Abstract
Transcription at most promoters is divergent, initiating at closely spaced oppositely oriented core promoters to produce sense transcripts along with often unstable upstream antisense transcripts (uasTrx). How antisense transcription is regulated and to what extent it is coordinated with sense transcription is not well understood. Here, by combining acute degradation of the multi-functional transcription factor CTCF and nascent transcription measurements, we find that CTCF specifically suppresses antisense but not sense transcription at hundreds of divergent promoters. Primary transcript RNA-FISH shows that CTCF lowers burst fraction but not burst intensity of uasTrx and that co-bursting of sense and antisense transcripts is disfavored. Genome editing, chromatin conformation studies and high-resolution transcript mapping revealed that precisely positioned CTCF directly suppresses the initiation of uasTrx, in a manner independent of its architectural function. In sum, CTCF shapes the transcriptional landscape in part by suppressing upstream antisense transcription.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Sequencing data of ChIP-seq and PRO-seq experiments are deposited in the Gene Expression Omnibus (GEO) under accession no. GSE173444. CTCF ChIP-seq and RAD21 ChIP-seq data were obtained from GEO under accession no. GSE150418 by Luan et al.27 (https://doi.org/10.1016/j.celrep.2021.108783). RNA-seq and ChIP-seq data are from GEO under accession no. GSE104334 by Rao et al.43 (https://doi.org/10.1016/j.cell.2017.09.026) and no. GSE98671 by Nora et al.32 (https://doi.org/10.1016/j.cell.2017.05.004). Source data are provided with this paper.
Code availability
The code used to process PRO-seq data is available at https://github.com/zhezhangsh/PROseqR.
References
Seila, A. C. et al. Divergent transcription from active promoters. Science 322, 1849–1851 (2008).
Core, L. J., Waterfall, J. J. & Lis, J. T. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 322, 1845–1848 (2008).
Preker, P. et al. RNA exosome depletion reveals transcription upstream of active human promoters. Science 322, 1851–1854 (2008).
Bagchi, D. N. & Iyer, V. R. The determinants of directionality in transcriptional initiation. Trends Genet. 32, 322–333 (2016).
Murray, S. C. et al. Sense and antisense transcription are associated with distinct chromatin architectures across genes. Nucleic Acids Res. 43, 7823–7837 (2015).
Seila, A. C., Core, L. J., Lis, J. T. & Sharp, P. A. Divergent transcription: a new feature of active promoters. Cell Cycle 8, 2557–2564 (2009).
Xu, Z. et al. Bidirectional promoters generate pervasive transcription in yeast. Nature 457, 1033–1037 (2009).
Wyers, F. et al. Cryptic Pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121, 725–737 (2005).
van Dijk, E. L. et al. XUTs are a class of Xrn1-sensitive antisense regulatory non-coding RNA in yeast. Nature 475, 114–117 (2011).
Rhee, H. S. & Pugh, B. F. Genome-wide structure and organization of eukaryotic pre-initiation complexes. Nature 483, 295–301 (2012).
Scruggs, B. S. et al. Bidirectional transcription arises from two distinct hubs of transcription factor binding and active chromatin. Mol. Cell 58, 1101–1112 (2015).
Andersson, R., Sandelin, A. & Danko, C. G. A unified architecture of transcriptional regulatory elements. Trends Genet. 31, 426–433 (2015).
Duttke, S. H. C. et al. Human promoters are intrinsically directional. Mol. Cell 57, 674–684 (2015).
Marquardt, S. et al. A chromatin-based mechanism for limiting divergent noncoding transcription. Cell 158, 462 (2014).
Kapranov, P. et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 316, 1484–1488 (2007).
Trinklein, N. D. et al. An abundance of bidirectional promoters in the human genome. Genome Res. 14, 62–66 (2004).
Core, L. J. et al. Analysis of nascent RNA identifies a unified architecture of initiation regions at mammalian promoters and enhancers. Nat. Genet. 46, 1311–1320 (2014).
Phillips, J. E. & Corces, V. G. CTCF: master weaver of the genome. Cell 137, 1194–1211 (2009).
