously treated solid tumours, non-Hodgkin lymphoma, and Hodgkin lymphoma (iMATRIX): a multicentre phase 1–2 study.Lancet Oncol. 21, 134–144 (2020).
Davis, K. L. et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 21, 541–550 (2020).
Havel, J. J., Chowell, D. & Chan, T. A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 19, 133–150 (2019).
Pich, O. et al. The mutational footprints of cancer therapies. Nat. Genet. 51, 1732–1740 (2019).
Angus, L. et al. The genomic landscape of metastatic breast cancer highlights changes in mutation and signature frequencies. Nat. Genet. 51, 1450–1458 (2019).
Kocakavuk, E. et al. Radiotherapy is associated with a deletion signature that contributes to poor outcomes in patients with cancer. Nat. Genet. 53, 1088–1096 (2021).
Behjati, S. et al. Mutational signatures of ionizing radiation in second malignancies. Nat. Commun. 7, 12605 (2016).
Gerstung, M. et al. The evolutionary history of 2,658 cancers. Nature 578, 122–128 (2020).
Mitchell, T. J. et al. Timing the landmark events in the evolution of clear cell renal cell cancer: TRACERx renal. Cell 173, 611–623.e17 (2018).
Rustad, E. H. et al. Timing the initiation of multiple myeloma. Nat. Commun. 11, 1917 (2020).
Coorens, T. H. H. et al. Inherent mosaicism and extensive mutation of human placentas. Nature 592, 80–85 (2021).
Schleiermacher, G. et al. Emergence of new ALK mutations at relapse of neuroblastoma. J. Clin. Oncol. 32, 2727–2734 (2014).
Althoff, K. et al. A Cre-conditional MYCN-driven neuroblastoma mouse model as an improved tool for preclinical studies. Oncogene 34, 3357–3368 (2015).
Schwab, M. et al. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour.Nature 305, 245–248 (1983).
Kohl, N. E. et al. Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell 35, 359–367 (1983).
Cobrinik, D. et al. Recurrent pre-existing and acquired DNA copy number alterations, including focal TERT gains, in neuroblastoma central nervous system metastases. Genes Chromosomes Cancer 52, 1150–1166 (2013).
Cheung, N.-K. V. et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA 307, 1062–1071 (2012).
Franks, L. M., Bollen, A., Seeger, R. C., Stram, D. O. & Matthay, K. K. Neuroblastoma in adults and adolescents: an indolent course with poor survival. Cancer 79, 2028–2035 (1997).
Ackermann, S. et al. A mechanistic classification of clinical phenotypes in neuroblastoma. Science 362, 1165–1170 (2018).
Carr-Wilkinson, J. et al. High frequency of p53/MDM2/p14ARF pathway abnormalities in relapsed neuroblastoma. Clin. Cancer Res. 16, 1108–1118 (2010).
DuBois, S. G. et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J. Pediatr. Hematol. Oncol. 21, 181–189 (1999).
Landau, H. J. et al. Accelerated single cell seeding in relapsed multiple myeloma. Nat. Commun. 11, 3617 (2020).
Berlanga, P. et al. Central nervous system relapse in high-risk stage 4 neuroblastoma: The HR-NBL1/SIOPEN trial experience. Eur. J. Cancer 144, 1–8 (2021).
Ishida, M. et al. Sprouty2 regulates growth and differentiation of human neuroblastoma cells through RET tyrosine kinase. Cancer Sci. 98, 815–821 (2007).
Gundem, G. et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357 (2015).
Keshelava, N. et al. Loss of p53 function confers high-level multidrug resistance in neuroblastoma cell lines. Cancer Res. 61, 6185–6193 (2001).
Koche, R. P. et al. Extrachromosomal circular DNA drives oncogenic genome remodeling in neuroblastoma. Nat. Genet. 52, 29–34 (2020).
