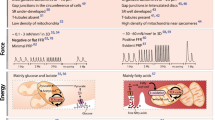
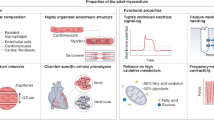
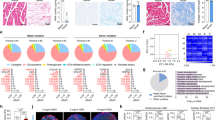
Abstract
Engineered cardiac tissues derived from human induced pluripotent stem cells offer unique opportunities for patient-specific disease modeling, drug discovery and cardiac repair. Since the first engineered hearts were introduced over two decades ago, human induced pluripotent stem cell-based three-dimensional cardiac organoids and heart-on-a-chip systems have now become mainstays in basic cardiovascular research as valuable platforms for investigating fundamental human pathophysiology and development. However, major obstacles remain to be addressed before the field can truly advance toward commercial and clinical translation. Here we provide a snapshot of the state-of-the-art methods in cardiac tissue engineering, with a focus on in vitro models of the human heart. Looking ahead, we discuss major challenges and opportunities in the field and suggest strategies for enabling broad acceptance of engineered cardiac tissues as models of cardiac pathophysiology and testbeds for the development of therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Langer, R. & Vacanti, J. P. Tissue engineering. Science 260, 920–926 (1993).
Kochanek, K. D., Xu, J. & Arias, E. Mortality in the United States, 2019. NCHS Data Brief 395, 1–8 (2020).
Murry, C. E. & MacLellan, W. R. Stem cells and the heart—the road ahead. Science 367, 854–855 (2020).
Vunjak-Novakovic, G., Ronaldson-Bouchard, K. & Radisic, M. Organs-on-a-chip models for biological research. Cell 184, 4597–4611 (2021).
Kim, H., Kamm, R. D., Vunjak-Novakovic, G. & Wu, J. C. Progress in multicellular human cardiac organoids for clinical applications. Cell Stem Cell 29, 503–514 (2022).
Thomas, D., Choi, S., Alamana, C., Parker, K. K. & Wu, J. C. Cellular and engineered organoids for cardiovascular models. Circ. Res. 130, 1780–1802 (2022).
Cho, S., Lee, C., Skylar-Scott, M. A., Heilshorn, S. C. & Wu, J. C. Reconstructing the heart using iPSCs: engineering strategies and applications. J. Mol. Cell. Cardiol. 157, 56–65 (2021).
Hofbauer, P., Jahnel, S. M. & Mendjan, S. In vitro models of the human heart. Development 148, dev199672 (2021).
Hofer, M. & Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 6, 402–420 (2021).
Weinberger, F., Mannhardt, I. & Eschenhagen, T. Engineering cardiac muscle tissue: a maturating field of research. Circ. Res. 120, 1487–1500 (2017).
Huang, N. F. et al. Big bottlenecks in cardiovascular tissue engineering. Commun. Biol. 1, 199 (2018).
Ogle, B. M. et al. Distilling complexity to advance cardiac tissue engineering. Sci. Transl. Med. 8, 342ps313 (2016).
Vunjak-Novakovic, G. et al. Challenges in cardiac tissue engineering. Tissue Eng. Part B Rev. 16, 169–187 (2010).
Stein, J. M., Mummery, C. L. & Bellin, M. Engineered models of the human heart: directions and challenges. Stem Cell Reports 16, 2049–2057(2020).
Guo, Y. & Pu, W. T. Cardiomyocyte maturation: new phase in development. Circ. Res. 126, 1086–1106 (2020).
Mills, R. J. et al. Drug screening in human PSC-cardiac organoids identifies pro-proliferative compounds acting via the mevalonate pathway. Cell Stem Cell 24, 895–907 (2019).
Ronaldson-Bouchard, K. et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556, 239–243 (2018).
Campostrini, G., Windt, L. M., van Meer, B. J., Bellin, M. & Mummery, C. L. Cardiac tissues from stem cells: new routes to maturation and cardiac regeneration. Circ. Res. 128, 775–801 (2021).
Hofbauer, P. et al. Cardioids reveal self-organizing principles of human cardiogenesis. Cell 184, 3299–3317 (2021).
Lewis-Israeli, Y. R. et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Commun. 12, 5142 (2021).
Skylar-Scott, M. A. et al. Orthogonally induced differentiation of stem cells for the programmatic patterning of vascularized organoids and bioprinted tissues. Nat. Biomed. Eng. 6, 449–462 (2022).
Radisic, M. et al. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol. Bioeng. 93, 332–343 (2006).
Puente, B. N. et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157, 565–579 (2014).
Rhee, S. et al. Endocardial/endothelial angiocrines regulate cardiomyocyte development and maturation and induce features of ventricular non-compaction. Eur. Heart J. 42, 4264–4276 (2021).
Miller, J. S. et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11, 768–774 (2012).
Redd, M. A. et al. Patterned human microvascular grafts enable rapid vascularization and increase perfusion in infarcted rat hearts. Nat. Commun. 10, 584 (2019).