Khoury, A. et al. Constitutively bound CTCF sites maintain 3D chromatin architecture and long-range epigenetically regulated domains. Nat. Commun. 11, 54 (2020).
Kubo, N. et al. Promoter-proximal CTCF binding promotes distal enhancer-dependent gene activation. Nat. Struct. Mol. Biol. 28, 152–161 (2021).
Nora, E. P. et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485, 381–385 (2012).
Thiecke, M. J. et al. Cohesin-dependent and -independent mechanisms mediate chromosomal contacts between promoters and enhancers. Cell Rep. 32, 107929 (2020).
Wutz, G. et al. Topologically associating domains and chromatin loops depend on cohesin and are regulated by CTCF, WAPL and PDS5 proteins. EMBO J. 36, 3573–3599 (2017).
Zuin, J. et al. Cohesin and CTCF differentially affect chromatin architecture and gene expression in human cells. Proc. Natl Acad. Sci. USA 111, 996–1001 (2014).
Busslinger, G. A. et al. Cohesin is positioned in mammalian genomes by transcription, CTCF and Wapl. Nature 544, 503–507 (2017).
Hyle, J. et al. Acute depletion of CTCF directly affects MYC regulation through loss of enhancer-promoter looping. Nucleic Acids Res. 47, 6699–6713 (2019).
Luan, J. et al. Distinct properties and functions of CTCF revealed by a rapidly inducible degron system. Cell Rep. 34, 108783 (2021).
Mattick, J. S. & Makunin, I. V. Non-coding RNA. Hum. Mol. Genet. 15, R17–R29 (2006).
Kwak, H., Fuda, N. J., Core, L. J. & Lis, J. T. Precise maps of RNA polymerase reveal how promoters direct initiation and pausing. Science 339, 950–953 (2013).
Bornelöv, S., Komorowski, J. & Wadelius, C. Different distribution of histone modifications in genes with unidirectional and bidirectional transcription and a role of CTCF and cohesin in directing transcription. BMC Genomics 16, 300 (2015).
Shen, Y. et al. A map of the cis-regulatory sequences in the mouse genome. Nature 488, 116–120 (2012).
Nora, E. P. et al. Targeted degradation of CTCF decouples local insulation of chromosome domains from genomic compartmentalization. Cell 169, 930–944 (2017).
Fudenberg, G. et al. Formation of chromosomal domains by loop extrusion. Cell Rep. 15, 2038–2049 (2016).
Sanborn, A. L. et al. Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc. Natl Acad. Sci. USA 112, E6456–E6465 (2015).
Dixon, J. R. et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380 (2012).
Tan-Wong, S. M. et al. Gene loops enhance transcriptional directionality. Science 338, 671–675 (2012).
Simonis, M. et al. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C). Nat. Genet. 38, 1348–1354 (2006).
van de Werken, H. J. G. et al. Robust 4C-seq data analysis to screen for regulatory DNA interactions. Nat. Methods 9, 969–972 (2012).
Krijger, P. H. L., Geeven, G., Bianchi, V., Hilvering, C. R. E. & de Laat, W. 4C-seq from beginning to end: a detailed protocol for sample preparation and data analysis. Methods 170, 17–32 (2020).
Geeven, G., Teunissen, H., de Laat, W. & de Wit, E. peakC: a flexible, non-parametric peak calling package for 4C and Capture-C data. Nucleic Acids Res. 46, e91 (2018).
Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Schwarzer, W. et al. Two independent modes of chromatin organization revealed by cohesin removal. Nature 551, 51–56 (2017).
Rao, S. S. P. Cohesin loss eliminates all loop. Cell Domains 4, 24–26 (2017).
Golding, I., Paulsson, J., Zawilski, S. M. & Cox, E. C. Real-time kinetics of gene activity in individual bacteria. Cell 123, 1025–1036 (2005).
Chubb, J. R., Trcek, T., Shenoy, S. M. & Singer, R. H. Transcriptional pulsing of a developmental gene. Curr. Biol. 16, 1018–1025 (2006).
Raj, A., Peskin, C. S., Tranchina, D., Vargas, D. Y. & Tyagi, S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol. 4, e309 (2006).
