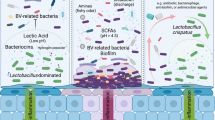
Abstract
We report the results of a first exploratory study testing the use of vaginal microbiome transplantation (VMT) from healthy donors as a therapeutic alternative for patients suffering from symptomatic, intractable and recurrent bacterial vaginosis (ClinicalTrials.gov NCT02236429). In our case series, five patients were treated, and in four of them VMT was associated with full long-term remission until the end of follow-up at 5–21 months after VMT, defined as marked improvement of symptoms, Amsel criteria, microscopic vaginal fluid appearance and reconstitution of a Lactobacillus-dominated vaginal microbiome. One patient presented with incomplete remission in clinical and laboratory features. No adverse effects were observed in any of the five women. Notably, remission in three patients necessitated repeated VMT, including a donor change in one patient, to elicit a long-standing clinical response. The therapeutic efficacy of VMT in women with intractable and recurrent bacterial vaginosis should be further determined in randomized, placebo-controlled clinical trials.
Similar content being viewed by others
Main
Bacterial vaginosis (BV) is a form of vaginal microbial community alteration in which the microbiome normally dominated by Lactobacillus species switches to one characterized by the emergence of anaerobes1,2,3,4. BV is prevalent in women of reproductive age, affecting from one-fourth to one-third of women5. It ranges from an asymptomatic finding in most cases to a clinically symptomatic entity characterized by an abnormal, often malodorous vaginal discharge in 16% of women diagnosed with BV, summing up to a prevalence of 4.4% for symptomatic BV in women aged 14–49 years5. BV may be associated with risk of upper genital tract infection6, complications of pregnancy (particularly preterm birth and lower success in fertility treatments7,8,9,10) and susceptibility to sexually transmitted infections11. At the clinically severe end of the BV spectrum, treatment with antibiotics (either systemic or vaginal) is associated with a 30% relapse rate within 3 months of initial treatment and a relapse rate of up to 50–70% within 1 year12. Therapeutic options are very limited in the subpopulation of women who experience persistent or recurrent BV despite multiple antibiotic treatment attempts13,14,15. Maintenance antimicrobial treatment16,17 is often the treatment suggested in these cases, but it can predispose to vaginal candidiasis18 and resistant infections19,20. Importantly, probiotic treatment of symptomatic patients with oral and/or vaginal administration of bacterial Lactobacillus strains has produced mixed results21,22, suggesting that the microbiome as a whole, rather than a single bacterial species, may be necessary for an effective cure at the clinically severe end of the BV spectrum. Fecal microbiome transplantation (FMT), in which feces from healthy donors are introduced into recipients’ intestines to replace their disease-associated microbiome, has recently been successfully used in treating severe and recurrent Clostridium difficile infection23. Although gastrointestinal microbiome interventions may offer a different ecological scenario than those related to a dysbiotic vaginal microbiome, we hypothesized that a similar use of VMT might be beneficial in treating the most severe cases of recurrent and antibiotics-nonresponsive BV.
Five patients were recruited (aged 27–47 years and referred to as patients A–E; Extended Data Fig. 1). All suffered from intractable BV, defined as four or more symptomatic episodes of BV during the previous year19, relapsing after repeated, prolonged and diverse antibiotic attempts requiring continuous maintenance antibiotic treatment to remain symptom free. All patients reported a substantial negative impact of BV symptoms on their quality of life, including devastating consequences to their relationships, sexual intimacy and self-esteem. All five patients were otherwise healthy. Patient screening, exclusion criteria and the consent process are described in the Methods. The three donors were premenopausal, healthy volunteers, aged 35–48 years (donors 1–3; Extended Data Fig. 1), who did not report having BV in the last 5 years or any history of recurrent BV. Donor selection and screening are detailed in the Methods. Repeated communication between the lead physician and the donors ensured that the behavioral requirements (e.g., abstinence from sexual activity for 1 week before donation in the sexually active donor) were strictly followed.
Before transplantation, all patients were treated with an intravaginal antibiotic regimen16 that previously resulted in a longer symptom-free period, which consisted of 5 g clindamycin cream (2%) for 7 d (recipients B, C and E) or 5 g metronidazole gel (0.75%) for 5 d (recipients A and D). VMT was performed 1 week after completion of antibiotic treatment24. During the procedure, vaginal fluid for transplantation was collected from the donors starting from the seventh day of the menstrual cycle (Methods) and taken from the upper half of the vagina and cervical fornices, while avoiding the cervix. The collected discharge was evaluated by pH and microscopy, diluted with 1 ml of sterile saline and transferred to the recipient’s posterior fornix, without the use of a speculum (Fig. 1a). VMT was completed within no more than 60 min of sample collection and was performed at any stage during the recipient’s menstrual cycle, except during menstruation. After the first VMT, repeat VMTs were performed in cases of symptom recurrence or with reappearance of one or more positive Amsel criteria during follow-up examinations (Extended Data Fig. 2). At each follow-up appointment (weekly for the first month and monthly to bimonthly thereafter), patients were interviewed and underwent a vaginal examination (including quantification of discharge, pH measurement, whiff test and microscopy). Remission of BV was defined at each appointment as disappearance of symptoms, normalization of all Amsel criteria and appearance of a normal Lactobacillus-dominated microbiome by light microscopy.