Zhang, B. et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater. 15, 669–678 (2016).
Brassard, J. A., Nikolaev, M., Hubscher, T., Hofer, M. & Lutolf, M. P. Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nat. Mater. 20, 22–29 (2021).
Skylar-Scott, M. A. et al. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 5, eaaw2459 (2019).
Rossi, G. et al. Capturing cardiogenesis in gastruloids. Cell Stem Cell 28, 230–240 (2021).
Wimmer, R. A. et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565, 505–510 (2019).
Kilpinen, H. et al. Common genetic variation drives molecular heterogeneity in human iPSCs. Nature 546, 370–375 (2017).
Ortmann, D. et al. Naive pluripotent stem cells exhibit phenotypic variability that is driven by genetic variation. Cell Stem Cell 27, 470–481 (2020).
Strano, A., Tuck, E., Stubbs, V. E. & Livesey, F. J. Variable outcomes in neural differentiation of human pscs arise from intrinsic differences in developmental signaling pathways. Cell Rep. 31, 107732 (2020).
Burnett, S. D. et al. Population-based toxicity screening in human induced pluripotent stem cell-derived cardiomyocytes. Toxicol. Appl. Pharmacol. 381, 114711 (2019).
Mannhardt, I. et al. Comparison of 10 control HPSC lines for drug screening in an engineered heart tissue format. Stem Cell Reports 15, 983–998 (2020).
Mannhardt, I. et al. Blinded contractility analysis in hIPSC-cardiomyocytes in engineered heart tissue format: comparison with human atrial trabeculae. Toxicol. Sci. 158, 164–175 (2017).
Frangogiannis, N. G. The extracellular matrix in myocardial injury, repair and remodeling. J. Clin. Invest. 127, 1600–1612 (2017).
Ott, H. C. et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat. Med. 14, 213–221 (2008).
Herum, K. M., Choppe, J., Kumar, A., Engler, A. J. & McCulloch, A. D. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol. Biol. Cell 28, 1871–1882 (2017).
Zhang, H. et al. Generation of quiescent cardiac fibroblasts from human induced pluripotent stem cells for in vitro modeling of cardiac fibrosis. Circ. Res. 125, 552–566 (2019).
Swift, J. et al. Nuclear lamin-a scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 (2013).
Martinez-Vidal, L. et al. Causal contributors to tissue stiffness and clinical relevance in urology. Commun. Biol. 4, 1011 (2021).
Mascharak, S. et al. Preventing engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 372, eaba2374 (2021).
Drakhlis, L. et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat. Biotechnol. 39, 737–746 (2021).
Silva, A. C. et al. Co-emergence of cardiac and gut tissues promotes cardiomyocyte maturation within human iPSC-derived organoids. Cell Stem Cell 28, 2137–2152 (2021).
Bao, X. et al. Directed differentiation and long-term maintenance of epicardial cells derived from human pluripotent stem cells under fully defined conditions. Nat. Protoc. 12, 1890–1900 (2017).
Burridge, P. W. et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 11, 855–860 (2014).
Lian, X. et al. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of wnt signaling. Stem Cell Reports 3, 804–816 (2014).
Lian, X. et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 8, 162–175 (2013).
Palpant, N. J. et al. Generating high-purity cardiac and endothelial derivatives from patterned mesoderm using human pluripotent stem cells. Nat. Protoc. 12, 15–31 (2017).
Shen, M., Quertermous, T., Fischbein, M. P. & Wu, J. C. Generation of vascular smooth muscle cells from induced pluripotent stem cells: methods, applications, and considerations. Circ Res. 128, 670–686 (2021).
Williams, I. M. & Wu, J. C. Generation of endothelial cells from human pluripotent stem cells. Arterioscler Thromb. Vasc. Biol. 39, 1317–1329 (2019).
Zhang, J. et al. Functional cardiac fibroblasts derived from human pluripotent stem cells via second heart field progenitors. Nat. Commun. 10, 2238 (2019).
Giacomelli, E. et al. Three-dimensional cardiac microtissues composed of cardiomyocytes and endothelial cells co-differentiated from human pluripotent stem cells. Development 144, 1008–1017 (2017).
Giacomelli, E. et al. Human-iPSC-derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell 26, 862–879 (2020).
Zhang, J. Z. et al. A human iPSC double-reporter system enables purification of cardiac lineage subpopulations with distinct function and drug response profiles. Cell Stem Cell 24, 802–811 (2019).
Boudou, T. et al. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng. Part A 18, 910–919 (2012).
Huebsch, N. et al. Miniaturized iPS-cell-derived cardiac muscles for physiologically relevant drug response analyses. Sci. Rep. 6, 24726 (2016).
Mathur, A. et al. Human iPSC-based cardiac microphysiological system for drug screening applications. Sci. Rep. 5, 8883 (2015).
Mohamed, T. M. A. et al. Regulation of cell cycle to stimulate adult cardiomyocyte proliferation and cardiac regeneration. Cell 173, 104–116 (2018).