Filippova, G. N. et al. An exceptionally conserved transcriptional repressor, CTCF, employs different combinations of zinc fingers to bind diverged promoter sequences of avian and mammalian c-myc oncogenes. Mol. Cell. Biol. 16, 2802–2813 (1996).
Shukla, S. et al. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature 479, 74–79 (2011).
Mayer, A. et al. Native elongating transcript sequencing reveals human transcriptional activity at nucleotide resolution. Cell 161, 541–554 (2015).
Booth, G. T., Wang, I. X., Cheung, V. G. & Lis, J. T. Corrigendum: divergence of a conserved elongation factor and transcription regulation in budding and fission yeast. Genome Res. 26, 1010–1011 (2016).
Tome, J. M., Tippens, N. D. & Lis, J. T. Single-molecule nascent RNA sequencing identifies regulatory domain architecture at promoters and enhancers. Nat. Genet. 50, 1533–1541 (2018).
Wu, A. C. K. et al. Repression of divergent noncoding transcription by a sequence-specific transcription factor. Mol. Cell 72, 942–954 (2018).
Sansó, M. et al. Cdk9 and H2Bub1 signal to Clr6-CII/Rpd3S to suppress aberrant antisense transcription. Nucleic Acids Res. 48, 7154–7168 (2020).
Baluapuri, A. et al. MYC recruits SPT5 to RNA polymerase II to promote processive transcription elongation. Mol. Cell 74, 674–687 (2019).
Xue, Y. et al. Mot1, Ino80C and NC2 function coordinately to regulate pervasive transcription in yeast and mammals. Mol. Cell 67, 594–607 (2017).
Tan-Wong, S. M., Dhir, S. & Proudfoot, N. J. R-loops promote antisense transcription across the mammalian genome. Mol. Cell 76, 600–616 (2019).
Nojima, T. et al. Deregulated expression of mammalian lncRNA through loss of SPT6 induces R-loop formation, replication stress and cellular senescence. Mol. Cell 72, 970–984 (2018).
Hou, C., Zhao, H., Tanimoto, K. & Dean, A. CTCF-dependent enhancer-blocking by alternative chromatin loop formation. Proc. Natl Acad. Sci. USA 105, 20398–20403 (2008).
Hsu, S. C. et al. The BET protein BRD2 cooperates with CTCF to enforce transcriptional and architectural boundaries. Mol. Cell 66, 102–116 (2017).
Bartman, C. R., Hsu, S. C., Hsiung, C. C.-S., Raj, A. & Blobel, G. A. Enhancer regulation of transcriptional bursting parameters revealed by forced chromatin looping. Mol. Cell 62, 237–247 (2016).
Lim, H. M., Lewis, D. E. A., Lee, H. J., Liu, M. & Adhya, S. Effect of varying the supercoiling of DNA on transcription and its regulation. Biochemistry 42, 10718–10725 (2003).
Peter, B. J. et al. Genomic transcriptional response to loss of chromosomal supercoiling in Escherichia coli. Genome Biol. 5, R87 (2004).
Lee, J., Krivega, I., Dale, R. K. & Dean, A. The LDB1 complex co-opts CTCF for erythroid lineage-specific long-range enhancer interactions. Cell Rep. 19, 2490–2502 (2017).
Baniahmad, A., Steiner, C., Köhne, A. C. & Renkawitz, R. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell 61, 505–514 (1990).
Weiss, M. J., Yu, C. & Orkin, S. H. Erythroid-cell-specific properties of transcription factor GATA-1 revealed by phenotypic rescue of a gene-targeted cell line. Mol. Cell. Biol. 17, 1642–1651 (1997).
Luppino, J. M. et al. Cohesin promotes stochastic domain intermingling to ensure proper regulation of boundary-proximal genes. Nat. Genet. 52, 840–848 (2020).
Stonestrom, A. J. et al. Functions of BET proteins in erythroid gene expression. Blood 125, 2825–2834 (2015).
Cong, L. & Zhang, F. Genome engineering using CRISPR-Cas9 system. Methods Mol. Biol. 1239, 197–217 (2015).
Reimer, K. A., Mimoso, C., Adelman, K. & Neugebauer, K. M. Co-transcriptional splicing regulates 3′ end cleavage during mammalian erythropoiesis. Mol. Cell 81, 998–1012 (2021).
