Abstract
Epithelial cells have an ability termed ‘cell competition’, which is an immune surveillance-like function that extrudes precancerous cells from the epithelial layer, leading to apoptosis and clearance. However, it remains unclear how epithelial cells recognize and extrude transformed cells. Here, we discovered that a PirB family protein, leukocyte immunoglobulin-like receptor B3 (LILRB3), which is expressed on non-transformed epithelial cells, recognizes major histocompatibility complex class I (MHC class I) that is highly expressed on transformed cells. MHC class I interaction with LILRB3 expressed on normal epithelial cells triggers an SHP2–ROCK2 pathway that generates a mechanical force to extrude transformed cells. Removal of transformed cells occurs independently of natural killer (NK) cell or CD8+ cytotoxic T cell-mediated activity. This is a new mechanism in that the immunological ligand–receptor system generates a mechanical force in non-immune epithelial cells to extrude precancerous cells in the same epithelial layer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All raw data in the manuscript can be disclosed, and all materials can be provided by appropriate ways. RNA-sequencing data that support the findings of this study have been deposited in BioProject (accession number PRJNA715932).
The prediction of the interaction between LILRB3 and HLA-G is available on VaPRoS (interaction model ID: 2dyp_D_1_A_1). Source data are provided with this paper.
References
Maruyama, T. & Fujita, Y. Cell competition in mammals—novel homeostatic machinery for embryonic development and cancer prevention. Curr. Opin. Cell Biol. 48, 106–112 (2017).
Kajita, M. et al. Filamin acts as a key regulator in epithelial defence against transformed cells. Nat. Commun. 5, 4428 (2014).
Hogan, C. et al. Characterization of the interface between normal and transformed epithelial cells. Nat. Cell Biol. 11, 460–467 (2009).
Kajita, M. et al. Interaction with surrounding normal epithelial cells influences signalling pathways and behaviour of Src-transformed cells. J. Cell Sci. 123, 171–180 (2010).
Leung, C. T. & Brugge, J. S. Outgrowth of single oncogene-expressing cells from suppressive epithelial environments. Nature 482, 410–413 (2012).
Watanabe, H. et al. Mutant p53-expressing cells undergo necroptosis via cell competition with the neighboring normal epithelial cells. Cell Rep. 23, 3721–3729 (2018).
Kon, S. et al. Cell competition with normal epithelial cells promotes apical extrusion of transformed cells through metabolic changes. Nat. Cell Biol. 19, 530–541 (2017).
Yamamoto, M., Ohsawa, S., Kunimasa, K. & Igaki, T. The ligand Sas and its receptor PTP10D drive tumour-suppressive cell competition. Nature 542, 246–250 (2017).
Yoh, K. E. et al. Repression of p63 and induction of EMT by mutant Ras in mammary epithelial cells. Proc. Natl Acad. Sci. USA 113, E6107–E6116 (2016).
Schwartz, M. et al. Genomic retargeting of p53 and CTCF is associated with transcriptional changes during oncogenic HRas-induced transformation. Commun. Biol. 3, 696 (2020).
Vivier, E. & Anfossi, N. Inhibitory NK-cell receptors on T cells: witness of the past, actors of the future. Nat. Rev. Immunol. 4, 190–198 (2004).
Chapman, T. L., Heikema, A. P., West, A. P. Jr. & Bjorkman, P. J. Crystal structure and ligand binding properties of the D1D2 region of the inhibitory receptor LIR-1 (ILT2). Immunity 13, 727–736 (2000).
Willcox, B. E., Thomas, L. M. & Bjorkman, P. J. Crystal structure of HLA-A2 bound to LIR-1, a host and viral major histocompatibility complex receptor. Nat. Immunol. 4, 913–919 (2003).
Held, W. & Mariuzza, R. A. Cis interactions of immunoreceptors with MHC and non-MHC ligands. Nat. Rev. Immunol. 8, 269–278 (2008).
van der Touw, W., Chen, H. M., Pan, P. Y. & Chen, S. H. LILRB receptor-mediated regulation of myeloid cell maturation and function. Cancer Immunol. Immunother. 66, 1079–1087 (2017).
Liang, S. et al. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6–STAT3 signaling pathway. Proc. Natl Acad. Sci. USA 105, 8357–8362 (2008).