Chen, V. C. et al. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res 15, 365–375 (2015).
Buikema, J. W. et al. Wnt activation and reduced cell–cell contact synergistically induce massive expansion of functional human ipsc-derived cardiomyocytes. Cell Stem Cell 27, 50–63 (2020).
Tavakol, D. N., Fleischer, S. & Vunjak-Novakovic, G. Harnessing organs-on-a-chip to model tissue regeneration. Cell Stem Cell 28, 993–1015 (2021).
Mawad, D. et al. A conducting polymer with enhanced electronic stability applied in cardiac models. Sci. Adv. 2, e1601007 (2016).
Wei, K. et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 525, 479–485 (2015).
Fei, P. et al. Cardiac light-sheet fluorescent microscopy for multi-scale and rapid imaging of architecture and function. Sci. Rep. 6, 22489 (2016).
Lind, J. U. et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater. 16, 303–308 (2017).
Michas, C. et al. Engineering a living cardiac pump on a chip using high-precision fabrication. Sci. Adv. 8, eabm3791 (2022).
McAleer, C. W. et al. Multi-organ system for the evaluation of efficacy and off-target toxicity of anticancer therapeutics. Sci. Transl. Med. 11, eaav1386 (2019).
Ronaldson-Bouchard, K. et al. A multi-organ chip with matured tissue niches linked by vascular flow. Nat. Biomed. Eng. 6, 351–371 (2022).
Ribeiro, A. J. et al. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl Acad. Sci. USA 112, 12705–12710 (2015).
Ma, Z. et al. Self-organizing human cardiac microchambers mediated by geometric confinement. Nat. Commun. 6, 7413 (2015).
Richards, D. J. et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 4, 446–462 (2020).
Bliley, J. M. et al. Dynamic loading of human engineered heart tissue enhances contractile function and drives a desmosome-linked disease phenotype. Sci. Transl. Med. 13, eabd1817 (2021).
Eschenhagen, T. et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J. 11, 683–694 (1997).
Hansen, A. et al. Development of a drug screening platform based on engineered heart tissue. Circ. Res. 107, 35–44 (2010).
MacQueen, L. A. et al. A tissue-engineered scale model of the heart ventricle. Nat. Biomed. Eng. 2, 930–941 (2018).
Nunes, S. S. et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat. Methods 10, 781–787 (2013).
Shadrin, I. Y. et al. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat. Commun. 8, 1825 (2017).
Zhao, Y. et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell 176, 913–927 e918 (2019).
Zimmermann, W. H. et al. Tissue engineering of a differentiated cardiac muscle construct. Circ. Res. 90, 223–230 (2002).
Huebsch, N. et al. Metabolically driven maturation of human-induced-pluripotent-stem-cell-derived cardiac microtissues on microfluidic chips. Nat. Biomed. Eng. 6, 372–388 (2022).
Legant, W. R. et al. Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. Proc. Natl Acad. Sci. USA 106, 10097–10102 (2009).
Kupfer, M. E. et al. In situ expansion, differentiation and electromechanical coupling of human cardiac muscle in a 3D bioprinted, chambered organoid. Circ. Res. 127, 207–224 (2020).
Lee, A. et al. 3D bioprinting of collagen to rebuild components of the human heart. Science 365, 482–487 (2019).
Pati, F. et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 5, 3935 (2014).
Acknowledgements
This work is supported by National Institutes of Health grants F32 HL152483 (to S.C.); R01 HL113006, R01 HL141371, R01 HL141851, R01 HL146690, R01 HL150693 and R01 HL163680 (to J.C.W.); and UH3 EB025765, P41 EB027062 and 3R01 HL076485 (to G.V.-N.).
Author information
Authors and Affiliations
Contributions
S.C. researched data, designed the figures and wrote the manuscript. S.C., D.E.D., K.W.L., G.V.-N. and J.C.W. contributed substantially to the discussion of content, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.C.W. is a cofounder of Greenstone Biosciences and G.V.-N. is a cofounder of Tara Biosystems; however, the work presented here is independent. The other authors report no competing interests.
Peer review
Peer review information
Nature Methods thanks Aitor Aguirre, Milena Bellin, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Nina Vogt, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cho, S., Discher, D.E., Leong, K.W. et al. Challenges and opportunities for the next generation of cardiovascular tissue engineering. Nat Methods 19, 1064–1071 (2022). https://doi.org/10.1038/s41592-022-01591-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-022-01591-3
This article is cited by
-
Ultrasound-assisted tissue engineering
Nature Reviews Bioengineering (2024)
-
3D Printing Approaches to Engineer Cardiac Tissue
Current Cardiology Reports (2023)
-
Control of the post-infarct immune microenvironment through biotherapeutic and biomaterial-based approaches
Drug Delivery and Translational Research (2023)
-
The heterocellular heart: identities, interactions, and implications for cardiology
Basic Research in Cardiology (2023)