Splinter, E., de Wit, E., van de Werken, H. J. G., Klous, P. & de Laat, W. Determining long-range chromatin interactions for selected genomic sites using 4C-seq technology: from fixation to computation. Methods 58, 221–230 (2012).
van de Werken, H. J. G. et al. 4C technology: protocols and data analysis. Methods Enzymol. 513, 89–112 (2012).
Femino, A. M., Fay, F. S., Fogarty, K. & Singer, R. H. Visualization of single RNA transcripts in situ. Science 280, 585–590 (1998).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Raj, A., Rifkin, S. A., Andersen, E. & van Oudenaarden, A. Variability in gene expression underlies incomplete penetrance. Nature 463, 913–918 (2010).
Acknowledgements
We are grateful to Hardison, Raj, Lis and Blobel laboratories for insightful discussions. We thank the staff at the flow cytometry core of The Children’s Hospital of Philadelphia. This work was supported by NIH grants no. R01 DK58044 to G.A.B., R24 DK106766 to G.A.B. and R.C.H., T32 HL07439 to C.M.S., R01GM121613 to R.C.H. and U01 DK127405 to G.A.B. and A.R., an EMBO long-term fellowship (ALTF 540-2018) to M.W.V. and an American Heart Association postdoctoral fellowship 836074 to M.W.V. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J.L. and G.A.B. conceived the study and designed experiments. J.L. and C.A.K. performed ChIP-seq experiments. J.L., M.W.V. and B.M.G. performed ChIP-seq analysis. J.L., C.M.S. and A.H. performed CRISPR editing experiments and 4C experiments. 4C results were analyzed by J.L., M.W.V. and S.Z. J.L. performed PRO-seq experiments. J.L., M.W.V. and Z.Z. analyzed PRO-seq results with advice from J.M.T. and J.T.L. C.M.S., M.G. and A.H. performed smFISH experiments, with data analyzed by C.M.S., J.L. and A.C. under the supervision of A.R. J.L., M.W.V. and G.A.B. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Elphège Nora and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Carolina Perdigoto, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
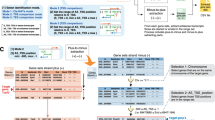
Extended Data Fig. 1 CTCF depletion leads to widespread uasTrx upregulation at divergent promoters.
a, Distribution of uasTrx lengths, grouped by changes in response to CTCF depletion. b, Row-linked heatmaps showing Pol II occupancy at active promoters, grouped by antisense changes (up, n = 376; unchanged, n = 9,632) upon CTCF depletion, sorted by occupancy level, and shown with respect to sense orientation. c, RT–qPCR of uasTrx for Ahcyl1 at indicated time points after CTCF depletion. Transcripts were normalized to Gapdh (error bar: SEM; n = 4). d, same as (c) but quantifying nascent sense transcripts. e, Scatterplot comparing transcriptional changes in gene body (GB) versus uasTrx. Data points grouped and colored based on uasTrx changes. P values were calculated by Spearman rank correlation test, r is the correlation coefficient. f, Log-transformed PRO-seq fold changes in GB after CTCF depletion, grouped by uasTrx changes. Lower and upper box ends represent the first and third quartiles with the median indicated as a horizontal line within the box. Mean is indicated by a circle within the box. Whiskers define the smallest and largest values within 1.5 times the interquartile range below the first or above the third quartile, respectively. Outliers are plotted as individual dots. g, Transcriptional changes in uasTrx and GB after CTCF depletion. h, Percentage of promoters with and without proximal (±100 bp) CBSs as a function of uasTrx changes. i, Correlation between PRO-seq changes and CTCF loss at uasTrx with proximal (±100 bp) CTCF binding. Linear regression line shown in magenta. P values were calculated by Spearman rank correlation test, r is the correlation coefficient.
