Abstract
The formation of new blood vessels and the establishment of vascular networks are crucial during brain development, in the adult healthy brain, as well as in various diseases of the central nervous system. Here, we describe a step-by-step protocol for our recently developed method that enables hierarchical imaging and computational analysis of vascular networks in postnatal and adult mouse brains. The different stages of the procedure include resin-based vascular corrosion casting, scanning electron microscopy, synchrotron radiation and desktop microcomputed tomography imaging, and computational network analysis. Combining these methods enables detailed visualization and quantification of the 3D brain vasculature. Network features such as vascular volume fraction, branch point density, vessel diameter, length, tortuosity and directionality as well as extravascular distance can be obtained at any developmental stage from the early postnatal to the adult brain. This approach can be used to provide a detailed morphological atlas of the entire mouse brain vasculature at both the postnatal and the adult stage of development. Our protocol allows the characterization of brain vascular networks separately for capillaries and noncapillaries. The entire protocol, from mouse perfusion to vessel network analysis, takes ~10 d.
Similar content being viewed by others
Introduction
The growth of tissues and organs during embryonic and postnatal development, tissue homeostasis in adulthood, and tumor growth require adequate vascularization via the establishment of complex 3D vascular networks and subsequent tissue perfusion and oxygenation1,2,3. In these physiological and pathological conditions, the formation of new blood vessels can occur via different mechanisms1,2,4,5,6. Vasculogenesis is defined as the de novo formation of blood vessels during which bone marrow—respectively blood islets—derived angioblasts (endothelial progenitor cells) differentiate into endothelial cells1,2. Occurring throughout life, vasculogenesis is most active during embryonic and early postnatal development. Sprouting angiogenesis describes the formation of new blood vessels from preexisting ones2,3, relying on a number of spatiotemporally tightly regulated cellular mechanisms involving cell adhesion and spreading, sprout initiation and formation, endothelial tip cell migration, endothelial stalk cell proliferation, tube formation and vessel anastomosis1,2. Another mechanism of physiological vessel formation that also occurs throughout the entire life span is intussusceptive angiogenesis, the process during which preexisting vessels split and give rise to daughter vessels without a corresponding increase in the number of endothelial cells1,7,8. Besides these three modes of physiological blood vessel formation, which can also occur in pathological settings, three additional mechanisms modifying blood vessels have been described in pathological settings (mainly tumors)4. These include vascular co-option, describing the organization of tumor cells into perivascular cuffs around existing microvessels of the surrounding healthy tissue to allow the oxygenation of the early tumor mass4,9,10; vascular mimicry, defined as the integration of tumor cells into the blood vessel wall mimicking endothelial cells11,12; and glioma stem-to-endothelial or glioma stem-to-pericyte transdifferentiation13,14,15,16. The relative importance of these modes of vessel formation and modification in the formation of a well-established 3D vascular network depends on the developmental stage and the pathology in the respective organs1,2,5 and is still not completely understood1.
Angiogenesis and vascular network formation in the postnatal and adult mouse brain
The human brain crucially depends on its vasculature for proper functioning as, despite accounting for only 2% of the body mass, it consumes 20% of the cardiac output and 20% of the oxygen and glucose of the entire body, corresponding to a tenfold higher rate of energy metabolism compared with an equivalent tissue volume in the rest of the body17,18. The brain vasculature is constituted of a complex 3D vascular network of 644 km (400 miles)19,20, anatomically organized so that each individual neuron is not more than 22–27 μm (in humans, depending on the cortical depth) away from the closest microcapillary, thereby assuring proper oxygen delivery21,22. In the mouse brain, the absolute numbers are obviously lower but show similar relative values22,23,24.
Cerebral angiogenesis in mice and humans (and in all mammals) is highly active during embryonic and postnatal brain development and almost quiescent in the adult healthy brain2,3,25, but is reactivated in vascular-dependent central nervous system (CNS) pathologies6,26,27. Thus, addressing how the brain vasculature network develops from the developmental to the adult stage is crucial for a better understanding of underlying vascular dynamics also in pathological conditions6,28.
In the developing mouse brain, the functional, perfused vascular network of the CNS parenchyma is established following multiple distinct steps: it starts at around embryonic day 7.5–8.5 (E7.5–E8.5) with the formation of the perineural vascular plexus (PNVP, responsible for the vascularization of the future meninges)29,30,31 via vasculogenesis in the ventral neural tube following a ventral-to-dorsal gradient6,32,33,34. In a subsequent step, at ~E9.5, vascular endothelial sprouts emanating from the PNVP invade the CNS parenchyma in a caudal to cranial direction, vascularizing the CNS tissue via sprouting angiogenesis, thereby forming the intraneural vascular plexus (INVP)3,6,28,32,35,36. The INVP is expanded and refined via subsequent vessel sprouting, branching and anastomosis6,32,35,36 while these vascular sprouts migrate from the PNVP radially inwards towards the ventricles3,28,35. These important angiogenic processes of blood vessel formation and remodeling start during embryonic CNS development32,35 and continue at the postnatal stage of brain development17,28,31,37,38,39. The expansion and remodeling of the intricate 3D CNS vascular network is required in order to adapt to local metabolic needs and neural activity28,37,38,40. To stabilize the newly formed blood vessels, perivascular cell types of the neurovascular unit, such as vascular smooth muscle cells, pericytes, neurons and astrocytes, are recruited to the vessel wall3,26,36,41,42,43, thereby establishing the mature, functional CNS vasculature3,6,26,41. The establishment of the CNS vascular network via sprouting angiogenesis requires a subset of specialized endothelial cell types: endothelial tip cells at the leading edge of the vascular sprout extend finger-like filopodia to sense environmental cues, thereby guiding the blood vessel sprout towards gradients of proangiogenic growth factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor1,2,3,44,45, platelet-derived growth factor46, ephrins47 and angiopoietins48, and away from gradients of antiangiogenic factors such as thrombospondin49,50, endostatin51, angioarrestin52, netrin-153, semaphorins 3A-B-E-F-G54 or Nogo-A38,39. Behind the leading tip cells, endothelial stalk cells elongate the growing vascular sprout as they proliferate and form the vascular lumen1,2,3,55,56,57. Neighboring sprouts then fuse and form vascular anastomoses, resulting in perfusion of the newly formed vessels1,2,3,56,58,59,60. While the new blood vessel further matures and reaches a functional stage, its endothelial cells acquire a quiescent state, called endothelial phalanx cells1,2,61. Perfusion is a prerequisite for the stabilization and maintenance of a functional vascular network, acting most likely via flow-induced shear-stress-related signaling pathways, such as the sphingosine-1-phosphate receptor-1 signaling62,63,64,65 and the VEGF receptor 2 (ref. 66) and flow-induced dose-dependent expression of the transcription factor Krüppel-like factor 2 (refs. 67,68), which regulates the expression of various antioxidative and antiinflammatory mediators to stabilize the vessel wall. Accordingly, the absence of perfusion of newly formed vascular sprouts leads to vessel regression, fine-tuned mainly by noncanonical Wnt signaling69,70,71. Whereas sprouting angiogenesis and vascular remodeling remain highly dynamic throughout the first postnatal month until around postnatal day 25 (P25)—with a peak in endothelial cell proliferation at around postnatal day 10 (P10)25—most of these redundant newly formed vessel sprouts undergo strategic pruning (most likely influenced by regional metabolic needs related to neural activity), and only a small fraction ends up forming mature perfused capillaries25. After the first month, the number of vessel segments remains largely constant and the vascular network stabilizes, followed by a progressively decreased turnover between formed and eliminated vessels during physiological aging25.