Kim, H. et al. Extrachromosomal DNA is associated with oncogene amplification and poor outcome across multiple cancers. Nat. Genet. 52, 891–897 (2020).
Kushner, B. H. et al. Efficacy of naxitamab in patients with refractory/relapse (R/R) high-risk neuroblastoma (HR-NB) by bone/bone marrow (BM) evaluation, potential sites of residual disease. J. Clin. Oncol. 39, 10022–10022. Preprint at https://doi.org/10.1200/jco.2021.39.15_suppl.10022 (2021).
Yarmarkovich, M. et al. Cross-HLA targeting of intracellular oncoproteins with peptide-centric CARs. Nature https://doi.org/10.1038/s41586-021-04061-6 (2021).
Diolaiti, D. et al. A recurrent novel MGA–NUTM1 fusion identifies a new subtype of high-grade spindle cell sarcoma.Cold Spring Harb. Mol. Case Stud. 4, a003194 (2018).
Zehir, A. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 23, 703–713 (2017).
Chakravarty, D. et al. OncoKB: Annotation of the oncogenic effect and treatment implications of somatic mutations in cancer.JCO Precis. Oncol. 2017, PO.17.00011 (2017).
Medina-Martínez, J. S. et al. Isabl Platform, a digital biobank for processing multimodal patient data.BMC Bioinformatics 21, 549 (2020).
Landrum, M. J. et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 44, D862–D868 (2016).
Forbes, S. A. et al. COSMIC: somatic cancer genetics at high-resolution. Nucleic Acids Res. 45, D777–D783 (2017).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020).
McLaren, W. et al. The Ensembl Variant Effect Predictor. Genome Biol. 17, 122 (2016).
Srivastava, A. et al. Alignment and mapping methodology influence transcript abundance estimation. Genome Biol. 21, 239 (2020).
Aran, D., Hu, Z. & Butte, A. J. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 18, 220 (2017).
Alexandrov, L. B., Nik-Zainal, S., Wedge, D. C., Campbell, P. J. & Stratton, M. R. Deciphering signatures of mutational processes operative in human cancer. Cell Rep. 3, 246–259 (2013).
Blokzijl, F., Janssen, R., van Boxtel, R. & Cuppen, E. MutationalPatterns: comprehensive genome-wide analysis of mutational processes. Genome Med. 10, 33 (2018).
Li, Y. et al. Patterns of somatic structural variation in human cancer genomes. Nature 578, 112–121 (2020).
Cortés-Ciriano, I. et al. Comprehensive analysis of chromothripsis in 2,658 human cancers using whole-genome sequencing. Nat. Genet. 52, 331–341 (2020).
Nik-Zainal, S. et al. The life history of 21 breast cancers. Cell 149, 994–1007 (2012).
Dang, H. X. et al. ClonEvol: clonal ordering and visualization in cancer sequencing. Ann. Oncol. 28, 3076–3082 (2017).
Kaufmann, T. L. et al. MEDICC2: whole-genome doubling aware copy-number phylogenies for cancer evolution. Genome Biol. 23, 241 (2022).
Shen, R. & Seshan, V. E. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res. 44, e131 (2016).