Extended Data Fig. 2 CTCF depletion in human HCT-116 and mESCs leads to antisense transcriptional changes.
a, Fraction of TSSs detected in the indicated numbers of mouse tissues where CTCF binds in proximity (within ± 100 bp), grouped by uasTrx changes. b, PRO-seq MA plot of control versus CTCF-depleted cells on the antisense strand (−1000 bp to +200 relative to annotated TSS) in human HCT-116 cells. Differentially expressed transcripts highlighted in color. c, Browser views of CTCF ChIP-seq (mm9 liftover from Rao et al., 2014) and PRO-seq signals at Gstp1 and Tap2 loci in HCT-116 cells. Arrows highlight location of CTCF-repressed uasTrx. Arrow color indicates uasTrx strandedness. kd, knockdown. d, RNA-seq MA plot of control versus CTCF-depleted cells on the antisense strand (−1000 bp to +200 relative to annotated TSS) in mESCs. Differentially expressed transcripts highlighted in color. e, Row-linked heatmaps showing CTCF occupancy at active promoters, grouped by uasTrx changes, sorted by binding enrichment levels, and shown with respect to sense orientation in HCT-116 cells and mESCs. f, Correlation between uasTrx and GB changes after CTCF depletion in PRO-seq data from HCT-116 cells, and RNA-seq data from mESCs. P value was calculated by Spearman rank correlation test; r is the correlation coefficient. g, Transcriptional changes in uasTrx and GB after CTCF depletion in PRO-seq from HCT-116 cells and RNA-seq data from mESCs. h, Log-transformed PRO-seq and RNA-seq fold changes in GB after CTCF depletion in HCT-116 cells and mESCs, respectively. Lower and upper box ends represent the first and third quartiles with the median indicated as a horizontal line within the box. Mean is indicated by a circle within the box. Whiskers define the smallest and largest values within 1.5 times the interquartile range below the first or above the third quartile, respectively. Outliers are plotted as individual dots. i, Log-transformed RNA-seq fold change in uasTrx in indicated conditions over control in mESCs. j, Brower views of CTCF ChIP-seq and RNA-seq signals at Ahcyl1, Azi2 and Rps3a1 loci in mESCs. Orange to yellow boxes and black arrow indicate (direction of) uasTrx.
Extended Data Fig. 3 Affected promoters are associated with architectural features.
a, Row-linked heatmaps showing RAD21 occupancy at sites with proximal (±100 bp) CTCF binding (up, n = 319; unchanged, n = 1,527), grouped by CTCF depletion-elicited uasTrx changes, sorted in the same order as Fig. 1i, and shown with respect to sense orientation. b, Distribution of looping frequencies of upregulated versus unchanged uasTrx with proximal (±100 bp) CTCF binding. P value calculated by Wilcoxon signed-rank test. c, Averaged insulation score centered at annotated TSS with proximal CTCF binding (up n = 319, unchanged n = 1,527) over 0.2 Mb window, plotted with respect to sense orientation, and grouped by uasTrx changes. d, Gene ontology terms enriched at genes with activated uasTrx. e, Genome browser views of CTCF ChIP-seq, PRO-seq and 4C-seq signals at Azi2. 4C-seq anchored at Azi2 promoter with (4 h auxin) and without (0 h auxin) CTCF degradation. Orange anchor indicates 4C-seq viewpoint. Sites of interest are indicated below the track and highlighted by dashed boxes. f, Same as in (e) for the Rps3a1 locus. g, Genome browser views of bulk CTCF ChIP-seq and PRO-seq at the Ahcyl1 locus. Predicted CTCF motif is highlighted in green and genotype of edited Ahcyl1 clones shown in Fig. 2c is depicted. h, Genotype of Azi2 TSS-proximally edited clones. Predicted CTCF motif highlighted in green. i, Left, CTCF ChIP-qPCR showing abrogation of CTCF binding at Ahcyl1 TSS-proximal CBS in mutants shown in Fig. 2c. Right, Ahcyl1 distal CBS served as a control for ChIP efficiency (error bar: SEM; n = 3). j, Same as in (i) for Azi2 TSS-proximal CBS.