Reactivation of developmentally active angiogenic signaling cascades in vascular-dependent CNS pathologies and the concept of the neurovascular link
Cerebral angiogenesis is highly active during embryonic and postnatal brain development and almost quiescent in the adult healthy brain2,3,25. In a variety of angiogenesis-dependent CNS pathologies such as brain tumors, brain arteriovenous malformations or stroke, developmental signaling programs driving angiogenic growth during development are reactivated1,2,6, thereby activating endothelial and perivascular cells of the neurovascular unit3,6,72. However, which (developmental) molecular signaling cascades are reactivated in these angiogenesis-dependent CNS pathologies and how they regulate angiogenesis and vascular network formation on a cellular and subcellular level is not well understood. Thus, more knowledge is needed to better define the mechanisms of angiogenesis and vascular network formation during brain development, in the adult healthy brain as well as in brain pathologies.
Directly related to this reactivation of developmentally active signaling cascades is the concept of the neurovascular link, defining the cellular and molecular basis of the parallel organization and alignment of arteries and nerves in the brain, which is of crucial importance to understand brain vascular development, both at the embryonic and at the postnatal stage6,26. At the (sub)cellular level, the growth cones of newly formed axons resemble the endothelial tip cells of sprouting blood vessels3. Microenvironmental stimulating and inhibiting signals result in extension and retraction of these structures thereby inducing directed movements of growing nerves and blood vessels6,26,42. At the functional level, numerous common molecular cues that guide both endothelial tip cells and axonal growth cones via these specialized cellular and subcellular structures have been discovered3,6,73. As angiogenesis is highly active during postnatal brain development37,38,74, it provides an adequate model system to address the process of sprouting angiogenesis, which is the predominant mode of vessel formation in the brain6,32. Molecularly, sprouting angiogenesis and endothelial tip cells are regulated via numerous signaling axes. For instance, endothelial tip cells are, similar to axonal growth cones, guided by the interplay of attractive and repulsive cues such as the classical axonal guidance molecules netrins, semaphorins, slits and ephrins, as well as the neural growth inhibitor Nogo-A3,4,6,26,38,39. We have previously shown that, in mice lacking functional Nogo-A, additional endothelial tip cells and vascular sprouts can result in an increased number of perfused blood vessels that integrate into the vascular network28,38.
It is further known that the transition from sprouting endothelial tip cells to functional, perfused blood vessels that integrate into vascular networks is mainly regulated by the VEGF-VEGFR-delta-like ligand 4-Jagged1-Notch pathway1,2,60,75,76,77,78,79,80, thought to be a central pattern generator of vascular sprout- and endothelial tip cell formation. The spatiotemporally tightly regulated balance between endothelial tip and stalk cell numbers and the balance between tip migration and stalk proliferation affect branching frequency and plexus density76. It was also shown that disturbance of these molecular processes may result in nonproductive angiogenesis with the formation of aberrant blood vessels78,79,80,81,82. Importantly, the remodeling of the final perfused vascular network exerting its crucial functions depends on many different factors that include tissue-specific metabolic and mechanical feedbacks from the microenvironment as well as blood-flow-related signaling pathways, respectively71. Nowadays, these processes of vascular remodeling from the developing to the adult, mature stage are incompletely understood60.
For instance, most of the numerously generated vascular sprouts in the first postnatal, highly angiogenic month in the mouse brain undergo pruning, and only a small fraction results in functional, perfused capillaries25. Thereafter, the brain vascular network remains relatively stable. Nevertheless, despite the vascular stability at the adult stage, some vessel formation and elimination continue throughout life25.
Another interesting aspect is that, while we observed a significantly (P < 0.01) increased vessel density in young postnatal mice lacking function Nogo-A, this effect was almost completely gone in the adult Nogo-A−/− mice38, raising questions regarding compensatory mechanisms by other molecules in the Nogo-A−/− mice but also regarding the plasticity of the vasculature in the young postnatal versus the adult stage.
These findings underline the need for a detailed protocol to describe how to quantitatively chart the 3D architecture of the brain vascular network of mice at any postnatal age. The protocol presented here will enable the mechanical and metabolic feedback that is finally responsible for maturation and stability of functional, perfused, 3D vascular networks to be investigated71. It allows direct and quantitative comparison of the brain vascular network tree between different postnatal and/or adult developmental stages as well as to distinguish capillaries and noncapillary blood vessels on the basis of various parameters such as vascular volume fraction, vascular branch point density and degree, vessel segment length, diameter, volume, tortuosity, directionality and extravascular distance.
Development of the protocol
Vascular network formation has previously been evaluated using vascular corrosion casting techniques in mice at the embryonic, postnatal and adult stages of development. In mice at the embryonic stage (8.5–13 d of gestation), vascular corrosion casting has been applied to investigate embryonic aortic development83 and the fetal circulation in relation to the placenta84. In adult mice, vascular corrosion casting techniques are widely used in all major organs, and have been shown, for example, to be particularly useful to study cochlear vasculature in age-related hearing loss85,86,87,88, the microvasculature in the urine bladder89, and alveolar anatomy during both physiological development90,91 and in several diseases92,93.
Vascular corrosion casting is a well-established technique to study cerebrovascular anatomy in physiological settings in mice39,94,95,96,97,98,99,100,101,102. Angiogenesis and vascular network morphology have previously been described by our group using vascular corrosion casting in the adult healthy mouse brain28,38, for instance using brain-specific VEGF165-overexpressing mice (age: 16 ± 2 weeks = P112) to study the effects of this proangiogenic stimulus on the adult brain vascular network103. Moreover, various vascular-dependent CNS pathologies have been investigated using the technique. As an example, we previously applied brain vascular corrosion casting to mouse models of Alzheimer’s disease using APP23 transgenic mice (age: ranging from 3 to 27 months of age = P90 to P810)104,105. Others described the application of the technique to pathologies such as brain arteriovenous malformations utilizing Alk-12f/2f (exons 4–6 flanked by loxP sites) VEGF stimulated mouse models106,107, microvascular networks in brain tumors in rats and primates using gliosarcoma xenotransplantation108, posttraumatic brain injury models109, transient focal cerebral ischemia in rats110 and traumatic brain injury in postmortem human patients111.
Recently, we have adapted the vascular corrosion casting technique enabling the effects of the well-known axonal growth-inhibitory protein Nogo-A to be described, which we have previously identified as a negative regulator of CNS angiogenesis and endothelial tip cell filopodia38, on the 3D vascular network in the postnatal mouse brain39.