Liu, X. et al. ANGPTL2/LILRB2 signaling promotes the propagation of lung cancer cells. Oncotarget 6, 21004–21015 (2015).
Shao, H. et al. Immune inhibitory receptor LILRB2 is critical for the endometrial cancer progression. Biochem. Biophys. Res. Commun. 506, 243–250 (2018).
Maruyama, T. et al. ZAK inhibitor PLX4720 promotes extrusion of transformed cells via cell competition. iScience 23, 101327 (2020).
Hamidouche, Z. et al. Priming integrin α5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc. Natl Acad. Sci. USA 106, 18587–18591 (2009).
Franceschi, R. T. & Xiao, G. Regulation of the osteoblast-specific transcription factor, Runx2: responsiveness to multiple signal transduction pathways. J. Cell Biochem. 88, 446–454 (2003).
Ito, Y., Bae, S. C. & Chuang, L. S. The RUNX family: developmental regulators in cancer. Nat. Rev. Cancer 15, 81–95 (2015).
Kang, X. et al. Inhibitory leukocyte immunoglobulin-like receptors: immune checkpoint proteins and tumor sustaining factors. Cell Cycle 15, 25–40 (2016).
Takai, T. Paired immunoglobulin-like receptors and their MHC class I recognition. Immunology 115, 433–440 (2005).
Rodgers, J. R. & Cook, R. G. MHC class Ib molecules bridge innate and acquired immunity. Nat. Rev. Immunol. 5, 459–471 (2005).
Miyamae, J. et al. Identification of novel polymorphisms and two distinct haplotype structures in dog leukocyte antigen class I genes: DLA-88, DLA-12 and DLA-64. Immunogenetics 70, 237–255 (2018).
Shiroishi, M. et al. Structural basis for recognition of the nonclassical MHC molecule HLA-G by the leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d). Proc. Natl Acad. Sci. USA 103, 16412–16417 (2006).
Isakov, N. ITIMs and ITAMs. The Yin and Yang of antigen and Fc receptor-linked signaling machinery. Immunol. Res 16, 85–100 (1997).
Garcia Fortanet, J. et al. Allosteric inhibition of SHP2: identification of a potent, selective, and orally efficacious phosphatase inhibitor. J. Med. Chem. 59, 7773–7782 (2016).
Lee, H. H. & Chang, Z. F. Regulation of RhoA-dependent ROCKII activation by Shp2. J. Cell Biol. 181, 999–1012 (2008).
Uehata, M. et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389, 990–994 (1997).
Javaherian, A., Vaccariello, M., Fusenig, N. E. & Garlick, J. A. Normal keratinocytes suppress early stages of neoplastic progression in stratified epithelium. Cancer Res. 58, 2200–2208 (1998).
Nakayama, J., Ito, E., Fujimoto, J., Watanabe, S. & Semba, K. Comparative analysis of gene regulatory networks of highly metastatic breast cancer cells established by orthotopic transplantation and intra-circulation injection. Int. J. Oncol. 50, 497–504 (2017).
Liao, N. S., Bix, M., Zijlstra, M., Jaenisch, R. & Raulet, D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science 253, 199–202 (1991).
Paul, S. & Lal, G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 8, 1124 (2017).
Stein-Douglas, K., Schwarting, G. A., Naiki, M. & Marcus, D. M. Gangliosides as markers for murine lymphocyte subpopulations. J. Exp. Med. 143, 822–832 (1976).
Lin, Q. et al. IFN-γ-dependent NK cell activation is essential to metastasis suppression by engineered Salmonella. Nat. Commun. 12, 2537 (2021).
Forkert, P. G. Mechanisms of lung tumorigenesis by ethyl carbamate and vinyl carbamate. Drug Metab. Rev. 42, 355–378 (2010).
O’Donnell, E. P., Zerbe, L. K., Dwyer-Nield, L. D., Kisley, L. R. & Malkinson, A. M. Quantitative analysis of early chemically-induced pulmonary lesions in mice of varying susceptibilities to lung tumorigenesis. Cancer Lett. 241, 197–202 (2006).
Li, S., MacAlpine, D. M., & Counter, C. M. Capturing the primordial Kras mutation initiating urethane carcinogenesis. Nat. Commun. 11, 1800 (2020).