Extended Data Fig. 4 CRISPR/Cas9-mediated deletion of TSS-proximal CBS leads to uasTrx activation.
a, Genome browser tracks of CTCF ChIP-seq and PRO-seq shown at the Azi2 locus on top. Representative 4C-seq profiles of control/mutant Azi2 clones. Regions of interest are indicated below tracks and highlighted by dashed boxes. Similar observations were made in 2 independent 4C-seq experiments. Orange anchor indicates 4C-seq viewpoint. Scissors indicate CRISPR/Cas9-edited region. b, RT–qPCR of Ahcyl1 uasTrx in control and edited clones. Transcripts were normalized to Gapdh (error bar: SEM; n = 4, except for uasTrx control, proximal and distal CBS deletion rep1 for which n = 3). c, same as in (b) for sense Ahcyl1 transcripts. d, RT–qPCR of Azi2 uasTrx in control and edited clones. Transcripts were normalized to Gapdh (error bar: SEM; n = 4 for primer pair 1, n = 2 for primer pair 2). e, same as in (d) for sense Azi2 transcripts. f, Genotype of Rps3a1 TSS-proximal CBS edited clones. Predicted CTCF motif highlighted in green. g, Left, CTCF ChIP-qPCR showing abrogation of CTCF binding at Rps3a1 TSS-proximal CBS in mutants. Right, distal CBS served as a control for ChIP efficiency (error bar: SEM; n = 3). h, RT–qPCR of Rps3a1 uasTrx in control and edited clones. Transcripts were normalized to Gapdh (error bar: SEM; n = 3). i, same as in (h) for sense Rps3a1 transcripts.
Extended Data Fig. 5 CRISPR/Cas9-mediated deletion of distal CBS does not lead to uasTrx activation.
a, Genotype of distal site A edited Ahcyl1 clones shown in Fig. 2c. Predicted CTCF motif is highlighted in green. b, Left, CTCF ChIP-qPCR showing abrogation of CTCF binding at distal anchor A in clones distal site A rep1 and 2 shown in Fig. 2c (error bar: SEM; n = 2). Right, proximal CBS served as a control for ChIP efficiency (error bar: SEM; n = 2). c, Same as in (a) for Ahcyl1 distal site B. d, same as in (a) for Ahcyl1 distal site C. e, same as in (b) for Ahcyl1 distal site B and C. f, Genotype of distal site A and B edited Azi2 clones.
Extended Data Fig. 6 Removal of chromatin-bound cohesin does not recapitulate CTCF-induced uasTrx changes.
a, PRO-seq MA plot of control versus NIPBL-depleted HCT-116 cells on uasTrx expression (−1000 bp to +200 relative to annotated TSS). Differentially expressed transcripts highlighted in color. b, Same as (a) but of RAD21-depleted HCT-116 cells. c, Scatterplot comparing log-transformed 5’ PRO-seq fold changes in uasTrx and GB. P value was calculated by Spearman rank correlation test; r is the correlation coefficient. d, Table showing the number and percentage of uasTrx and GB changes after RAD21 depletion in HCT-116 cells. e, Box plot showing log-transformed PRO-seq fold changes in GBs after RAD21 depletion in HCT-116 cells. Lower and upper box ends represent the first and third quartiles with the median indicated as a horizontal line within the box. Mean is indicated by a circle within the box. Whiskers define the smallest and largest values within 1.5 times the interquartile range below the first or above the third quartile, respectively. Outliers are plotted as individual dots. f, Left, row-linked heatmap showing CTCF occupancy at active promoters, grouped by uasTrx changes after RAD21 depletion, sorted by occupancy levels, and shown with respect to sense orientation. Right, same as left, but plotting RAD21 occupancy. Note that neither CTCF nor RAD21 is enriched at genes with upregulated uasTrx.
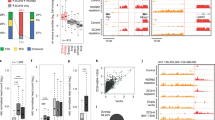
Extended Data Fig. 7 CTCF inhibits antisense burst fraction; sense/antisense co-bursting is disfavored.
a, Table showing raw smFISH allele counts. b, Experimental outline for RNA half-life estimation. c, Representative smFISH images of 3 independent replicates before and after DRB treatment at Ahcyl1 and Rps3a1. d, Left, box plot showing uasTrx and sense burst fractions at Ahcyl1 before and after DRB treatment. Right, same as left but quantifying burst sizes. Lower and upper box ends represent the first and third quartiles with the median indicated as a horizontal line within the box. Mean is indicated by a circle within the box. Whiskers define the smallest and largest values within 1.5 times the interquartile range below the first or above the third quartile, respectively. Outliers are plotted as individual dots. P values were calculated by two-sample t-test. e, Same as (d) but for Rps3a1. f, RT–qPCR measuring nascent sense and uasTrx levels at Ahcyl1 and Rps3a1 before and after DRB treatment. Transcripts were normalized to Gapdh and plotted relative to time 0 h (error bar: SEM; n = 4).