Here, we demonstrate an optimized adaptation and extension of these previously described methods, allowing hierarchical imaging and computational analysis of the vascular network tree during any postnatal brain developmental stage in the mouse brain. Using vascular corrosion casting, hierarchical, desktop and synchrotron radiation microcomputed tomography (SRµCT)-based imaging and image analysis, we provide a refined, quantitative 3D analysis generating absolute parameters, characterizing the entire 3D mouse brain vessel network at a micrometer resolution.
Overview of the procedure
Here, we present a technique for the visualization and computational analysis of the 3D vascular network in the postnatal and adult mouse brain. Figure 1 illustrates the different steps of the procedure with the corresponding timescale. A more detailed description of the procedure is provided in ‘Experimental design’, under ‘Detailed description of the procedure’. Briefly, the protocol starts with transcardial perfusion of artificial cerebrospinal fluid (ACSF) containing heparin, followed by 4% (wt/vol) paraformaldehyde (PFA) in PBS and the resin PU4ii (vasQtec) at the desired day of postnatal development (in our case P10, corresponding to the peak of endothelial cell proliferation in the mouse brain according to Harb et al.25) or adulthood (in our case P60) (Steps 1–8) (Figs. 2 and 3 and Extended Data Fig. 1). This is followed by resin curing (overnight) (Step 9), soft-tissue maceration (for 24 h) (Step 11), decalcification of the surrounding bone structures (overnight) (Step 12) and dissection of the cerebral vascular corrosion casts from the remaining extracranial vessels (Step 13), followed by subsequent washing (Step 14), sputter-coating with gold on a subset of casts (Step 15), contrasting (osmium tetroxide) (Step 16), another round of washing (Step 17), freeze-drying (lyophilization) (Step 18), mounting and evaluation by scanning electron microscopy (SEM) of a subset of casts (Steps 19–20) (Extended Data Fig. 2 and Supplementary Fig. 1), optional low-resolution desktop µCT scanning for ROI selection (Steps 21–22), high-resolution SRµCT or desktop µCT scanning (Steps 23–24), computational analysis (Steps 25–49) and statistical analysis (Step 50) (Fig. 4).
The different sections of the protocol are indicated in color-coded boxes. White boxes indicate preparation steps, green boxes describe experimental steps, blue boxes indicate analysis steps and brown boxes describe quality control steps. Black arrows indicate the order in which the different steps of the protocol are performed. A time scale in days is shown on the left. KOH, potassium hydroxide.
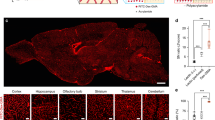
a,b, Schematic drawing illustrating the experimental setup before (a) and after (b) resin perfusion. c–f, Surgical opening (c,d), angle of 30° in the sagittal plane (e) and site of needle insertion (e) for intracardial perfusion with 10–20 ml ACSF containing 25,000 U/L heparin via the left ventricle, followed by 4% (wt/vol) PFA in PBS, and by the polymer resin PU4ii (vasQtec) (f), were all infused at the same rate (4 ml/min and 90–100 mmHg) in a P10 mouse pup (see also Supplementary Video 1). Successful perfusion is indicated by a bluish aspect of the animal, which can be well observed at the paws (white arrow), snout (white arrowhead) or tail (white outlined arrowhead). g–j, Schematic drawings depicting the individual steps visible in c–f. k,l, Resin cast of a P10 animal after soft-tissue maceration, before (k) and after (l) bony tissue decalcification (paw, white arrow and snout, white arrowhead). m,n, The cerebral vasculature is sharply dissected from the extracranial vessels, resulting in the isolated brain vascular corrosion cast of the P10 mouse brain (white arrow, m) (n). Scale bars, 5 mm (k–n).
a–g, Photograph (a) and schematic drawing (b) of a whole adult (P60) mouse after perfusion of resin PU4ii. Insets showing a schematic illustration of the principle of vascular corrosion casting (c,d), in which the vascular corrosion casts represents the inner volume of perfused blood vessels in blue; defined by the inner vessel diameter, before (c) and after (d) maceration of soft tissues. Maceration of the surrounding CNS tissue including the blood vessel wall and decalcification of bony structures, exposes the entire vascular corrosion cast (e,f), with a schematic drawing of the casted brain vasculature in more detail (g).
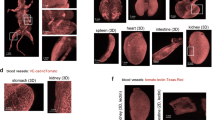
a–l, Schematic visualization of the ROI placement in both postnatal (P10) (a–d) and adult (P60) (e–h) mouse brains with 3D overviews (a,e) and detailed panels for the respective anatomical plane (b,f coronal, c,g sagittal, d,h axial). The µCT scanning procedure of a vascular corrosion cast includes the placement of ROIs, here depicted in the 3D rendering of a whole postnatal (P10) WT brain, shown in the different anatomical planes (i–l) (red: cortex, blue: hippocampus, yellow: superior colliculus, green: corpus callosum, and black: brain stem). m,n, Raw images of single µCT projections in the x-plane, y-plane and z-plane (from left to right) of a ROI placed in the cortex of a P10 WT animal (m), followed by the same z-plane for which the edges of the segmented regions are shown as red outlines (Otsu’s thresholding method) (n). o, Visualization of the image segmentation and skeleton tracing process. In the imaging process, the original vessel structure (left) is converted into a digital approximation that is segmented using the Otsu method (middle). The centerlines of the vessels are found using skeletonization (middle, highlighted pixels). The pixel-accurate centerline (middle, black dotted line) is smoothed for better representation of the centerlines of the individual vessel branches (right, colored lines). For each branch, its length is measured as a sum of distances between the smoothed centerline points (right, colored points), and its diameter as the average diameter of the branch (right, arrows). p, Three-dimensional visualization of the original data (P10 WT cortex) showing the CT plane (grayscale), the segmented vessels (red, left side) and the vessel centerlines (blue lines, right side). Part of segmentation has been cut off in the front of the CT plane. Scale bars: 250 µm (i–l), 100 µm (m,n).