Hilton, H. G. & Parham, P. Missing or altered self: human NK cell receptors that recognize HLA-C. Immunogenetics 69, 567–579 (2017).
Karre, K., Ljunggren, H. G., Piontek, G. & Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319, 675–678 (1986).
Ljunggren, H. G. & Karre, K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today 11, 237–244 (1990).
Gupta, A., Cao, W. & Chellaiah, M. A. Integrin αvβ3 and CD44 pathways in metastatic prostate cancer cells support osteoclastogenesis via a Runx2/Smad5/receptor activator of NF-κB ligand signaling axis. Mol. Cancer 11, 66 (2012).
Campoli, M. & Ferrone, S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene 27, 5869–5885 (2008).
Bicknell, D. C., Rowan, A. & Bodmer, W. F. β2-microglobulin gene mutations: a study of established colorectal cell lines and fresh tumors. Proc. Natl Acad. Sci. USA 91, 4751–4755 (1994).
Douglass, J. et al. Bispecific antibodies targeting mutant RAS neoantigens. Sci. Immunol. 6, eabd5515 (2021).
Kantak, S. S. & Kramer, R. H. E-cadherin regulates anchorage-independent growth and survival in oral squamous cell carcinoma cells. J. Biol. Chem. 273, 16953–16961 (1998).
Barkal, A. A. et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat. Immunol. 19, 76–84 (2018).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016).
Hsu, P. D. et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827–832 (2013).
Maruyama, T. et al. Increasing the efficiency of precise genome editing with CRISPR–Cas9 by inhibition of nonhomologous end joining. Nat. Biotechnol. 33, 538–542 (2015).
Aurnhammer, C. et al. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum. Gene Ther. Methods 23, 18–28 (2012).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 (2013).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Acknowledgements
This work was supported by Advanced Research & Development Programs for Medical Innovation (Prime) (grant number 21gm6210019h0001) from the Japan Agency for Medical Research and Development (AMED), the Precursory Research for Embryonic Science and Technology (PRESTO) (grant number JPMJPR168C) from the Japan Science and Technology Agency, Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (B) 18H02675, the Kato Memorial Bioscience Foundation, the Naito Foundation, the Takeda Science Foundation, the Mitsubishi Foundation, Terumo Life Science Foundation, SGH Foundation and MSD Life Science Foundation (to T.M.). We acknowledge J. Livet (Sorbonne University) for providing pPB∞CAG-MCS vector.
Author information
Authors and Affiliations
Contributions
T.M. conceptualized the study. S.A., N.K., J.N., R.T., N.P., K.O., N.S., K.K., H.A. and T.M. conducted all investigations. S.A. and T.M. wrote the original draft, and Y.F. and T.M. reviewed and edited the manuscript. T.M. acquired funding. The study was supervised by K.S., N.G., Y.F. and T.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Immunology thanks Tristan Rodriguez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Zoltan Fehervari was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Extended data for Fig. 1.
a, RUNX2 mRNA was induced under the mixed condition. Normal and/or RasV12-HaCaT cells were single/mixed-cultured and treated with doxycycline for 8 h. The cell lysates were subjected to quantitative (q) PCR analyses (Method 1). b, Knockdown efficiency of RUNX2 mRNA in HaCaT cells. The knockdown efficiency of RUNX2 mRNA is shown as a graph. HaCaT cells were transfected with siRNA and then cultured for 48 h. The cell lysates were subjected to qPCR analyses. c, RUNX2 knockdown in normal HaCaT cells suppressed apical extrusion. RasV12 and siRNA-transfected normal HaCaT cells were mixed and treated with doxycycline for 16 h. Cells were subjected to immunohistochemistry with phalloidin (white) and Hoechst (blue). Representative XZ images of fluorescence staining and quantification of apical extrusion of RasV12 cells (Method 1); NT, Not transfected; scale bars, 10 μm. Broken lines indicate the apical lines of the cell monolayers. d,e, Schematics of method to narrow-down function-unknown Canis transmembrane genes containing the RUNX2-binding motif (Method 2). Gene expression profiles using MDCK cells found 41 genes that contain the RUNX2-binding motif in their promoter regions (d, green inverted triangles) among the 241 function-unknown transmembrane genes (d, blue). 2 out of the 14 genes that are generic in humans and canines (d, magenta and Supplementary Table 1), human LILRB3 and SPATA31A6, are function-unknown (e). f,g, LILRB3 and ALTR mRNA were upregulated under the mix condition. Normal/RasV12-MDCK (f) or -HaCaT (g) cells were single/mixed-cultured and treated with doxycycline for 8 h. And the cell lysates were subjected to qPCR analyses (Method 1). h, Depletion of RUNX2 in normal cells suppressed LILRB3 induction under the mix condition. siRNA-transfected normal/RasV12-HaCaT cells were single/mixed-cultured and treated with doxycycline for 8 h. The cell lysates were subjected to qPCR analyses (Method 1); NT, not transfected. a-c,f-h, Data are presented as mean ± s.d.; n = 4 (a,g) or 3 (b,c,f,h) biologically independent experiments; *P < 0.05, **P < 0.01 by paired two-sided Student’s t-test; ns., not significant. c,j,m, Scale bars, 10 μm.