Extended Data Fig. 8 CTCF inhibits antisense transcription initiation through precise positioning.
a, 5’ end mapping in a 100 bp window of sense reads on a training set of 1,395 TSSs with the highest PRO-seq reads mapped to ±50 bp around the TSS and no other start sites within 1000 base pairs. b, Metaplot of sense and antisense 5’ end PRO-seq mapping, centered at annotated sense TSSs and plotted with respect to sense orientation for genes with upregulated uasTrx. Solid lines and shades show average signals and the 12.5/87.5 percentiles, respectively. c, Same as in (b) for unchanged uasTrx genes. d, heatmap of 5’ end mapping at unchanged promoters with a portion of sites (10%; ‘downstream proximal’) manually picked from the rest (‘others’), which demonstrates a CTCF distribution similar to that at uasTrx up genes. e, Related to (d), plotting PRO-seq changes in uasTrx at unaffected promoters, grouped based on CTCF positioning relative to 5’ PRO-seq signals. Lower and upper box ends represent the first and third quartiles with the median indicated as a horizontal line within the box. Whiskers define the smallest and largest values within 1.5 times the interquartile range below the first or above the third quartile, respectively. Outliers are plotted as individual dots. f, Related to (d), comparing uasTrx changes and CTCF binding loss at unaffected promoters, grouped based on CTCF positioning relative to 5’ PRO-seq signals.
Extended Data Fig. 9 Sense transcription initiation mostly unaffected upon uasTrx increase.
a, Heatmap of 5’ end mapping at genes with unchanged uasTrx (n = 1,201) that exhibit proximal CTCF binding and high-confidence CTCF motif(s) (motif prediction score>75), centered on CTCF motifs, sorted by mean antisense signal densities over the center 200 bp and shown with respect to sense orientation. Black line highlights CTCF motif locations. b, Metaplot of data in (a). c, 5’ end mapping of sense and uasTrx transcription at the Eif2s1 gene. Yellow star indicates annotated sense TSS, CTCF motif indicated in green. d, Same as in (c) for Nsmce4a. e, Same as in (c) for Rbm17. f, Same as in (c) for Stk4. g, Violin plot showing sense changes at TSS −50 to +150 bp. P value comparing conditions was calculated using a Wilcoxon rank sum test. Significant differentially enriched TSSs are indicated in colors. Boxes within violins represent first and third quartiles with the median indicated as an horizontal line within the box. Whiskers define 1.5× the interquartile range. Outliers are plotted as individual dots.
Supplementary information
Supplementary Tables 1–5
1- Differential uasTrx expression analysis between control and auxin-mediated CTCF depletion in G1E-ER4 cells. 2- Differential uasTrx and gene expression analysis between control and CTCF or NIPBL knock down in HCT-116 cells. 3- Predicted promoter-proximal CTCF motif coordinates and scores. 4- Oligonucleotide sequences used for CRISPR, RT–qPCR, ChIP-qPCR and 4C-seq. 5- RNA-FISH probe coordinates and sequences.
Source data
Source Data Fig. 1
Characteristics of uasTrx that go up or are unchanged after auxin-mediated CTCF degradation.
Source Data Fig. 4
Predicted CTCF motif coordinates and score for CBSs proximal to uasTrx that go up or are unchanged after auxin-mediated CTCF degradation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luan, J., Vermunt, M.W., Syrett, C.M. et al. CTCF blocks antisense transcription initiation at divergent promoters. Nat Struct Mol Biol 29, 1136–1144 (2022). https://doi.org/10.1038/s41594-022-00855-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-022-00855-y
This article is cited by
-
Interplay between coding and non-coding regulation drives the Arabidopsis seed-to-seedling transition
Nature Communications (2024)