Applications of the protocol
The protocol comprises vascular corrosion casting, hierarchical imaging and computational analysis of individual vessel segments of the 3D vascular network in postnatal and adult mouse brains. The method distinguishes between vessels of different diameters, for instance between capillaries (inner diameter <7 µm) and noncapillaries (inner diameter ≥7 µm). The protocol allows the influence of any angiogenic molecule to be studied, including pharmacological compounds and blocking antibodies, and to test their pro- or antiangiogenic effects on vascularization and various specific parameters of the vascular network in postnatal as well as adult wild-type or genetically modified mice38,112. Importantly, the method provides a detailed quantitative description of the 3D vasculature, allowing precise comparison of different experimental settings or genetic modifications. Quantitative information gained includes vascular volume fraction, vascular branch point density and degree, and vessel segment length, diameter, volume, tortuosity, directionality and extravascular distance, on both the level of global vascular network morphometry as well as local vascular network topology. This is quite different from the classical use of the corrosion cast technique for which in general only gross visual comparisons have been made and described. Importantly, the method could be used with laboratory/desktop μCT scanners only, which substantially increases the applicability of the protocol as compared with combined desktop μCT plus SRμCT setups. The protocol can also be applied to the analysis of the 3D vascular network in the entire mouse brain by creating artifact-free, high-resolution images of the complete and intact vascular corrosion cast, allowing the vascular network morphology of any given ROI to be visualized within the mouse brain. This improves precision and is a substantial simplification in contrast to the two-step ROI-based approach during which the ROIs first have to be placed on a lower-resolution overview μCT scan with subsequent high-resolution SRμCT or desktop μCT scanning of selected ROIs. Finally, given the crucial role of angiogenesis and vascular networks during both tissue development as well as in various pathologies in- and outside the CNS1,2,6,113,114, and referring to previous vascular corrosion casting-based analyses of Alzheimer’s disease, brain tumors and other pathologies105,106,108,109, the improved method presented here can, after validation in other tissues or pathological conditions, in principle be applied to virtually any physiological or pathological setting.
Although corrosion casts represent only a snapshot in time, they can also serve as templates for blood flow simulations that can, in addition to simulating cerebral blood flow in healthy brains, be used to simulate impaired oxygen and nutrient supply as a consequence of altered vascular network architecture in different cerebrovascular pathologies, such as brain tumors, brain vascular malformations and stroke.
Advantages of the protocol compared with alternative methods
The hierarchical imaging of vascular corrosion casts combined with the computational analysis at the level of individual vessel segments up to entire vascular networks in both the postnatal and adult mouse brain as described here has several key advantages as compared with alternative methods.
This method enables visualization and quantitative assessment of the structure of blood vessel networks in the developing postnatal as well as in the entire adult mature mouse brain down to the level of capillaries, with yet unmet precision.
The brain microvasculature has proven hard to image owing to the small size of the capillaries and large ROIs required for network-level analysis. Traditional, noninvasive, in vivo imaging modalities such as Doppler tomography115,116, μMRI117 and magnetic resonance angiography118 have a resolution in the range of tens of micrometers, but that is not high enough to detect the microvasculature (compared with the near 1 μm resolution in μCT imaging). Confocal single-photon and two-photon imaging can provide the required resolution but have depth limitations22,25, whereas other, classical (automated) histology-based methods are often limited to smaller regions, can be disturbed by deformations of the tissue slices especially at high resolutions (thin slices) and are therefore not suitable for quantification of the brain vasculature on a network level119,120,121. Recently, light-sheet fluorescence microscopy (LSFM) of the stained blood vessel network in both cleared non-CNS122,123 and in cleared CNS tissue124,125,126,127 has received increasing attention. LSFM has proved to be a very attractive and efficient method for imaging of large vessel networks with relatively high resolution. Contrary to corrosion casting, the LSFM technique requires optically clear or cleared samples (for the excitation wavelength). In corrosion casting, there are no such requirements and therefore no possibility of typical LSFM artifacts such as shadowing or photobleaching. Routinely achievable isotropic resolution of µCT techniques is near 1 µm, but in LSFM the resolution is often anisotropic. For example, here we have an isotropic voxel size of 0.65 µm, compared with 1.625 × 1.625 × 3 µm achieved through multidye vessel staining, tissue clearing and 3D LSFM in ref. 126. For analysis, anisotropic voxels are often resampled to isotropic voxel size corresponding to the lowest-resolution component, as was also done in ref. 126, resulting in isotropic voxel size of 3 µm for convolutional neural network vessel segmentation and subsequent analysis of the vasculature of the whole mouse brain126.
Micro-CT imaging with use of intravascular contrast agents is used both in vivo and ex vivo and made it possible to image the vasculature in whole organs in three dimensions with increasing resolution102,128,129,130,131. Ex vivo, vascular corrosion casts have shown to be an excellent method to investigate brain vasculature in particular39,99,101,102,104,105,132. Corrosion casting, in combination with recent advances in imaging techniques for both for SRμCT and desktop μCT setups, as used in this protocol, results in a resolution that is at least three times higher (0.65 μm versus 2 μm pixel size) than that reported in another recent paper that used a similar approach to study the mouse brain vasculature102.
The protocol used here allows the postnatal and adult CNS blood vessel network to be quantitatively described in detail down to the capillary level, (Figs. 5–14, Extended Data Figs. 3–6, Supplementary Figs. 2–21), including visualization and quantification of vessel directionality in the x-, y- and z-planes (Fig. 12 and Supplementary Figs. 12–16, 21), which provides important additional information. Moreover, it permits us to distinguish between capillaries and noncapillaries using clearly defined morphological parameters. In this paper, we classified a vessel as a capillary if its inner diameter was <7 µm (Figs. 3 and 6–10). Similar morphological classification is more difficult and tedious with other, classical (automated) histology-based methods owing to dye color variations, staining artifacts and distortion of the true vessel diameter by perivascular cells119,120,121. Histology-based methods including recently published LSFM-based methods122,123,124,125,126 are also less precise in addressing inner vessel diameters.
a–d, Computational 3D reconstructions of the vascular network using surface rendering based on the SRµCT data showing a ROI as volume mask (a), after the vessel segmentation process (b), and after the placement of centerlines (c), with a zoom of both stages combined (d). Blood vessel structures of all sizes are well defined. e–g, Computational 3D reconstructions of µCT scans of vascular networks of the postnatal (P10) WT and adult (P60) WT mouse brains displayed with color-coded vessel thickness. The increased vessel density in the P60 WT cortices (e), hippocampi (f) and superior colliculi (g) is obvious. Color bar indicates vessel radius (µm). The boxed areas are enlarged at right. h–j, Quantification of the 3D vascular volume fraction in P10 cortex (h), hippocampus (i) and superior colliculus (j), by computational analysis using a global morphometry approach. The vascular volume fraction in all these brain structures was significantly higher in the P60 WT animals than that in the P10 WT animals (n = 4–6 for P10 WT; n = 8–12 for P60 WT animals; and on average three ROIs per animal and brain region have been used). All data are shown as mean distributions, where the open dot represents the mean. Boxplots indicate the 25% to 75% quartiles of the data. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars: 100 µm (e–g, overview), 50 µm (e–g, zoom).
a,b, Scheme illustrating a capillary bed and the distinction between capillaries and noncapillaries based on the radius of the inner volume of resin-perfused blood vessels after maceration of the surrounding CNS tissue including the blood vessel wall. A capillary is defined as a blood vessel with an inner diameter (a,b, green) of <7 µm, whereas a noncapillary vessel is defined as a blood vessel with an inner diameter (a,b, magenta) of ≥7 µm, according to ref. 172. c,d, Three-dimensional computational reconstruction of µCT scans of cortical vascular networks in a P60 WT sample highlighting a vessel tree with noncapillaries in magenta and capillaries in green.