Extended Data Fig. 2 Schematics for knockout of AltR and LILRB3/5.
a,c,g, Direct sequencing results of CRISPR/Cas9-mediated gene disruption in MDCK or HaCaT cells. Sequences of MDCK- (a) or HaCaT-wild-type (-WT) (c,g) cells are shown in top rows, and those of MDCK-ALTR KO (a), HaCaT-LILRB3 KO (c), or -LILRB5 KO (g) cells are shown in lower two sequences. The protospacer-adjacent motif (PAM) is underlined. The red spacing and arrows indicate a deleted nucleotide and the guide sequences, respectively. b, MDCK-ALTR KO cells were rescued with exogenous AltR-V5-mCherry using iOn system (Method 3), and mCherry of exogenous AltR localized at the boundary with RasV12 cells in normal MDCK cells. A box with broken lines or bold lines shows the original domain structure of AltR or AltR-V5 construct used for the rescue experiment; ss, signal sequence region; TM, transmembrane region. Representative XY images of fluorescence staining of MDCK-ALTR KO-AltR-V5-OE cells (bottom) shows the localization of exogenous AltR using mCherry (red) and phalloidin (white). The areas indicated by broken squares are magnified as in lower left squares; scale bars, 10 μm. d, HaCaT-LILRB3 KO cells had no expression of LILRB3 protein. Immunoblotting analysis using an anti-LILRB3 and HaCaT-WT or -LILRB3 KO cell lysate. e, Disruption of LILRB3 gene in RasV12 cells had no effect on apical extrusion. Representative XZ fluorescence images (left) and the apical extrusion efficiency of RasV12 cells (right) are shown. Normal/RasV12-HaCaT cells were mixed and treated with doxycycline for 16 h. Cells were subjected to immunohistochemistry with phalloidin (red) and Hoechst (blue). Broken lines indicate the apical lines of the cell monolayers. Data are presented as mean ± s.d.; n = 3 biologically independent experiments were analyzed by unpaired two-sided Student’s t-test; ns., not significant; scale bars, 10 μm. f, Canis AltR is homologous to human LILRB3 and LILRB5. AltR has 4 immunoglobulin-like domains (D1−D4) in the extracellular region shown as a blue circle. D1 and D2 (D1/D2) domains of AltR shown as blue-filled circles are most homologous to D1/D2 of human LILRB3 and LILRB5. In the intracellular region, ITIM domains are shown as blue squares.
Extended Data Fig. 3 Extended data for Fig. 2.