a, Schematic depiction of the VMT study. b, Amsel criteria. c, Wet-mount microscopy before VMT: clue cells (black arrow). The vaginal microbiome comprised of abnormal coccid bacteria (white arrow). Wet-mount microscopy after VMT: normal, mature vaginal squamous epithelial cells (black arrow) and Lactobacillus morphotypes (white arrow) are present. This wet mount represents normavaginal discharge (original magnification, ×400). d, Discrete clinical features during post-VMT follow-up. A–E are the individual VMT recipients. BL, borderline; BV, bacterial vaginosis; IM, intermediate; N, negative (whiff test, discharge), normal (microscopy); P, positive.
Four patients (patients A–D) had long-lasting improvements in their Amsel scores (Fig. 1b), microscopic vaginal fluid appearance (Fig. 1c,d), whiff test, discharge and vaginal fluid pH (Fig. 1d) after 1–3 VMT sessions (Extended Data Figs. 2 and 3). The fifth patient (patient E) had partial remission, manifesting as a subjective reduction of symptoms, negative whiff test, cumulative Amsel scoring of 0–1 and an intermediate microscopic vaginal fluid appearance. Patients A and B underwent a single VMT each from donors 1 and 2, respectively. Both reported immediate clinical improvement, with the disappearance of odor within 1 week of transplantation, and a gradual decrease in discharge, resulting in no symptoms 1 month after VMT. In both patients, normalization of all Amsel criteria as well as normal Lactobacillus-dominated microscopic appearance was documented 1 week after transplantation and persisted on follow-up examinations (11.5 months in patient A and 5.5 months in patient B). Patient C received the microbiome of donor 1. She reported an improvement of symptoms after VMT and became BV negative according to Amsel criteria, but her microscopic findings were consistent with persistence of BV. She therefore underwent a repeat VMT from the same donor (donor 1) without preceding antibiotic treatment. For 4 months, the patient reported an improvement of symptoms and BV status was negative according to Amsel criteria. However, 4.5 months after the first VMT, she experienced a recurrence of odor, positive Amsel criteria and a BV microbiome appearance on microscopy, all consistent with recurring BV. She, therefore, underwent a third VMT, this time using a sample from a different donor (donor 3) after vaginal antibiotic treatment. After this VMT, she reported complete resolution of symptoms; Amsel criteria were normalized; and microscopy showed a normal Lactobacillus-dominated appearance for 11 months of follow-up. Patients D and E likewise had a fluctuant course. After a first VMT from donor 1, patient D experienced a recurrence of symptoms, positive Amsel criteria and microscopic findings consistent with BV. She underwent a second VMT from the same donor (donor 1), after which she reported clinical improvement of symptoms and was BV negative according to Amsel criteria. However, she exhibited an intermediate vaginal microbial appearance on microscopy and therefore underwent a third VMT from the same donor (donor 1), after which she reported clinical improvement with the disappearance of odor and improvement of discharge, associated with negative Amsel criteria and a normal Lactobacillus-dominated appearance on microscopy. On evaluation 21 months after the third transplant, the patient reported no recurrences, had negative Amsel criteria and exhibited normal microscopy. After VMT from donor 2, patient E reported a partial symptomatic improvement, associated with negative Amsel criteria and a normal Lactobacillus-dominated appearance on microscopy, for 4 weeks of follow-up. She then took systemic antibiotics for pharyngitis and soon after reported a recurrence of odor, accompanied by positive Amsel criteria and BV-characteristic microscopic appearance. She underwent a repeat VMT from the same donor (donor 2), resulting in the normalization of all Amsel criteria and improvement of her microscopic vaginal appearance to an intermediate microbiome configuration, coupled with partial symptomatic improvement, for 6.5 months of follow-up.
To characterize the genus-level changes associated with VMT, we sequenced all donors’ and recipients’ vaginal microbiome samples using 16S ribosomal DNA (rDNA) sequencing (Methods). Interestingly, healthy microbiomes clustered differently from the microbiomes of BV-diagnosed patients (Extended Data Fig. 4a) after applying principal-coordinates analysis (PCoA) with UniFrac distances25. Using Bray–Curtis (BC) dissimilarity, we followed recipients’ microbiomes before and after VMT and observed a rapid change in the composition of all microbiomes after VMT, correlated with recovery in all of the Amsel criteria (Extended Data Fig. 4b).