Acknowledgements
We thank T. Heaton and J. Gerstle for their surgical expertise in specimen collections; D. Wedge and M. Ni Leathlobhair of Big Data Institute, University of Oxford, UK, for support and interesting discussions; and members of MSKCC Integrative Genomics Operation core for sample processing and sequencing. N-K.V.C. was partly supported by the Enid Haupt Endowed Chair, the Robert Steel Foundation, Katie Find a Cure, and the Catie Hoch Foundation in building the neuroblastoma tumor tissue archive. E.P. is a Josie Robertson Investigator and is supported by the European Hematology Association, American Society of Hematology, Gabrielle’s Angels Foundation, V Foundation and The Geoffrey Beene Foundation and a Damon-Runyon Rachleff Innovator Award recipient. Funding for this study was supported by the Olayan Fund for Precision Pediatric Cancer Medicine. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
E.P., N-K.V.C. and G.G. designed the study. G.G., M.F.L., J.S.M-M., J.E.A-O. and J.Z. developed algorithmic infrastructure and G.G. performed bioinformatic analysis with support from L.C., M.R. and G.A. N-K.V.C., S.S.R., B.S., M.P.L, B.H.K., S.M. and N.S. performed the clinical management of the patients. N-K.V.C. and N.B. performed patient consent. N-K.V.C. oversaw biospecimen banking performed by I.Y.C. and Y.F., whereas D.Y. and F.D.C executed laboratory processing of patient-derived xenograft specimens. N-K.V.C. collected clinical data for the patients. C.A.I.O. led the clinical donation program. G.G. prepared figures and tables. G.G., N-K.V.C. and E.P. reviewed analysis results and interpretation of findings and wrote the manuscript with input from D.B.S., B.H.K., S.M., C.A.I.D. and A.L.K. All authors reviewed and approved the manuscript for submission.
Corresponding authors
Ethics declarations
Competing interests
G.G. is a consultant in Isabl Inc. E.P., A.L.K. and J.S.M-M. are founders, equity holders and hold fiduciary roles in Isabl Inc. N-K.V.C. reports receiving commercial research grants unrelated to this study, from Y-mAbs Therapeutics and Abpro Labs; holding ownership interest/equity in Y-mAbs Therapeutics and Abpro-Labs and owning stock options in Eureka Therapeutics. N-K.V.C. is the inventor and owner of issued patents, some licensed by MSKCC to Y-mAbs Therapeutics, Biotec Pharmacon and Abpro Labs. MSKCC also has financial interest in Y-mAbs Therapeutics. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Johannes Schulte, David Gisselsson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Tumor cohort, summary of mutation calling and genomic landscape.
(a) (left) Barplots give a breakdown of the tumors sequenced by WGS (n = 247) and MSK-IMPACT panel (n = 223) according to sample types and disease subtypes. (Middle) Barplot gives a breakdown of 94 patients with > =2 tumors according to the availability of WGS and/or MSK-IMPACT data. (Right) Panel lists the number of patients included in the evolutionary analyses. For n = 247 WGS tumors, barplots show (b) the number of SNVs, indels and SVs and (c) the prevalence of segmental CNAs and genes affected by mutations and SVs. Only genes affected in > =2 patients are shown. Bottom barplot gives a summary of the mutation types for each CNA/gene. CNA, copy-number aberration. Complex, small complex insertion/deletion. Del, small deletion. Ins, small insertion. SNV, single-nucleotide variant. SV, structural variant. (d) Survival plot shows the clinical outcome of MYCN-A patients (n = 68) with TERTp substitutions, TERT-SV or no TERT events with 95% confidence intervals shown as the shaded area. P-value from a multivariate analysis taking into account age at diagnosis is shown (coxph function in R). (e) Heatmap gives a summary of co-mutation patterns in the current cohort (n = 470 tumors) with the frequency of events in the upper triangle and odds ratios in the lower triangle, respectively. Only odds ratios with p-values <0.05 are colored in shades of blue for co-mutation or red for mutually exclusive interactions. Significant interactions after multiple testing correction are indicated with a star or a dot according to the significance level of different correction metrics: FDR, false discovery rate. FWER, family-wise error rate. The data and script for the figure are available in Supplementary Tables 1, 2 and the GitHub repository.
Extended Data Fig. 2 Summary of mutational signature analyses in WGS data.
(a) 96 mutational contexts for the single-nucleotide variant (SNV) signatures identified de novo are shown as the barplots on the left while the reference signatures from COSMIC.v3 are shown in the barplot in the middle. Right barplot shows the prevalence of different types of indels amongst the indel signatures identified de novo. (b) Heatmap shows the proportions of substitutions at 96 mutational contexts for each WGS tumor (n = 247) shown in rows together with sample type, disease subtype, platinum, temozolomide and radiotherapy status on the left and number of SNVs and indels and exposure to identified signatures in substitution and indel data on the right. The data and script for the figure are available Supplementary Table 1 and at the GitHub repository.