a, RasV12 expression induced HLA-B mRNA in MCF10A cells. The raw meta-data reported in9,10 were re-analyzed. In non-malignant mammary epithelial cells, MCF10A, HLA-B mRNA was induced in a RasV12 expression-dependent manner. Data of study #1 and Study #2 were extracted from9,10, respectively. b, RasV12 expression did not affect HLA-A or HLA-C on the cell surface. HaCaT-RasV12 cells were treated with doxycycline for the indicated time period and followed by immunohistochemistry under the non-permeabilized condition. Samples were stained with an anti-HLA-A or anti-HLA-C (white) and phalloidin (blue). Representative XY images of fluorescence staining are shown. c, Schematic of sgRNA targeting HLA-A/B/C exon 1. The guide sequence targeting human HLA-B is indicated in green. PAM is in bold typeface. d, HaCaT-RasV12-HLA TKO cells had no MHC class Ia proteins. HaCaT-RasV12 WT or -HLA TKO cells were single-cultured. The cell lysates were subjected to immunoblotting. Arrowhead: target band. *: non-specific band. e-g, Direct sequencing results of CRISPR/Cas9-mediated gene disruption in MDCK cells. Sequences of MDCK WT cells are shown in top rows, and those of MDCK-DLA12 KO (e), -DLA64 KO (f), or -DLA88 KO (g) cells are shown in lower two rows. PAM is underlined. The red spacing and arrows indicate a deleted nucleotide and the guide sequences, respectively. h, Disruption of TAP1 suppressed the efficiency of apical extrusion. Normal/Rasv12-MDCK cells were mixed and treated with doxycycline for 24 h. Cells were subjected to immunohistochemistry with phalloidin (red) and Hoechst (blue) (Method 1). Representative XZ images of fluorescence staining (top) and quantification data of apical extrusion of RasV12 cells (bottom) are shown. Data are presented as mean ± s.d.; n = 3 biologically independent experiments; **P < 0.01, ***P < 0.001 by unpaired two-sided Student’s t-tests over MDCK-RasV12 WT cells; scale bars, 10 μm. Broken lines indicate the apical lines of the cell monolayers.
Extended Data Fig. 4 Extended data for Fig. 3.
a, A computational search using VaPRoS predicted the binding model between MHC class I and LILRB3. The predicted complex of LILRB3 (red) and HLA-G (green) were show in a ribbon diagram and the interaction site in a blue circle, and 8 amino acids (8mer peptides) are further shown and highlighted as blue squares. Amino acid sequences of Human HLA-B and Canis DLA88 (MHC class Ia), which are most similar to the region of HLA-G including the predicted interaction site are shown below HLA-G. Same as MHC class I, amino acid sequence of Canis AltR, which is most similar to the region of LILRB3 including the predicted interaction site is shown below as well. b, Constructs of recombinant proteins and predicted 8mer synthetic peptides. Gray boxes with broken lines show original domain structure of Canis MHC class Ia and LILRB3/AltR. Bold-lined boxes represent structures of recombinant protein or synthetic peptide of Canis MHC class Ia (blue) or AltR (pink). Rec.GST–MHC-α3-Δ8mer–Flag is a truncated mutant of rec.GST–MHC-α3–Flag which lacks the region of the predicted interaction site (amino acid sequence: PISDHEVT). Amino acid sequences of 8mer synthetic peptides are listed as follows: α3-8mer PISDHEVT; AltR-D1-8mer VLYKDGYP; LILRB3-D1-8mer RLHKEGSP; SS, signal sequence region; TM, transmembrane region. c,e, The effects of rec.MHC-α3 and rec.AltR-D1/D2 proteins on apical extrusion. Normal/RasV12-MDCK cells were mixed and cultured with the indicated concentration of rec.GSTDα1/α2–Flag (α1/2) or rec.GST–MHC-α3–Flag (α3) (c), or rec.GST–AltR-D1/D2–HA (D1/D2) or rec.GST–AltR-D3/D4–HA (D3/D4) (e) upon doxycycline treatment for 24 h. The efficiency of apical extrusion is shown in graphs. d, Deletion of the predicted minimal binding site in Canis MHC class Ia cancelled the promotion of apical extrusion by the rec.MHC-α3 treatment. Nomal/RasV12-HaCaT cells were mixed and cultured with 3 μM of rec.GST-α3-Flag (α3) or rec.GST-α3-Δ8mer-Flag protein upon the doxycycline treatment for 16 h. The efficiency of apical extrusion is shown in a graph. f, 8mer synthetic peptides, α3-8mer and D1-8mer, promoted and suppressed apical extrusion, respectively. HaCaT and HaCaT-RasV12 cells were mixed and cultured with α3-8mer, AltR-D1-8mer, or LILRB3-D1-8mer upon the doxycycline treatment for 16 h. The efficiency of apical extrusion is shown in a graph. c-f, Data are presented as mean ± s.d.; n = 3 biologically independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001 by unpaired two-sided Student’s t-test; ns., not significant.