To study the effect of VMT on vaginal microbiome species-level composition and metagenomic function, all donors’ and recipients’ samples underwent shotgun metagenomic sequencing. As expected, the microbiomes of donors and recipients were found in two distinct clusters using PCA (Fig. 2a). The effect of VMT on global microbiome composition over the follow-up period was assessed by BC dissimilarity on the species level, as compared to patients’ baseline BV configuration. Four of five VMT recipients exhibited a drastically changed microbiome composition already at the first month after VMT, which correlated with a notable recovery of their Amsel criteria (Fig. 2b) as well as with every discrete clinical criterion (Extended Data Fig. 5a). Patient C experienced the same trend, only after the third and successful VMT (Fig. 2b). The post-VMT microbiome of one patient (patient E) relapsed to her baseline microbiome BV composition after failure of the first VMT (Fig. 2b). However, the repeat successful VMTs in this patient induced a distinctively different configuration, mirrored by a marked species-level BC distance from baseline, similarly to the post-VMT trend observed with the other four patients after a successful VMT (Fig. 2b). In four of the five VMT recipients, the vaginal microbiome configuration remained distinct from the baseline BV configuration over a period of 5–21 months after a successful VMT (Fig. 2c). Notably, the post-VMT vaginal microbiome composition became significantly more similar to that of the collective donor vaginal microbiome configuration, as compared to the corresponding similarity between the pre-VMT and donor configurations (Euclidean distances, P = 0.0012; Fig. 2c and Extended Data Fig. 5b). This similarity was present after a successful VMT and through the follow-up period (Fig. 2d). Notably, the current preliminary case series is underpowered to statistically test a person-specific donor contribution to a recipient’s specific clinical features or microbiome configuration after VMT. Larger future cohorts may enable better resolution or, alternatively, demonstrate that distinctions can be made between only a ‘healthy’ versus a BV microbiome configuration.
a, Metagenomic PCA performed on the donors’ and recipients’ baselines. b, Metagenomic BC distance from baseline, correlated with the Amsel criteria scores. c, Metagenomic PCA performed on samples from the donors and the baseline and last collected samples from each participant. Arrows depict the conversion of VMT recipients from baseline samples to samples after successful VMT and are colored by the respective donor’s color. Dots unconnected by the arrows represent the microbiome configurations of donors. Inset, bar plot displaying the Euclidean distances of pre-VMT (full red bar) and post-VMT (empty red bar) samples to samples from each donor; **P = 0.0012, paired two-tailed t-test. Error bars are s.d. from the mean; n = 5 recipients. d, Metagenomic BC distance from the respective donor, correlated with the Amsel criteria scores. e, Metagenomic assessment of the change in the microbiome composition at the genus level after VMT. Arrows indicate a VMT, with color corresponding to the donor. f, Metagenomic bar plot denoting the species contributing the most to the first PC. Arrows indicate a VMT, with colors corresponding to the donor; triangles indicate an antibiotic treatment. Note that patient D recieved an antibiotic treatment also prior to her second VMT procedure, but the corresponding triangular mark was not included owing to space limitations. g,h, Metagenomic KEGG gene annotated PCA (n = 50 total recipient samples), colored by Amsel criteria (g) and by relative abundance of the Bifidobacterium genus and the Lactobacillus genus (h). A–E are the individual VMT recipients.
This post-VMT compositional change was mostly dominated by an expansion in members of the Lactobacillus genus, combined with a decrease in members of the Bifidobacterium genus, closely related to the Gardnerella genus. (The reference that was used for taxonomic annotations classifies Gardnerella genus and Gardnerella vaginalis specie as Bifidobacterium and Bifidobacterium vaginale, accordingly; Fig. 2e and Extended Data Fig. 5c.) Other genera, including Fannyhessea and Prevotella, were reduced upon successful VMT-induced remission of BV (Extended Data Fig. 5d). We further used species-level PCA in reducing the complex microbiome dimensionality (Extended Data Fig. 5e). Indeed, the PCA clustered the samples into a BV cluster, containing mostly samples with one or more Amsel-diagnosed BV features, and a healthy cluster, with mostly no diagnosed clinical features (Extended Data Fig. 5e). We applied a k-means algorithm (k = 2), using the coordinates of the first and second PCs, to define the two clusters that were visually identified (Extended Data Fig. 5e). We then calculated the purity score for each Amsel criteria score division. Considering the purity scores, we classified our samples into two groups according to their Amsel score (i.e., the first group comprised all samples having Amsel criteria = 0 and the second group comprised all samples with Amsel criteria > 0). To see whether the groups were indeed different, we conducted a permutational analysis of variance test using the BC dissimilarity matrix (P < 0.05). The difference between the two clusters could be explained by the relative levels of Bifidobacterium and Lactobacillus genera in each sample (Extended Data Fig. 5f). The most dominant features that contributed to the change in the first PC, which differentiated between the clusters, consisted mostly of Lactobacillus crispatus specie in the healthy cluster and Bifidobacterium vaginale in the BV cluster (Fig. 2f), demonstrating the 15 overall most PC1-influential taxa, as represented in each vaginal microbiome configuration). Interestingly, recipient E, who was the only partial clinical responder, featured a different dominant post-VMT lactobacillus strain (Lactobacillus gasseri), that was not one of the top 15 PC1-influential strains in the other four VMT recipients).
