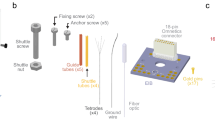
Abstract
How dynamic activity in neural circuits gives rise to behavior is a major area of interest in neuroscience. A key experimental approach for addressing this question involves measuring extracellular neuronal activity in awake, behaving animals. Recently developed Neuropixels probes have provided a step change in recording neural activity in large tissue volumes with high spatiotemporal resolution. This protocol describes the chronic implantation of Neuropixels probes in mice and rats using compact and reusable 3D-printed fixtures. The fixtures facilitate stable chronic in vivo recordings in freely behaving rats and mice. They consist of two parts: a covered main body and a skull connector. Single-, dual- and movable-probe fixture variants are available. After completing an experiment, probes are safely recovered for reimplantation by a dedicated retrieval mechanism. Fixture assembly and surgical implantation typically take 4–5 h, and probe retrieval takes ~30 min, followed by 12 h of incubation in probe cleaning agent. The duration of data acquisition depends on the type of behavioral experiment. Since our protocol enables stable, chronic recordings over weeks, it enables longitudinal large-scale single-unit data to be routinely obtained in a cost-efficient manner, which will facilitate many studies in systems neuroscience.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
Data availability
Source data for Fig. 11 are available in our GitHub repository (https://github.com/nerf-common/chronic-neuropixels-protocol).
References
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Steinmetz, N. A., Zatka-Haas, P., Carandini, M. & Harris, K. D. Distributed coding of choice, action and engagement across the mouse brain. Nature 576, 266–273 (2019).
Hartley, T., Lever, C., Burgess, N. & O’Keefe, J. Space in the brain: how the hippocampal formation supports spatial cognition. Philos. Trans. R. Soc. B Biol. Sci. 369, 20120510 (2014).
Krupic, J., Bauza, M., Burton, S. & O’Keefe, J. Local transformations of the hippocampal cognitive map. Science 359, 1143–1146 (2018).
Steinmetz, N. A. et al. Neuropixels 2.0: a miniaturized high-density probe for stable, long-term brain recordings. Science 372, eabf4588 (2021).
Berényi, A. et al. Large-scale, high-density (up to 512 channels) recording of local circuits in behaving animals. J. Neurophysiol. 111, 1132–1149 (2014).
Chung, J., Sharif, F., Jung, D., Kim, S. & Royer, S. Micro-drive and headgear for chronic implant and recovery of optoelectronic probes. Sci. Rep. 7, 2773 (2017).
Michon, F. et al. Integration of silicon-based neural probes and micro-drive arrays for chronic recording of large populations of neurons in behaving animals. J. Neural Eng. 13, 046018 (2016).
Van Daal, R. J. J. et al. System for recording from multiple flexible polyimide neural probes in freely behaving animals. J. Neural Eng. 17, 016046 (2020).
Juavinett, A. L., Bekheet, G. & Churchland, A. K. Chronically implanted Neuropixels probes enable high-yield recordings in freely moving mice. eLife https://doi.org/10.7554/eLife.47188 (2019).
Cohen, J. Y., Haesler, S., Vong, L., Lowell, B. B. & Uchida, N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482, 85–88 (2012).
Krynitsky, J. et al. Rodent Arena Tracker (RAT): a machine vision rodent tracking camera and closed loop control system. eNeuro 7, 1–9 (2020).
Siegle, J. H. et al. Open Ephys: an open-source, plugin-based platform for multichannel electrophysiology. J. Neural Eng. 14, 045003 (2017).
Jun, J. J. et al. Real-time spike sorting platform for high-density extracellular probes with ground-truth validation and drift correction. Preprint at bioRxiv https://doi.org/10.1101/101030 (2017).
Pachitariu, M., Steinmetz, N., Kadir, S., Carandini, M. & Harris, K. Fast and accurate spike sorting of high-channel count probes with KiloSort. in Advances in Neural Information Processing Systems 29 (NeurIPS 2016).
Esquivelzeta Rabell, J., Mutlu, K., Noutel, J., Martin del Olmo, P. & Haesler, S. Spontaneous rapid odor source localization behavior requires interhemispheric communication. Curr. Biol. 27, 1542–1548.e4 (2017).
Acknowledgements
We thank H. den Bakker, G. Vincente Ortiz and M. D’Andola for providing their electrophysiological data recorded with the Neuropixels probes and the fixtures. We thank M. Broux for performing the histology. This work was supported by Cohen Veterans Bioscience through a generous grant COH-0011 from S. A. Cohen. R.J.J.v.D. is supported by the Hermesfonds (VLAIO Baekeland mandate, HBC.2018.2114). C.A. is supported by KU Leuven Postdoctoral Mandate (PDM/19/173). S.H. is funded by Research Foundation Flanders (FWO) grant G096219N and KU Leuven C1 grant C14/17/109. F.K. is funded through Flemish Research Project FWO G0D7516N, KU Leuven C1 grant C14/17/042 and European FET-Open grant STARDUST (agreement 767092).
Author information
Authors and Affiliations
Contributions
R.J.J.v.D.: conceptualization of the fixtures; design, prototyping and fabrication of the fixtures; preparing the figures; writing of the original manuscript. C.A.: conceptualization of the fixtures; design and prototyping of the fixture; implantations to animals; data acquisition and analysis; preparing the figures; writing of the original manuscript. F.M.: conceptualization of the fixtures; design and prototyping of the fixtures; implantations to animals; data acquisition and analysis; preparing the figures; writing of the original manuscript. A.A.A.A.: conceptualization of the fixtures; revising the manuscript; funding acquisition. M.K.: conceptualization of the fixtures; revising the manuscript; funding acquisition. F.K.: conceptualization of the fixtures; writing of the original manuscript; funding acquisition. S.H.: conceptualization of the project; writing of the original manuscript; funding acquisition. All authors revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
R.J.J.v.D. is an employee of ATLAS Neuroengineering BV, Leuven, Belgium. A.A.A.A. is managing director of ATLAS Neuroengineering BV, Leuven, Belgium. The remaining authors declare no conflict of interests.
Additional information
Peer review information Nature Protocols thanks Ying Fang, Bozhi Tian and the other, anonymous reviewer(s) for their contribution to the peer review of this work
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Steinmetz, N. A. et al. Science 372, eabf4588 (2021): https://doi.org/10.1126/science.abf4588
Supplementary information
Supplementary Video 1
Freely behaving male C57BL/6J mouse (16 weeks old) in box
Supplementary Video 2
Freely behaving male C57BL/6J mouse (20 weeks old) in open field
Supplementary Data 1
CAD files single probe fixture
Supplementary Data 2
CAD files dual-probe fixture
Supplementary Data 3
CAD files movable-probe fixture
Supplementary Data 4
CAD files stereotaxic adapters
Rights and permissions
About this article
Cite this article
van Daal, R.J.J., Aydin, Ç., Michon, F. et al. Implantation of Neuropixels probes for chronic recording of neuronal activity in freely behaving mice and rats. Nat Protoc 16, 3322–3347 (2021). https://doi.org/10.1038/s41596-021-00539-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00539-9
This article is cited by
-
Acute head-fixed recordings in awake mice with multiple Neuropixels probes
Nature Protocols (2023)
-
Modified Neuropixels probes for recording human neurophysiology in the operating room
Nature Protocols (2023)
-
Framework for automated sorting of neural spikes from Neuralynx-acquired tetrode recordings in freely-moving mice
Bioelectronic Medicine (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.