Extended Data Fig. 5 Extended data for Fig. 4.
a,b, AltR-V5 expressed in normal cells was phosphorylated under the mixed condition, and the phosphorylation was promoted by MHC class I. MDCK-ALTR KO-AltR-V5-OE cells were cultured alone (a Normal), or mix-cultured with MDCK-RasV12 WT cells (a,b Mix) or -DLA88 KO cells (b) with doxycycline and Na3VO4 for 2 h (b) or 4 h (a,b). The cell lysates were subjected to immunoprecipitation assay with an anti-phospho-tyrosine and followed by detection with an anti-V5. Numeric value below bands is relative intensities of phosphorylated AltR normalized with total AltR. Arrowheads: phosphorylated V5-AltR. *: non-specific band. c,d, An SHP2-specific inhibitor suppressed apical extrusion using MDCK and HaCaT cells. Co-cultured HaCaT or MDCK cells were treated with DMSO or an SHP2-specific inhibitor, SHP099, upon doxycycline treatment for 16 (c, HaCaT cells) or 24 (d, MDCK cells) h. The extrusion efficiency was quantified and shown as graphs (Method 1). e, LILRB3 knockout suppressed the accumulation of filamin in surrounding normal cells. HaCaT WT or -LILRB3 KO cells were mixed with HaCaT-RasV12 cells and cultured with doxycycline for 12 h, followed by immunohistochemistry with an anti-filamin (red) and phalloidin (white). Representative XY images of fluorescence staining (left) and quantification data of filamin accumulation in surrounding HaCaT cells (right) are shown; scale bars, 10 μm. f, The primed normal cells by contact with RasV12 cells induces LILRB3/AltR, which promotes apical extrusion via filamin accumulation. Neighboring normal cells sense physical changes, eg. membrane tension, of RasV12 cells and induce RUNX2, and thereby the induced RUNX2 drives LILRB3 expression (left). The expressed LILRB3 on the cell surface of normal cell further interacts with MHC class I of RasV12 cells. Activated LILRB3 by interaction with MHC class I is phosphorylated and recruits SHP2, leading to activation of SHP2 − ROCK2 signaling pathway, which eventually promotes filamin accumulation (right). c-e, Data are presented as mean ± s.d.; n = 3 biologically independent experiments (c,d); n = 56 (WT) or 61 (LILRB3 KO) cells examined over three biologically independent experiments (e); *P < 0.05, **P < 0.01, ****P < 0.0001 by unpaired two-sided Student’s t-test.
Extended Data Fig. 6 Normal cells eliminate RasV12 cells from pile-up colony via the interaction between LILRB3 and MHC class I.
a, LILRB3 knockout in normal cells retained RasV12 cell in the epithelial monolayer, and the remaining RasV12 cells formed tumor like cell mass in vitro. Schematic of in vitro pile-up colony formation assay (top) and representative XY images of fluorescence staining (bottom) are shown. HaCaT-RasV12 cells were mixed with HaCaT-LILRB3 KO cells to retain RasV12 cells in an epithelial monolayer. After 48 h incubation, pile-up colonies were cultured for the indicated time period, Day 2 to Day 6. The cells were subjected to immunohistochemistry using phalloidin (red) and Hoechst (blue). b-d, The rec.MHC-α3 treatment suppressed pile-up colony formation and outcompeted RasV12 cells undergo apoptosis. Schematic of in vitro pile-up colony formation assay (b,c top). Normal HaCaT and HaCaT-RasV12 cells were mixed. The cells were treated with doxycycline and rec.AltR-D1/D2 protein to retain RasV12 cells in an epithelial monolayer (Day 0). After 48 h incubation (Day 2), pile-up colonies were cultured with or without rec.MHC-α3 protein for the indicated period from Day 2 to Day 6. The samples were subjected to immunohistochemistry using phalloidin (red), Hoechst (blue) (b-d), and an anti-cleaved Caspase-3 (white and white arrowhead) (d). Representative XY images of fluorescence staining (b,c, bottom and d, left) and quantification data of the number of active Caspase-3 positive cell in outmost RasV12 cells on Day 4 (d, right) are shown; broken line, the position of pile-up colony on Day 4. Data are presented as mean ± s.d; n = 6 [α3 (-)] or 15 [α3 (+)] cells examined over three biologically independent experiments; ***P < 0.001 by unpaired two-sided Student’s t-test. a-d, Scale bars, 20 μm.