Functional microbiome changes after VMT, as assessed using the Kyoto Encyclopedia of Genes and Genomes (KEGG), revealed two distinct functional clusters that separated the BV microbiome from the healthy one (Fig. 2g), corresponding to the taxonomic differences noted between these conditions (Fig. 2h). Upon recipient follow-up, functional BC distances from the baseline microbiome correlated with a decrease in Amsel criteria (Extended Data Fig. 6a). Changes in the functional potential of the microbiome shifted at the time of VMT (Extended Data Fig. 6b) and remained unaltered over the follow-up period (Extended Data Fig. 6c). Similarly to the taxonomic analysis, the current preliminary case series was underpowered to statistically test person-specific donor and recipient similarities in functional microbiome characteristics, and these could only cluster as BV, healthy donor and post-VMT groups. An alternative functional analysis using Gene Ontology (GO) terms demonstrated that the post-VMT BC distance from the baseline likewise correlated with the decrease in Amsel criteria in three patients (patients A–C; Extended Data Fig. 7a), whereas in two patients (patients D and E) the distances remained unchanged. Nonetheless, a clear PCA cluster could be observed (Extended Data Fig. 7b) between the BV and healthy microbiome configurations, and these could be clearly linked to the different taxonomic composition of the BV and healthy clinical states (Extended Data Fig. 7c). The most dominant GO terms remained stable throughout VMT and the follow-up period, potentially explaining the low BC changes we observed using this analytical method (Extended Data Fig. 7d), whereas the second PC exhibited a substantial change in GO term signatures over the course of the follow-up period (Extended Data Fig. 7e).
Collectively, we report the feasibility of using VMT as a long-term treatment for recurrent, antibiotics-nonresponsive and intractable BV. Although we did not observe adverse effects associated with VMT in this study, we cannot completely exclude potential risks associated with any microbiome transfer procedure. The transplant of antimicrobial-resistant microbes has been reported in immunocompromised patients undergoing FMT26, and the long-term consequences of VMT remain unknown. Gynecologic and obstetric complications, however unlikely, are also possible. Additionally, the risks of unintended pregnancy due to the transfer of sperm or the transfer of undetected pathogens with the vaginal fluid are not negligible. Ιn our study, donor selection followed stringent criteria to minimize the risks, yet these criteria may not be applicable in other settings. The use of contraception by recipients as a mandatory criterion in future studies, associated with the development of a ‘vaginal fluid bank’ in which samples from suitable donors will be kept for a period allowing for repeated screening, and verification of the absence of HIV sero-conversion before VMT, may be recommended. Finally, BV may be asymptomatic or readily treatable with antibiotics in most women, and therefore VMT should be considered only in cases of multiple treatment failures and substantial disruption of the patient’s quality of life due to chronic and intractable symptoms. Although all patients enrolled in this exploratory study benefited from the procedure, the efficacy of VMT as a treatment in intractable BV needs to be determined in randomized, placebo-controlled trials.
Methods
Human study cohort
This study was conducted at the Hadassah Medical Center in Jerusalem, Israel. All participants provided written informed consent. The research protocol was approved by the ethics committee at the Hadassah Medical Center (HMO-0667-13) and the Weizmann Institute of Science (603-1, 680-1) with ClinicalTrials.gov ID NCT02236429.
Bacterial vaginosis
BV was diagnosed by the Amsel criteria, requiring three of the four following symptoms or signs: homogeneous, thin, white discharge; pH >4.5; a fishy odor of vaginal discharge before or after addition of 10% potassium hydroxide (i.e., the whiff test); and >20% vaginal epithelial cells studded with adherent coccobacilli (clue cells) on microscopic examination16,27. On microscopy, bacterial microbiome appearance was defined as normal (lactobacilli dominated), BV (coccid-bacillary dominated) or intermediate, as quantified by using the Hay–Ison criteria28.
Recipients
Inclusion criteria
Recipients were included if they were aged 18–50 years with recurrent BV, defined as ≥4 symptomatic episodes of BV during the previous year, and required maintenance antibiotic treatment (twice weekly) to remain symptom free or if they experienced recurrence of BV in ≤2 months after treatment, with a documented history of recurrent BV. Exclusion criteria included pregnancy or planned pregnancy in the upcoming year or infection with hepatitis B, hepatitis C, HIV or syphilis. Study candidates underwent screening for cervicovaginal infection with Chlamydia trachomatis, Neisseria gonorrhea, Mycoplasma genitalium or Trichomonas vaginalis, using a PCR assay. Any patient who presented a positive result for any of these infections received the standard recommended treatment, with a documented negative assay result deemed mandatory for inclusion in the study. All patients underwent a cervical cytology screening test (Pap test) and PCR-based screening for human papilloma virus (HPV). In the case of an abnormal cytology test or a positive HPV test, patients were referred for colposcopy. In addition, vaginal cultures for yeast and bacteria (streptococci groups A, B, C and G), urine cultures, urinalysis, and serology analysis for HIV, hepatitis A, B and C, Treponema pallidum, herpes viruses and cytomegalovirus (CMV) were performed in all cases.