a–c, Histograms showing the distribution of vessel diameter in P10 WT and P60 WT animals. P60 WT animals show an increased absolute frequency of capillaries as compared to the P10 WT situation, whereas the number of noncapillaries remains largely unchanged in the cortex (a), hippocampus (b) and superior colliculus (c) (bin width = 0.36 µm; number of bins = 55). Black dashed line marks the separation between capillaries (<7 µm) and noncapillaries (≥7 µm). d,e, Computational 3D reconstructions of µCT images of vascular networks of P10 WT and P60 WT mice in the cortices separated for noncapillaries (magenta) and capillaries (green). The increased density of capillaries (green) in the P60 WT samples for the cortices is evident; density in noncapillaries (magenta) is also increased. The boxed areas are enlarged at the right for capillaries (d,e). f–h, Quantification of the 3D vascular volume fraction for all vessels (f), noncapillaries (g) and capillaries (h) in P10 WT and P60 WT calculated by local topology analysis. The significant increase of the vascular volume fraction for all vessels in the P60 WT animals (f) was mainly due to a significant increase at the level of capillaries (h) (n = 4–6 for P10 WT; n = 8–12 for P60 WT animals were used; and on average three ROIs per animal and brain region were used). All data are shown as mean distributions where the open dot represents the mean. Boxplots indicate the 25% to 75% quartiles of the data. The shaded blue and red areas indicate the SD. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars: 100 µm (d,e, overviews), 50 µm (d,e, zooms).
a, Scheme illustrating a capillary bed and the distinction between capillaries and noncapillaries based on the radius of the inner volume of resin-perfused blood vessels (see legend for Fig. 6a). b, Scheme depicting the definition of vascular branch points. Each voxel of the vessel center line (black) with more than two neighboring voxels was defined as a vascular branch point. This results in branch point degrees (number of vessels joining in a certain branch point) of minimally three. In addition, two branch points were considered as a single one if the distance between them was <2 µm (b). Branch point are depicted as black dots in a and b. c, Histogram showing the distribution of branch point diameter in P10 WT and P60 WT animals. P60 WT animals show an increased branch point density as compared with P10 WT mice mainly at the capillary level (bin width 0.38 µm; number of bins 40). Black dashed line marks the separation between capillaries (<7 µm) and noncapillaries (≥7 µm), as defined in Fig. 6. d,e, Computational 3D reconstructions of µCT images of cortical vascular networks of P10 WT and P60 WT mice with visualizations of the vessel branch points displayed as dots, separately for noncapillaries (magenta) and capillaries (green). The higher density of branch points in the P60 WT cortices especially at the capillary level (green) is obvious; a slight increase of branch point density can be observed at the noncapillary level (magenta). The boxed areas are enlarged at the right. f–h, Quantitative analysis of the branch point density for all vessels (f), noncapillaries (g) and capillaries (h) in P10 WT and P60 WT cortices by local morphometry analysis. The significant increase of the branch point density for all vessels in the P60 WT animals (f) was mainly due to a significant increase at the level of capillaries (h) and in part due to a significant increase at the level of non-capillaries (g). (n = 4–6 for P10 WT; n = 8–12 for P60 WT animals were used; and on average three ROIs per animal were used). All data are shown as mean distributions where the open dot represents the mean. Boxplots indicate the 25% to 75% quartiles of the data. The shaded blue and red areas indicate the SD. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars: 100 µm (d,e, overviews), 50 µm (d,e, zooms).
a,b, Schemes showing the definition of vessel diameter (a), vessel length (a) and vessel tortuosity (b). The segment diameter is defined as the average diameter of all single elements of a segment (a). The segment length is defined as the sum of the length of all single elements between two branch points (a). The segment tortuosity is the ratio between the effective distance le and the shortest distance ls between the two branch points associated to this segment (b). The segment density is defined as the number of segments, classified into global, capillary and noncapillary segments, divided by the network volume. c, Graph depicting the relationship between segment diameter and segment length in P10 WT and P60 WT animals. The ratio ‘segment diameter-to-segment length’ was higher in P10 WT mice compared with P60 WT mice (bin width 4.93; number of bins 14). d–f,g–n, Quantification of the 3D vessel network parameters segment density (d–f), segment diameter (g–j) and segment length (k–n) for all vessels, noncapillaries and capillaries in P10 WT and P60 WT cortices calculated by local morphometry analysis. The segment density (d) was significantly increased for all vessels in the cortices of P60 WT mice as compared with the P10 WT mice, whereas the segment diameter (h) and segment length (l) were significantly decreased for all vessels in the P60 WT mice as compared with the P10 WT mice. In line with the other parameters, these differences were mainly due to a highly significant increase respectively decrease at the level of capillaries (f,j,n) and in part due to an increase respectively decrease at the level of noncapillaries (e,i,m) (n = 4–6 for P10 WT; n = 8–12 for P60 WT animals; and on average three ROIs per animal and brain region were used). (g, bin width 0.36 µm, number of bins 55; k, bin width 2.5 µm, number of bins 40). All data are shown as mean distributions where the open dot represents the mean. Boxplots indicate the 25% to 75% quartiles of the data. The shaded blue and red areas indicate the SD. *P < 0.05, **P < 0.01, ***P < 0.001.
a–h, Quantification of the 3D vessel network parameters segment tortuosity and segment volume (a–h) for all vessels, noncapillaries and capillaries in P10 WT and P60 WT cortices calculated by local morphometry analysis. The segment tortuosity (a,b) and segment volume (e,f) were significantly increased for all vessels in the cortices of P60 WT mice as compared with the P10 WT mice. In line with the other parameters, these differences were mainly due to a highly significant increase respectively decrease at the level of capillaries (d,h) and in part due to an increase respectively decrease at the level of noncapillaries (c,g) (n = 4–6 for P10 WT; n = 8–12 for P60 WT animals; and on average three ROIs per animal and brain region were used). (a, bin width 0.013, number of bins 80; e, bin width 2,957.74 µm3, number of bins 55). All data are shown as mean distributions where the open dot represents the mean. Boxplots indicate the 25% to 75% quartiles of the data. The shaded blue and red areas indicate the SD. *P < 0.05, **P < 0.01, ***P < 0.001.
a, Schematic defining the extravascular distance as the shortest distance of any given voxel in the tissue to the next vessel structure. b, Histogram showing the distribution of the extravascular distance in P10 WT and P60 WT animals. P60 WT animals show an increased relative frequency of shorter extravascular distances as compared to the P10 WT situation (bin width 10; number of bins 20). c–h, Color map indicating the extravascular distance in the cortices, hippocampi and superior colliculi of P10 WT and P60 WT mice. Each voxel outside a vessel structure is assigned a color to depict its shortest distance to the nearest vessel structure. The reduced extravascular distance in P10 WT animals as compared with the P60 WT animals is obvious. The color bars indicate the shortest distance to the next vessel structure. i–k, Quantification of the extravascular distance in P10 WT and P60 WT in the three brain regions calculated by global morphometry analysis. The extravascular distance in the cortices (i), hippocampi (j) and superior colliculi (k) of P60 WT animals was significantly decreased as compared with the P10 WT animals (n = 4–6 for P10 WT; n = 8–12 P60 WT animals were used; on average three ROIs per animal and brain region were analyzed). All data are shown as mean distributions where the open dot represents the mean. Boxplots indicate the 25% to 75% quartiles of the data. The shaded blue and red areas indicate the SD. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars: 100 µm (c–h overviews), 50 µm (c–h zooms).