Extended Data Fig. 7 Extended data for Fig. 5.
a, The mix proportion of normal and HaCaT-RasV12 cells used in tumorigenesis assay in Fig. 5b,c,e are described. ‘Figure#’ indicates the figure number in Fig. 5. ‘Genotype’ indicates genotypes of HaCaT or HaCaT-RasV12 cells. ‘HaCaT:RasV12’ means a ratio of the actual cell number used for the experiments as a unit of 105 cells. ‘Mix rate’ indicates ratio of HaCaT cell number over the RasV12 cell number. ‘Rec.α3’ indicates whether rec.MHC-α3 protein was coinjected (+) or not (–). b, High magnification fluorescence images of Fig. 5d. Tumors collected from mice at 2 week were fixed, and the frozen sections of the collected tumors were subjected to immunohistochemistry with phalloidin (white) and Hoechst (blue). c, LILRB3 was induced in the normal cells contacting with RasV12-HLA TKO cells. HaCaT and/or HaCaT-RasV12-HLA TKO cells were single/mixed-cultured and treated with doxycycline for 12 h. Immunohistochemistry were conducted under the non-permeabilized condition using an anti-LILRB3 (red) and phalloidin (white). Representative XY images of fluorescence staining are shown. d, Rec.MHC-α3 protein promoted apical extrusion of HaCaT-RasV12-HLA TKO cells under the mix condition. Normal/RasV12-HaCaT cells were mixed and cultured with or without rec.MHC-α3 protein upon doxycycline treatment for 16 h. The extrusion efficiency was quantified and shown as a graph. Data are presented as mean ± s.d; n = 3 biologically independent experiments; **P < 0.01 by unpaired two-sided Student’s t-test. e, Tumor formed by coinjection of normal and RasV12 cells recruited macrophage at the peripheral of tumors. HaCaT WT or -LILRB3 KO cells were mixed with HaCaT-RasV12 cells at a ratio of 10:1 and coinjected with rec.MHC-α3 protein into nude mice. After 2 weeks, tumors were collected and fixed, followed by frozen sectioning. Frozen sections of tumors were subjected to immunohistochemistry with an anti-F4/80 (Macrophage marker, red), an anti-CK19 (Epithelial cell marker, white) and Hoechst (blue). Representative XY images of fluorescence staining are shown. b,c,e, Scale bars, 200 μm (b) or 10 μm (c,e).
Extended Data Fig. 8 Extended data for Fig. 6.
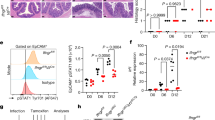
a, Mouse Pirb is the homologous gene of Canis ALTR. PirB has 6 immunoglobulin-like domains (D1 − D6) in the extracellular region shown as a circle. D1/D2 and D3/D4 domains of PirB have high homology with D1/D2 domain of AltR shown as blue-filled circles. In the intracellular region, ITIM domains are shown as blue squares. b, Rec.AltR-D1/D2 protein interacted with mouse MHC class I (mMHC-I). Cell lysates of mouse Raw264 cells were mixed with GST or rec.GST–AltR-D1/D2–HA protein (rec.AltR-D1/D2) and subjected to pulldown assay with glutathione-conjugated beads. Interacted mouse MHC class I was detected with an anti-MHC-I (clone: OX-18). White arrowhead: rec.AltR-D1/D2 protein. Black arrowhead: GST. *: mMHC-I. c, The interaction analysis of rec.GST–MHC-α3–Flag with PirB-D1-8mer using Bio-Layer Interferometry (BLI). Kd = 2.88 ± 0.20 nM. d, PirB was upregulated in surrounding normal cells. NMuMG and/or NMuMG-pTRE3G-GFP–RasV12 (NMuMG-RasV12) cells were single/mixed-cultured treated with doxycycline for 12 h. The cells were stained with an anti-PirB (red), phalloidin (white) and Hoechst (blue). e, Images of air-evacuated lungs. Collected lungs were fixed and then air-evacuated under the low-pressure condition. Arrowhead: tumorigenic nodule. f, Images of H&E staining (top) and immunofluorescence staining (bottom) of a nodule; E-cadherin, red; CK19, white; Hoechst, blue. g, Urethane induced Kras-mutated nodules in lungs. The genomic DNAs of nodules microdissected from the lungs were extracted and subjected to PCR analysis. The amplicons around the Kras exon 2 (codon 12, G12V) or exon 3 (codon 61, Q61L/R) were used for the direct-sequencing analysis. The observed Kras mutation and mutation rate are shown in a graph; ND., not detected. h, HaCaT-RasQ61L cells were extruded from the epithelial monolayer. HaCaT cells were mixed with HaCaT-RasV12 or -pTRE3G-Myc-RasQ61L cells and cultured with doxycycline for 16 h. The cells were stained with an anti-Myc (green), phalloidin (red), and Hoechst (blue) (Method 1). Representative XZ images of fluorescence staining (left) and quantification data of apical extrusion (right). Broken lines indicate the apical lines of the cell monolayers. i, Knockdown efficiency of Pirb by AAV6-shPirb infection. Mouse Raw 264 cells were infected with AAV6-shRNA for control (shCtrl) or Pirb (shPirb) and cultured for 48 h. The cells were subjected to qPCR analysis. The knockdown efficiency is shown as relative expression to AAV6-shCtrl-infected cells. d-f,h, Scale bars, 10 μm (d,h), 2 mm (e), or 100 μm (f). h,i, Data are presented as mean ± s.d.; n = 3 biologically independent experiments; **P < 0.01 by unpaired two-sided Student’s t-test.