Donors
Inclusion criteria
Donors were included if they were aged 18–50 years and premenopausal. Exclusion criteria included history of BV in the last 5 years or any history of recurrent BV; presence of a cervico-vaginal sexually transmitted infection (C. trachomatis, N. gonorrhea, M. genitalium or T. vaginalis); a positive HPV test; vaginal presence of streptococci groups A, C or G; history of recurrent candida vulvovaginitis; history of recurrent urinary tract infections; use of any antibiotics in the month preceding vaginal fluid collection; use of systemic medication; use of probiotics (orally or vaginally); consumption of herbal or homeopathic remedies; acute illness; history of cancer; history of anogenital dysplasia; history of anogenital HPV; history of anogenital herpes; vulvar or vaginal disease (acute or chronic); pregnancy; abnormal urinalysis or infection; or seropositivity to HIV, hepatitis C, hepatitis B, herpes or syphilis.
Donors’ long-term medical and sexual history was familiar to the lead clinician. Two were non-sexually active for 8 years or more; one was engaged in a 25-year monogamous relationship. Donors had a negative history of vaginal symptoms and underwent an examination to verify the absence of BV and other vaginitis, using a thorough history, gynecologic exam, vaginal fluid microscopic assessment, cultures and PCR assays. All donors answered a questionnaire that is used at our blood bank (see below) to screen for possible risk factors for acquiring an infection that we potentially missed using PCR assays, cultures and serology. Donors were screened for potentially important infections, including group B Streptococcus (GBS) and CMV, so as not to expose women who were GBS or CMV negative to potential future complications of pregnancy and delivery caused by GBS or CMV. Before vaginal fluid collection, it was explicitly verified with the donors that they did not have sexual intercourse in the week preceding the intervention.
Donor history questionnaire
-
1.
Are you generally healthy?
-
2.
Do you suffer from any health problem? If you do, please specify.
-
3.
Have you taken any medication during the past month? If so, please indicate each one.
-
4.
Have you traveled abroad during the past 12 months? If so, please specify where and when.
-
5.
Did you get a tattoo/ear or body piercing/accidental needlestick during the last 6 months?
-
6.
Were you bitten by an animal in the last few months?
-
7.
Did you suffer from hepatitis?
-
8.
Did you live with a person who has hepatitis in the past 6 months?
-
9.
Did you suffer from tuberculosis during the past two years?
-
10.
Did you receive antibiotic treatment for a sexually transmitted infection (e.g., Chlamydia, gonorrhea, trichomonas) in the past year?
-
11.
Did you live in a malaria-infected area or suffer from malaria?
-
12.
Did you undergo any surgery? If yes, please specify which? When?
-
13.
Did you receive a blood transfusion? If so, when?
-
14.
Were you pregnant or delivered during the past 6 months?
If an answer to any of the following questions is positive, we ask that you do not participate as a donor:
-
1.
Had sexual contact with a new partner during the past 6 months (even if since then he became a regular partner)?
-
2.
Used needles to take drugs?
-
3.
Are you a carrier of hepatitis B or C?
-
4.
Are you a carrier of genital herpes or HPV?
-
5.
Did you ever have syphilis, HIV or any other sexually transmitted disease?
-
6.
Did you have a vaginal culture showing positive group B streptococcus?
-
7.
Did you have recurrent urinary tract infections?
-
8.
Did you have complaints of vaginal discharge or bad odor during the past 5 years?
-
9.
Did you or are you suffering from recurrent vaginal infections?
-
10.
Do you have a history of abnormal PAP tests, or been found positive for HPV?
-
11.
Were you ever diagnosed with cancer?
-
12.
Did you have any fever during the past month?
-
13.
Are you using oral or vaginal probiotics?
-
14.
Did you use any local treatment for vaginal inflammation during the past month? (anti-fungal, antibiotics, steroids, homeopathic or herbal)?
-
15.
Are you suffering from excessive vaginal discharge, itching or unpleasant odor?
-
16.
Did you have infectious mononucleosis during the past year?
Consent process
All patients underwent a thorough and detailed consent process, including being informed of the hazards potentially mitigated by this experimental approach. They received a highly detailed explanation of the study procedures. They were also informed about the limited ability to completely predict future gynecologic and obstetric complications; the possible transfer of infectious agents, including those that cannot be screened for; as well as the small but non-negligible risk of inadvertent sperm transfer. For patients who did not use an intrauterine device, the use of hormonal contraception was recommended. A time interval of 7–14 d was set after the detailed explanation was given and before deciding on participation to let the patient consider all of her options.