a, Computational 3D reconstruction of µCT scans of cortical vascular networks of P60 WT displayed with color-coded directionality obtained by attributing every vessel segment to its main direction in the x (red), y (green) and z (blue) planes within the selected ROIs. b,c, Computational 3D reconstruction of µCT scans color-coded for vessel directionality depicting the P10 WT (b) and P60 WT (c) cortex. Cortical renderings clearly show that the superficial PNVP extended in the x- and y-directions, whereas the INVP exhibited a radial sprouting pattern into the brain parenchyma along the z-axis, perpendicular to the PNVP. d–f, Quantitative analysis showing histograms for the distribution of the relative angles of the vascular segments emanating from a certain branch point (segment angle for adjacent vessels) in P10 and P60 animals in the cortex. Noncapillaries revealed two main angles of orientation, namely ~90° and at ~170°, and these two main angles of orientation were more pronounced at the adult P60 as compared with the developing P10 stage (e). The capillaries derived at angles between around 75° and 150° at both the developing P10 and mature P60 stages in the cortex, (f). (d–f, bin width 5; number of bins 55). Scale bars, 100 µm (b,c).
a–h, Cross sections of the whole-brain scan in both P10 (a–d) and P60 (e–h) mouse brains with 3D overviews (a,e) and detailed panels for the respective anatomical planes (b,f coronal, c,g sagittal, d,h axial). i,j, Computational 3D reconstructions of µCT scans of vascular networks of the P10 WT and P60 whole brain scans, displayed with color-coded vessel thickness. The increased vessel density in the P60 WT (j) cortex, hippocampus, thalamus, cerebellum, medulla and pons, as compared with the P10 WT (i) samples is obvious. Color bar indicates vessel radius (µm). k–r, Quantification of the 3D vascular volume fraction in P10 cortex (k), hippocampus (l), superior colliculus (m), thalamus (n), hypothalamus (o), cerebellum (p), pons (q) and medulla (r) by computational analysis using a global morphometry approach. The vascular volume fraction in all these brain structures was higher in the P60 WT animals than that in the P10 WT animals (the anatomical regions were divided into ROI-sized sections for analysis: between n = 5, dentate gyrus, and n = 126, cortex for P10 WT; and between n = 4, CA3, and n = 76, cortex, of these sections for P60 WT were analyzed, highly dependent on the size of the anatomical region). All data are shown as mean distributions, where the open dot represents the mean. Boxplots indicate the 25% to 75% quartiles of the data. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars: 2.5 µm (a–h, overviews), 0.25 µm (a,e, zooms), 1 µm (b–d, f–h, zooms).
a–d, Schematic illustrations of the postnatal and adult mouse brain vasculature, both as 3D whole brains (a) and as enlargements specified for the different anatomical regions, namely cortex (b), hippocampus (c) and superior colliculus (d). e,f, From the P10 to the P60 stage, an increased vascular volume fraction of functional blood vessels established via increased vascular endothelial sprouting, branching and migration mainly at the capillary level can be observed (e,f). g, Schematics summarizing the most important differences in the 3D vascular network architecture of P10 and P60 mouse brains: The vascular volume fraction (percentage of tissue volume occupied by the entire vascular network), the branchpoint and vessel/segment density, the branchpoint degree, and the vessel/segment tortuosity are increased in the mature P60 as compared with the developing P10 animals. On the other hand, the vascular parameters vessel/segment length, vessel/segment volume, vessel/segment and branchpoint diameter, and the extravascular distance are decreased in the mature P60 as compared with the developing postnatal P10 mice. The vessel directionality showed more pronounced angles of orientation for noncapillaries at the P60 stage as compared with P10 stage, whereas the orientation of the capillaries seemed largely unchanged between the two developmental stages.
Hierarchical imaging and computational reconstruction of the 3D vascular network via global vascular network morphometry and local vascular network topology allow accurate, complete and quantitative analysis and description of the 3D vessel structure in the postnatal and the adult mouse brain at the network level. These computational 3D analyses result in vessel parameters (Figs. 5–14, Extended Data Figs. 3–6, Supplementary Figs. 2–21) with direct biological relevance instead of single measures, such as ‘percentage of section area occupied by vessels’, that are obtained in classical 2D-section analysis methods.
For instance, maximum intensity projections of tissue slices are often used, even though they lead to an overestimation of the vessel density, as the vascular volume fractions of multiple optical sections are summed up, an error that adds up with increasing section thickness. A 3D analysis resulting in true vessel volume fractions, therefore, provides more accurate quantifications of the vascular networks and also allows better comparison between different studies. Three-dimensional analyses can be either obtained by combining vascular corrosion casting techniques with computational analysis as described here, or by stereological methods as we28 and others133 have previously reported.
In contrast to section analysis (2D, maximum intensity projections), computational analysis of 3D hierarchical images allows characterization of additional parameters of the vascular network such as vessel volume fraction, branch point density and degree and vessel density, diameter, length, tortuosity, volume, directionality and extravascular distance (Figs. 5–12 and Extended Data Figs. 3–6). This is similar to the image analysis obtained from confocal z-stacks using stereological methods28.
Our protocol allows clear identification of capillary (inner vessel diameter <7 µm, Fig. 6a,b) and noncapillary (inner vessel diameter ≥7 µm, Fig. 6a,b) blood vessels on the basis of the morphological criteria of the inner vessel diameter (Figs. 3, 6–10, Extended Data Figs. 4–6, Supplementary Figs. 2–8, 17–19). This biologically important distinction is more difficult to address with classical immunofluorescence119,120,121, in vivo imaging modalities such as μMRI117, μCT131,134, contrast-enhanced digital subtraction and CT angiography135, as well as with more recently developed methods such as LSFM analyses, with which vessel diameter cannot be determined as adequately. Moreover, there is also a lack of reliable capillary markers123,124,125,126 that otherwise might solve this issue for staining-based methods. Indeed, markers for capillary endothelial have been proposed in recent years, for instance, Mfsd2a136, TFRC, SKC16a2, CA4, CXCL12, Rgcc, SPOCK2 and others that were recently identified in large endothelial single-cell-RNA sequencing datasets137,138,139. However, to the best of our knowledge, there is currently no established cellular marker that selectively labels capillaries, as all the proposed and above-mentioned markers also label noncapillary blood vessels, though to a lesser extent138,139. Accordingly, the most adequate method for distinguishing capillary and noncapillary blood vessels is still based on the morphological criteria of the inner vessel diameter, e.g., <7 µm for capillaries versus ≥7 µm for noncapillary blood vessels (Figs. 3, 6–10, Extended Data Figs. 4–6 and Supplementary Figs. 2–8, 17–19).