Extended Data Fig. 3 Biological and clinical correlates of mutational patterns.
(a) Scatter plots show the association between exposure to SBS18 (left) or SBS40 (right) and age at diagnosis amongst the diagnostic/t-resection tumors (n = 132) (Pearson correlation). (b) Box plot shows the mean expression of the genes in glutaminolysis signature associated with ROS accumulation22 across diagnostic tumors (n = 59) of different disease subtypes (left) and MYCN-A tumors (n = 56) from diagnosis, t-resection and relapse and further relapses (right). Median, upper and lower quartiles as well as comparisons with significant p-values from a two-sided Wilcoxon test are shown according to the significance levels in the legend. (c) For n = 151 tumors, scatterplots (left) shows correlation between number of SNVs and the number of predicted neoantigens, and (right) shows the relationship between the number of predicted neoantigens and immune infiltrates in the surrounding tumor microenvironment as assessed from RNAseq (Pearson correlation). (d) Barplots show the proportion of genome-wide SNVs for n = 45 tumors (left) and oncogenic driver SNVs for n = 54 tumors (right) attributed to different mutational signatures broken down by presence in post-therapy relapse tumors. For all scatterplots Pearson correlation and associated p-value with a blue linear regression line and 95% confidence interval in grey is shown. The data and script for the figure are available in Supplementary Table 1 and the GitHub repository.
Extended Data Fig. 4 Subclone trees for MYCN-A, ATRX and MDM2-CDK4 patients.
Each tree shows the subclonal structure in an individual patient. Patients are organized according to disease subtype and the availability of tumors from primary site (diagnosis/reresection/t-resection) and relapses. Branches are annotated with recurrent CNAs and oncogenic mutations/SVs and colored according to the latest tumor they were identified in 1) blue for subclones specific to a diagnostic tumor 2) light blue for subclones seen in reresections 3) green for subclones seen in a t-resection tumor 4) orange for subclones seen in a relapse tumor and 5) red for subclones seen in a further relapse tumor. Subclonal events at MYCN, TERT and ATRX loci are shown in red font. Events with which clonal status cannot be determined are indicated with a question mark. Different evolutionary patterns are indicated with an icon next to the patient id. Tumor sites and the type of sequencing are indicated below the trees. G, whole-genome sequencing. T, targeted sequencing. B, both. For H103207, H118706 and H134819 a simplified version of the tree is shown due to space. Detailed analysis of subclonal structure for 94 patients is provided in Supplementary Data Files 1–69. The data for the figures are available in Supplementary Table 5 and the scripts are available through the ISABL platform.
Extended Data Fig. 5 Subclone trees for TERT-SV, SEG-CNA and NUM-CNA patients.
Each tree shows the subclonal structure in an individual patient. Patients are organized according to disease subtype and the availability of tumors from primary site (diagnosis/reresection/t-resection) and relapses. Branches are annotated with recurrent CNAs and oncogenic mutations/SVs and colored according to the latest tumor they were identified in 1) blue for subclones specific to a diagnostic tumor 2) light blue for subclones seen in reresections 3) green for subclones seen in a t-resection tumor 4) orange for subclones seen in a relapse tumor and 5) red for subclones seen in a further relapse tumor. Subclonal events at MYCN, TERT and ATRX loci are shown in red font. Events with which clonal status cannot be determined are indicated with a question mark. Different evolutionary patterns are indicated with an icon next to the patient id. Tumor sites and the type of sequencing are indicated below the trees. G, whole-genome sequencing. T, targeted sequencing. B, both. For H103207, H118706 and H134819 a simplified version of the tree is shown due to space. Detailed analysis of subclonal structure for 94 patients is provided in Supplementary Data Files 1–69. The data for the figures are available in Supplementary Table 5 and the scripts are available through the ISABL platform.