VMT sample collection and transfer
The flat, broad part of Ayre’s spatula was used for sample collection. This device, originally designed for vaginal sample collection for the Pap test, does not absorb the fluid, is shaped for vaginal use and does not wound the vagina. In parallel to VMT sample collection, samples were taken, using the same technique, for molecular analysis, using the ESwab Multiple Specimen Collection and Transport System (COPAN) and stored at −80 °C. After VMT, recipients were instructed to avoid sexual intercourse for 1 month; avoid bathing (in a bath, hot tub, swimming pool, etc.) for 1 week; avoid douching, intravaginal medications and systemic antibiotics for 1 month; and avoid probiotics for the entire follow-up period of 1 year.
Follow-up after VMT
At each appointment, patients underwent a gynecologic examination that consisted of an evaluation by the Amsel criteria including microscopy of vaginal discharge. In the case of an abnormal cytology test or a positive HPV test at recruitment (four patients), patients underwent colposcopy before VMT and were followed as recommended by American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines, including colposcopy, HPV test and cytology screening. One patient with a normal cytology test and a negative HPV test had repeated cytology screening at the end of 1 year of follow-up and was instructed to continue follow-up as recommended by ASCCP guidelines. As recipients continued to have sexual intercourse after VMT, an infection possibly detected in the time after VMT could not necessarily be attributed to the VMT and was therefore not routinely assessed.
16S sequencing and analysis
For 16S amplicon sequencing, PCR amplification was performed of the 16S rDNA gene and subsequently sequenced using 500-bp paired-end sequencing (Illumina MiSeq). Amplicons spanning variable region 4 (V4) of the 16S rDNA gene were generated by using the following barcoded primers: Fwd 515F, AATGATACGGCGACCACCGAGATCTACACTATGGTAATTGTGTGCCAGCMGCCGCGGTAA; Rev 806R, CAAGCAGAAGACGGCATACGAGATXXXXXXXXXXXXAGTCAGTCAGCCGGACTACHVGGGTWTCTAAT; where X represents a barcode base. W = A or T; M = A or C; H = A or C or T. The reads were then processed using the QIIME 2.2019-1 pipeline29. In brief, fastq quality files and a mapping file indicating the barcode sequence corresponding to each sample were used as inputs; reads were split by sample according to the barcode, then they were denoised by the dada2 plugin and taxonomic classification was performed using the Greengenes database and the naive Bayes pretrained QIIME2 classifier. Rarefaction was used to exclude samples with insufficient count of reads per sample. For beta diversity, UniFrac measurements were plotted on the basis of 70,000 reads per sample.
Shotgun metagenomics sequencing and processing
Genomic DNA was purified using a Purelink Microbiome DNA purification kit (Invitrogen) optimized for a Tecan automated platform. For shotgun sequencing, Illumina libraries were prepared using a Nextera DNA Sample Prep kit (Illumina, FC-121-1031), according to the manufacturer’s protocol, and sequenced on the Illumina NextSeq platform with a read length of 80 bp. We then used Illumina’s bcl2fastq script to make the fastq files. Host reads were removed using KneadData with default parameters and the hg19 reference. Taxonomic assignment was performed with Kraken230, using this recommended prebuilt index database31. From the resulting table, we extracted the counts for both genus and species levels separately. We then subsampled the count tables so each sample had 100,000 reads in total. Three samples that did not reach 100,000 reads were excluded from the taxonomic analysis. On top, we filtered out all bacteria whose total abundance after rarefaction was 10-4. In all clinical–microbiome distance correlations, only time points that included both clinical and microbiome measurements were used. Functional annotation was performed using the Humann232 pipeline, with the same input reads we used for the taxonomic analysis. The output was normalized to counts per million, combined and annotated to KEGG and GO terms using Humann’s built-in scripts (humann2_regroup_table with UniRef90ko and UniRef90go, respectively, together with humann2_rename_table). These datasets were later filtered to remove all genes and terms for which the abundance was <10-3.
Statistical analysis
Paired t-tests were used to compare baseline and post-VMT distances to the centroid of the three donors. The distances were computed on all PCs. PCA was performed using the scikit-learn package in Python, as were all the analyses, after performing a log transformation in all cases. Figure 2f shows the 15 leading loadings (in absolute value) for the first and second PCs. k-means clustering was also performed using scikit-learn, for 100 iterations with random_state = iteration’s index.
Permutation tests were performed in the following manner. Under the null hypothesis that the microbiome profiles of samples originating from both groups have the same distribution, we let μ0 denote the mean of pairwise dissimilarity between the original groups. For i = 1 to 105, we shuffled the labels of the groups stratifying the permutations so that labels were switched only within the same subject’s samples. We then denoted the mean of pairwise dissimilarity of the relabeled groups by μ1. The probability of the null hypothesis is \({\it{p}} = \frac{{\# \left\{ {\mu _i \ge {\it{\mu }}_0} \right\}_{i = 1}^{10^5}}}{{10^5}} \cdot\) For example, we counted each iteration that had a higher mean of pairwise dissimilarity, and the P value is the sum of these divided by the number of iterations.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The sequencing data has been deposited at the European Nucleotide Archive with accession number PRJEB34085. All other requests for raw and analyzed data, generated as part of a clinical trial, will be promptly reviewed by the Hadassah-Hebrew University Medical Center and the Weizmann Institute of Science to verify whether the request is subject to intellectual property confidential obligations or affects patient confidentiality.