Extended Data Fig. 9 Patients with high expression of LILRB3 show the better prognosis.
The correlation between the prognosis of cancer patients and the mRNA levels of LILRB3. mRNA expression profiles and survival rates in clinical data were obtained from cBioPortal and analyzed by Log-rank test. Red and blue lines represent survival rates of High expression group (LILRB3-High group) and Low expression group (LILRB3-Low group), respectively. Skin Melanoma (LILRB3-High group: n = 28, LILRB3-Low group: n = 398) and Pancreatic Adenocarcinoma (LILRB3-High group: n = 34, LILRB3-Low group: n = 143). Details of analyzing methods are described in the method section.
Supplementary information
Supplementary Information
Supplementary Tables 1–4.
Supplementary Video 1
GFP–RasV12 fluorescence images. HaCaT-RasV12 cells were mixed with normal HaCaT cells (top left), with normal HaCaT cells and treated with rec.MHC-α3 protein (top right), with HaCaT-LILRB3 KO cells (bottom left) or with HaCaT-LILRB3 KO cells and treated with rec.MHC-α3 protein (bottom right). Cells were mixed and seeded on a thin collagen-coated bottom dish. The images were acquired using a live-imaging system every 5 min for 48 h (8–56 h) after 8 h of incubation with doxycycline with or without rec.MHC-α3 protein. Movies from 20 to 44 h are provided as representative videos. At 32 h, most of the RasV12 cells apically extruded from an epithelial monolayer. Following treatment with rec.MHC-α3 protein, apical extrusion was observed at the earlier time points (24–28 h), and the number of extruding RasV12 cells increased. However, disruption of LILRB3 suppressed apical extrusion of RasV12 cells even in the presence of rec.MHC-α3 protein.
Supplementary Video 2
Merge with GFP–RasV12 fluorescence and bright-field images. HaCaT-RasV12 cells were mixed with normal HaCaT cells (top left), with normal HaCaT cells and treated with rec.MHC-α3 protein (top right), with HaCaT-LILRB3 KO cells (bottom left) or with HaCaT-LILRB3 KO cells and treated with rec.MHC-α3 protein (bottom right).
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed gels.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Ayukawa, S., Kamoshita, N., Nakayama, J. et al. Epithelial cells remove precancerous cells by cell competition via MHC class I–LILRB3 interaction. Nat Immunol 22, 1391–1402 (2021). https://doi.org/10.1038/s41590-021-01045-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-021-01045-6
This article is cited by
-
CaSSiDI: novel single-cell “Cluster Similarity Scoring and Distinction Index” reveals critical functions for PirB and context-dependent Cebpb repression
Cell Death & Differentiation (2024)
-
Epithelial recognition and elimination against aberrant cells
Seminars in Immunopathology (2024)
-
Gut microbiota in colorectal cancer development and therapy
Nature Reviews Clinical Oncology (2023)
-
Cell competition in development, homeostasis and cancer
Nature Reviews Molecular Cell Biology (2023)
-
Pleiotropic effects of cell competition between normal and transformed cells in mammalian cancers
Journal of Cancer Research and Clinical Oncology (2023)