High-resolution hierarchical imaging and computational analyses enable the 3D visualization, quantification and characterization of the entire vascular brain network. These analyses permit various quantitative parameters to be obtained at the level of both the entire vascular network as well as of individual vessel segments, such as the volume fraction; extravascular distance; branch point density and degree; and vessel density (entire vascular network), diameter, length, tortuosity, volume and directionality (individual vessel segment) (Figs. 5–10, 12, 13, Extended Data Figs. 5, 6, and Supplementary Figs. 2–21). Moreover, the number of vessel branch points enables better understanding of the 3D aspects of vessel branching within the process of sprouting angiogenesis (Fig. 8, Extended Data Fig. 5, 6, and Supplementary Figs. 9–11), which constitutes the predominant model of vessel formation in the brain3,28,39. Interestingly, the combination of vessel branch point analyses with the above-mentioned distinction between capillaries and noncapillaries allows revealing of underlying mechanisms of sprouting angiogenesis in different brain regions such as cortex, hippocampus and superior colliculus (Fig. 8, Extended Data Figs. 5, 6, Supplementary Figs. 9–11). For instance, branching events emanating from the penetrating vessels of the INVP in the cortex can clearly be visualized and quantified (Figs. 7, 8). Such computational/quantitative analyses are not commonly feasible using either classical staining-based methods119,120,121 or novel methods including LSFM122,123,124,125,126.
This protocol enables functional consequences of the vascular network formation to be addressed, such as the extravascular distance, e.g., the shortest distance of any given point in the tissue to the next vessel structure (Fig. 11, Supplementary Figs. 2,20), which are more difficult to address throughout the whole brain with alternative methods.
In principle, for simultaneous analysis of vasculature and the surrounding tissue, our protocol can also be combined with immunofluorescence using various vascular markers, e.g., pericyte markers (platelet-derived growth factor receptor β, CD13), astrocyte markers (glial fibrillary acidic protein) or neuronal markers (Nf160 and Tub-βIII), to visualize perivascular cell types. This can be done by sectioning the resin-perfused brains without tissue maceration and bony structure decalcification, thereby leaving the brains intact, followed by immunofluorescence staining. By mixing in specific fluorescent dyes with the resin solution, it is also possible to improve and specify the inherent fluorescence of PU4ii (Extended Data Fig. 7). Accordingly, virtually any protein of interest can be analyzed in the morphological context of the developing postnatal and adult vascular network, without the risk of collapse or deformation of vascular structures that is often encountered with a staining-only, histological approach119,121.
Recent technical improvements allow scanning of the whole postnatal and adult brain vascular corrosion cast at once with the same resolution as the method involving ROI selection prior to the scan. This whole-brain scan allows analysis of any ROI, even after SRμCT scanning (Fig. 13 and Supplementary Figs. 17–21).
Limitations of the method
Despite the advantages described above, this method has some disadvantages and limitations.
In contrast to classical immunofluorescent-based methods, the technique described here is more labor-intensive. For instance, the imaging and analysis of the superficial vascular plexus of the postnatal retina140,141 or of the embryonic hindbrain33—which both form a 2D flat vascular structure and are the most commonly used in vivo angiogenesis models—can easily be assessed, in principle even with a standard fluorescence microscope. The quantitative analysis of angiogenesis in the postnatal mouse brain28—in which the vasculature develops in 3D with vessel sprouting occurring in all directions—requires confocal imaging for proper analysis, thus resulting in longer image acquisition times and larger datasets as compared with the postnatal retina and embryonic hindbrain models33,140. The method using confocal imaging is similar regarding labor intensiveness to the protocol described here but cannot be applied at once to the whole brain vasculature.
Comparable to the immunofluorescent-based analysis of the postnatal mouse brain28, the structural complexity of the postnatal and adult mouse brain angioarchitecture requires meticulous navigation within each individual sample to ensure that truly equivalent brain areas are selected. This is crucial as vascular density and angiogenic activity vary considerably among different brain regions37,39,102,126. Navigation within postnatal mouse brain samples as prepared with the present protocol is hampered because, even though the combination of vascular corrosion castings with histological staining (e.g., Nissl or H&E stains, which would disturb the immunofluorescence) is feasible (Extended Data Fig. 7), these different kinds of sample preparation preclude the 3D hierarchical imaging as described. Therefore, for detailed navigation (and ROI placement, see Fig. 4a–l) throughout the postnatal and adult mouse brain, we recommend referring to the Developing- and Adult Allen Mouse Brain Atlas or to other commonly used mouse brain atlases (Fig. 4a–l).
Radiopaque CT contrasts agents (e.g., radiopaque resin PU4ii) do not require soft tissue to be macerated prior to imaging and do not require staining with osmium tetroxide. This is in contrast to the resin PU4ii employed here. We emphasize that a direct comparison of the imaging quality of different contrast agents/technologies is impossible to make without a dedicated comparative study of the different technologies. However, we could speculate that in the intact (not macerated), resin-perfused brain tissue, contrast arises from X-ray absorption differences between tissues and the resin PU4ii-perfused vessels, whereas in our case (macerated tissue), contrast arises from the difference in X-ray absorption between air and resin PU4ii-perfused vessels142. The nonmacerated tissue is known to attenuate X-rays to a greater extent than air, thereby resulting in a less contrasted image as compared with techniques including tissue maceration142.
This protocol for vascular corrosion casting and computational 3D analysis does not enable endothelial tip cells or newly formed but yet immature, nonperfused blood vessels to be directly identified as a percentage of functional, perfused vessels since only the perfused part of the vessel tree can be analyzed. Endothelial tip cells and immature nonperfused blood vessels can, however, be identified by combining vascular corrosion casting with classical immunofluorescence staining techniques (Extended Data Fig. 7). Moreover, blood flow in the functional vessels cannot be measured. In light of the finding that an increased density of perfused blood vessels does not necessarily result in higher blood flow, as it has for instance been shown for transgenic overexpression of VEGF165 (= VEGF-A) in the adult mouse brain103, this needs to be taken into account.
The protocol described here provides only snapshots of brain angiogenesis at given time points for individual mice and, in consequence, requires a relatively large number of animals in order to study the dynamics of angiogenesis and vascular network architecture over time. We have partially addressed this aspect here by comparing the P10 with the P60 mouse brain. Although methods to study in vivo angiogenesis in the developing postnatal and adult mouse brain are currently not widely available, methods for real-time imaging of the brain vasculature exist25,40 and may, in the future, complement our protocol.