References
Nasioudis, D., Linhares, I. M., Ledger, W. J. & Witkin, S. S. BJOG. 124, 61–69 (2017).
Ravel, J. et al. Proc. Natl Acad. Sci. USA 108(Suppl. 1), 4680–4687 (2011).
Gajer, P. et al. Sci. Transl. Med. 4, 132ra52 (2012).
Ravel, J. & Brotman, R. M. Genome Med. 8, 35 (2016).
Koumans, E. H. et al. Sex. Transm. Dis. 34, 864–869 (2007).
Peipert, J. F., Montagno, A. B., Cooper, A. S. & Sung, C. J. Am. J. Obstet. Gynecol. 177, 1184–1187 (1997).
Babu, G., Singaravelu, B., Srikumar, R., Reddy, S. V. & Kokan, A. J. Clin. Diagnostic Res. 11, DC18–DC22 (2017).
Koedooder, R. et al. Hum. Reprod. 34, 1042–1054 (2019).
Haahr, T. et al. Hum. Reprod. 31, 795–803 (2016).
Paavonen, J. & Brunham, R. C. N. Engl. J. Med. 379, 2246–2254 (2018).
Taha, T. E. et al. AIDS 12, 1699–1706 (1998).
Bradshaw, C. S. et al. J. Infect. Dis. 193, 1478–1486 (2006).
Marrazzo, J. M. et al. Sex. Transm. Dis. 37, 732–744 (2010).
Oduyebo, O. O., Anorlu, R. I. & Ogunsola, F. T. Cochrane Database Syst. Rev. 3, CD006055 (2009).
Joesoef, M. R. & Schmid, G. P. Clin. Infect. Dis. 20(Suppl. 1), S72–S79 (1995).
Workowski, K. A. & Bolan, G. A. Centers for Disease Control and Prevention. MMWR Recomm. Rep. 64, 1–137 (2015).
Hay, P. Curr. Opin. Infect. Dis. 22, 82–86 (2009).
Sanchez, S., Garcia, P. J., Thomas, K. K., Catlin, M. & HolmesK. K. Am. J. Obstet. Gynecol. 191, 1898–1906 (2004).
Sobel, J. D. et al. Am. J. Obstet. Gynecol. 194, 1283–1289 (2006).
Beigi, R. H., Austin, M. N., Meyn, L. A., Krohn, M. A. & Hillier, S. L. Am. J. Obstet. Gynecol. 194, 1283–1289 (2006).
Mastromarino, P., Vitali, B. & Mosca, L. New Microbiol. 36, 229–238 (2013).
Bohbot, J. M. et al. J. Gynecol. Obstet. Hum. Reprod. 47, 81–86 (2018).
Malikowski, T., Khanna, S. & Pardi, D. S. Curr. Opin. Gastroenterol. 33, 8–13 (2017).
Eriksson, K., Larsson, P. G., Nilsson, M. & Forsum, U. APMIS 119, 373–376 (2011).
Lozupone, C. & Knight, R. Appl. Environ. Microbiol. 71, 8228–8235 (2005).
Food and Drug Administration. Important Safety Alert Regarding Use of Fecal Microbiota for Transplantation and Risk of Serious Adverse Reactions Due to Transmission of Multi-Drug Resistant Organisms (US Food and Drug Administration, 2019).
Amsel, R. et al. Am. J. Med. 74, 14–22 (1983).
Ison, C. A. & Hay, P. E. Sex. Transm. Infect. 78, 413–415 (2002).
Bolyen, E. et al. Nat. Biotechnol. 37, 852–857 (2019).
Wood, D. E. & Salzberg, S. L. Genome Biol. 15, R46 (2014).
Méric, G., Wick, R. R., Watts, S. C., Holt, K. E. & Inouye, M. Preprint at https://www.biorxiv.org/content/10.1101/712166v1 (2019)
Franzosa, E. A. et al. Nat. Methods 15, 962–968 (2018).