Extended Data Fig. 6 CN state and SVs at MYCN locus.
For each patient, integrated CN/SV plots showing the details of MYCN locus, boxplots showing the aberrant read support for SV clusters across all tumors, barplots showing the number SVs in each SV cluster and body maps with the tumors are shown. The data for this figure are available as raw data at the dbGAP and scripts are available through the ISABL platform.
Extended Data Fig. 7 CN state and SVs at MYCN locus.
For each patient, integrated CN/SV plots showing the details of MYCN locus, boxplots showing the aberrant read support for SV clusters across all tumors, barplots showing the number SVs in each SV cluster and body maps with the tumors are shown. The data for this figure are available as raw data at the dbGAP and scripts are available through the ISABL platform.
Extended Data Fig. 8 CN state and SVs at MYCN locus.
For each patient, integrated CN/SV plots showing the details of MYCN locus, boxplots showing the aberrant read support for SV clusters across all tumors, barplots showing the number SVs in each SV cluster and body maps with the tumors are shown. The data for this figure are available as raw data at the dbGAP and scripts are available through the ISABL platform.
Extended Data Fig. 9 Timing of metastasis.
Signatures trees as described in Fig. 6 are shown for 13 patients with one or more tumors from the primary site and two or more tumors from locoregional and/or distant metastasis. Patients in the top row have at least one tumor from distant metastatic site while patients in the bottom row have locoregional relapses only. The subclones involved in disease spread from the primary are indicated with a black arrow while the daughter clones are shown with red arrows. The data for the figure are available in Supplementary Table 4 and the scripts are available through the ISABL platform.
Extended Data Fig. 10 Complex seeding patterns in H132374.
Subclonal structure for patient H132374 is shown with treatment timeline and signature tree as described in Figs. 4a and 6, respectively. Body maps depict two different scenarios that explain the subclonal structure in H132374: The left body map shows possible polyclonal seeding in the CNS by a mixture of subclones 3 and 4. In this scenario lung metastasis is caused by subclone-4 after platinum chemotherapy. Shown on the right body map is the second scenario of met-to-met seeding from CNS to lung by subclone-4 after therapy. Detailed description of the patient is provided in Supplementary Information and Supplementary Data File 19. The data for the figure are available in Supplementary Table 4 and as raw data at dbGAP and scripts are available through ISABL platform.
Supplementary information
Supplementary Information
Supplementary methods and information, Supplementary Figures 1–5
Supplementary Data 1
Table-1: This table contains stage and age of diagnosis and survival information about patients as well as tumor specific information such as sample type, disease subtype and type of sequencing performed (n = 470). Table-2: This table contains putative oncogenic SNV, Indel, SV and CNAs for the whole cohort (n = 470). Table-3: This table contains information about the different clonality analyses performed in patients with multiple tumors (n = 94). Table-4: This table contains information about the clusters obtained using DPClust in patients with multi-WGS data (n = 45). Table-5: This table contains information about the subclones identified in patients with multiple tumors sequenced by WGS and/or MSK-IMPACT (n = 94). Table-6: This table contains information about the clonal transitions from diagnosis to relapses (n = 47 patients) and across consecutive relapses (n = 43 patients). Table-7: This table contains information about the limit of detection for n = 32 patients with at least one primary and one relapse tumor.
Supplementary Data 2
All legends are in the PDF file.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gundem, G., Levine, M.F., Roberts, S.S. et al. Clonal evolution during metastatic spread in high-risk neuroblastoma. Nat Genet 55, 1022–1033 (2023). https://doi.org/10.1038/s41588-023-01395-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-023-01395-x
This article is cited by
-
A human neural crest model reveals the developmental impact of neuroblastoma-associated chromosomal aberrations
Nature Communications (2024)