Acknowledgements
We thank the members of the Hadassah-Hebrew University Medical Center Department of Obstetrics and Gynecology, the Elinav lab at the Weizmann Institute of Science and members of the DKFZ Cancer–Microbiome division for insightful discussions. We thank S.M. Cohen for expert editing. All cartoons were created, under a license, using BioRender software. The study was supported by a grant from the Joint Research Fund of the Hebrew University of Jerusalem and Hadassah Medical Center. H.S. is the incumbent of the V.R. Schwartz Research Fellow Chair. E.E. is supported by Yael and Rami Ungar, the Leona M. and Harry B. Helmsley Charitable Trust, the Adelis Foundation, the Pearl Welinsky Merlo Scientific Progress Research Fund, the Lawrence and Sandra Post Family Foundation, the Daniel Morris Trust, the Park Avenue Charitable Fund, the Hanna and Dr. Ludwik Wallach Cancer Research Fund, the Howard and Nancy Marks Charitable Fund, Aliza Moussaieff, the estate of Malka Moskowitz, the estate of Myron H. Ackerman, the estate of Bernard Bishin for the WIS-Clalit Program, Donald and Susan Schwarz, and grants funded by the European Research Council, the Israel Science Foundation, the Israel Ministry of Science and Technology, the Israel Ministry of Health, the Helmholtz Foundation, the Else Kroener Fresenius Foundation, the Garvan Institute, the European Crohn’s and Colitis Organization, the Deutsch-Israelische Projektkooperation and the Wellcome Trust. E.E. is the incumbent of the Sir Marc and Lady Tania Feldmann Professorial Chair, a senior fellow at the Canadian Institute of Advanced Research and an international scholar at the Bill & Melinda Gates Foundation and the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
A.L.-S., D.G.-W. and E.E. conceived the study. A.L.-S. recruited and supervised the participants and performed all clinical procedures. Y.C. performed all computational analyses. M.D.-B. performed sample preparation, processing and sequencing. A.L. and U.M. assisted with the computational analysis. J.S. and A.E.M. provided microbiology insights and support. S.Y. contributed to study conception and design. H.S. supervised all lab work. A.L.-S, D.G.-W., Y.C. and E.E. interpreted the experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
E.E. is a paid consultant at DayTwo and BiomX. None of this work is related to, funded or endorsed by, shared or discussed with or licensed to any commercial entity. None of the other authors has any competing interest.
Additional information
Peer review information Joao Monteiro was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
Clinical background of donors and recipients.
Extended Data Fig. 2
Recipient clinical parameters over time.
Extended Data Fig. 3
Recipient pre-VMT and post-VMT range of clinical values throughout the follow-up period.
Extended Data Fig. 4 Genus-level 16S recombinant DNA assessment of the vaginal microbiome following VMT.
(a) 16S principal coordinates analysis using UniFrac distances colored by Amsel criteria scores, n = 50 total recipient samples; (b) Bray-Curtis distances from baseline, correlated with the Amsel criteria scores measured on the same day. A–E are the individual VMT recipients.
Extended Data Fig. 5 Metagenomic compositional assessment of the vaginal microbiome following VMT.
(a) Bray-Curtis distances from baseline, correlated with the Amsel criteria scores measured on the same day; (b) Bray-Curtis distances from respective donor, correlated with the Amsel criteria scores measured on the same day; (c) Metagenomic assessment of the change in the microbiome composition at the Genus level following VMT in absolute values; (d) Change in microbiome in the genus level following VMT; (e) PCA performed on the metagenomic taxonomic data colored by Amsel criteria scores and divided into cluster using 2-means algorithm (n = 47 total recipient samples); (f) PCA colored by relative abundance of Bifidobacterium and of Lactobacillus genus (n = 47 total recipient samples). A–E are the individual VMT recipients.
Extended Data Fig. 6 Metagonomic functional (KEGG) assessment of the vaginal microbiome following VMT.
(a) Bray-Curtis distances from baseline, correlated to Amsel criteria scores and their components, measured on the same day; (b) Change in microbiome functional KEGG gene annotated following VMT; (c) Metagenomic bar plot denoting the KEGG genes that most contributed to the first principal component. A–E are the individual VMT recipients.
Extended Data Fig. 7 Metagonomic functional (GO) assessment of the vaginal microbiome following VMT.
(a) Bray-Curtis distances from baseline, correlated to Amsel criteria scores measured on the same day; (b), (c), principal component analysis, n = 50 total recipient samples, colored by (b), Amsel criteria score, (c) relative abundance of Bifidobacterium and of Lactobacillus genus; (d) Change in microbiome functional GO terms annotated following VMT; (e) Metagenomic bar plot denoting the GO terms that most contributed to the second principal component. A–E are the individual VMT recipients.
Supplementary information
Supplementary Information
Supplementary Methods
Rights and permissions
About this article
Cite this article
Lev-Sagie, A., Goldman-Wohl, D., Cohen, Y. et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med 25, 1500–1504 (2019). https://doi.org/10.1038/s41591-019-0600-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-019-0600-6
This article is cited by
-
Comparative analysis of the vaginal bacteriome and virome in healthy women living in high-altitude and sea-level areas
European Journal of Medical Research (2024)
-
Effect of stress urinary incontinence on vaginal microbial communities
BMC Microbiology (2024)
-
Cervicovaginal microbiota: a promising direction for prevention and treatment in cervical cancer
Infectious Agents and Cancer (2024)
-
Markers of fertility in reproductive microbiomes of male and female endangered black-footed ferrets (Mustela nigripes)
Communications Biology (2024)
-
Utilization of the microbiome in personalized medicine
Nature Reviews Microbiology (2024)