In contrast to immunofluorescent-based methods evaluating angiogenesis in the postnatal retina, in the embryonic hindbrain and in the postnatal brain28,140, the identification of and distinction between endothelial tip cells, trailing stalk cells and filopodia is not possible with vascular corrosion casting and computational 3D analysis, thereby limiting the ability to characterize patterns of sprouting angiogenesis and to differentiate between active and quiescent vessel sprouts. Our protocol allows the distinction between capillaries and noncapillaries on the basis of their inner diameter (Figs. 3, 6–10), and this is in principle possible for every other vessel of a given size (Fig. 5 and Extended Data Fig. 3). However, it does not permit direct differentiation between arteries and veins or between arterioles and venules, which have similar inner vessel diameters but different functionalities102. The differentiation between arteries and veins is possible using SEM images revealing different shapes of nuclei impressions on the vascular cast depending on arterial versus venous flow (e.g., elongated nuclei impression in arteries versus more round nuclei impressions in veins), as we have previously described100. SEM, does, however, not allow for computational analysis of the vascular parameters. To circumvent these problems, the combination of vascular corrosion casting and immunofluorescence using classical markers for arteries (such as EphrinB2143, Jagged-1144, Alk-1145, etc.) and veins (such as VCAM-1146, COUP-TFII147, EphB4148, etc.) (Extended Data Fig. 7) allows both 3D computational analysis of the vascular network as well as parameters and arteriovenous differentiation to be undertaken.
Extravasation of intravascularly infused resin has been described in experimental liver149 and mammary150 tumor models. Although not described in brain vascular corrosion casting, and also not observed by us in the postnatal mouse brain with more angiogenic active vessels that are supposed to be leakier than mature vessels, some resin extravasation, for example, in vascular-dependent CNS pathologies associated with an impaired blood–brain barrier such as stroke, brain trauma or brain tumors, cannot be excluded.
Another limitation of the described protocol is the invasive nature of the technique as the mice need to be sacrificed for resin perfusion (Fig. 2 and Extended Data Fig. 1); similar to the other, above-mentioned techniques of mouse postnatal retina, embryonic hindbrain and postnatal brain angiogenesis28,35,140. Vessel size index-MRI (VSI-MRI) has been proposed as a quantitative, noninvasive MRI-derived imaging biomarker, for the noninvasive assessment of tumor blood vessel architecture and to evaluate the effects of vascular targeted therapy. The technique was validated in high-grade human gliomas151, and a direct comparison between in vivo MRI and postmortem μCT vessel calibers showed excellent agreement in vessel size measurements152. However, the parameters addressed with VSI-MRI are limited to an estimation of fractional blood volume and blood vessel size (resolution of 1.875 × 1.875 mm with a total matrix size of 240 × 218 mm), and the additional vascular parameters such as vessel length, tortuosity, vessel directionality, branch point density and extravascular distance addressed with our protocol are not measurable with VSI-MRI techniques152.
Experimental design
Detailed description of the procedure
As a first step, after the animal is properly anesthetized, the chest is opened in order to gain access to the left ventricle (Fig. 2a–f and Extended Data Fig. 1a,b) (Steps 1–2). To this end, the skin is lifted with the forceps and then cut perpendicular to expose the sternum (Fig. 2c,d,g,h and Extended Data Fig. 1b) (Step 3). The sternum is then lifted, and lateral cuts through the rib cage are made exposing the chest cavity. The rib cage flap is pulled back and fixed using a pin/needle, and the pericardium is carefully removed using rounded forceps. The heart is lifted slightly using forceps, and then a winged perfusion needle is inserted into the left ventricle through the apex, and the point of insertion is sealed with instant glue (Fig. 2e,i and Extended Data Fig. 1c) (Steps 4–5). Note the insertion at a flat angle of ~30° (Fig. 2e,i). Care must be taken during this procedure to avoid inserting the needle too deep into the heart in order to avoid damage of the atrioventricular valve. After proper placing and securing of the needle (using the wings of the needle and pins/needles) in the left ventricle, the right atrium is opened to allow the blood and perfused fluid to leave the circulation, providing unlimited outflow (Fig. 2b,f,j, Extended Data Fig. 1d, Supplementary Video 1) (Step 6). Perfusion of 5 ml (for P10 animals) and 20 ml (for adult animals) of ACSF containing 25,000 U/L heparin through the left ventricle is followed by perfusion with 4% (wt/vol) PFA (dissolved in PBS) in order to fixate the vasculature and prevent the blood vessels from collapsing, and the resin PU4ii solution (Steps 6–7). All perfusion solutions are perfused at a pressure of 90–100 mmHg (4 ml/min) for P10 mice and 100–120 mmHg (8 ml/min) for P60 mice. Successful perfusion is indicated by sufficient outflow of the solutions from the opening in the right atrium and by the bluish appearance of the paws, snout and tail immediately after starting the resin perfusion (Fig. 2f,j, Extended Data Fig. 1d, Supplementary Video 1) (Step 8). After 24 h of resin curing, soft tissue is macerated in 300–400 ml 7.5% (wt/vol) potassium hydroxide (KOH) for at least 24 h, followed by incubation in 300–400 ml 5% (vol/vol) formic acid for another 24 h to decalcify bony structures (Steps 9–12). The brain vascular corrosion cast is dissected out of the skull and freed from extracranial vessels (Fig. 2k–n, Extended Data Fig. 1e–h, Supplementary Video 1) (Step 13).
The principle of vascular corrosion casting is schematically shown in Fig. 3. The step-by-step procedure of intracardial polymer resin perfusion, tissue maceration, bone decalcification and brain dissection is illustrated in Fig. 2, Extended Data Fig. 1 and Supplementary Video 1.
As an additional sidestep, not part of the main protocol pipeline, a combination of resin perfusion and immunofluorescence staining and imaging can be performed (Extended Data Fig. 7) (Step 10). For this purpose, resin curing is immediately followed by removal of bony structures (without tissue maceration), to preserve soft tissue required for immunofluorescence staining. After cutting, the soft-tissue slices are then incubated with any biological markers of interest such as Fluoro Myelin Red (Molecular Probe Inc) for myelin and DAPI. Additional to the inherent fluorescence of PU4ii, specific fluorescent dyes can be used (Extended Data Fig. 7). For conventional immunofluorescence microscopy, we recommend a slice thickness of 100–150 µm.
For quality control, random samples of vascular corrosion casts are mounted on stubs and sputter-coated with gold104,132 for SEM (SEM, Hitachi S4000) (see Extended Data Fig. 2 for sufficiently perfused casts; see Supplementary Fig. 1 for insufficiently perfused casts) (Steps 14–15, Step 20). Optionally, scanning helium ion microscopy that does not require sputter coating could be used for quality control. In this case, the same casts that will be μCT imaged can be used for quality control.
The casts that are to be μCT imaged are stained using osmium tetroxide to increase X-ray absorption, which leads to higher signal-to-noise ratio (SNR) and, thus, better contrast in X-ray imaging103 (Steps 16–17). Afterwards, the osmicated casts are lyophilized overnight and mounted on plexiglas stubs or other suitable sample holders using cyanoacrylate glue (Steps 18–19). Optionally, the entire corrosion casts are first imaged with low-resolution desktop µCT to select desired ROIs (Steps 21–22). This step is not required if the whole casts are to be imaged.
The corrosion casts are scanned with high-resolution desktop or SRμCT (either preselected ROIs or the whole brain) and analyzed using a computational imaging processing pipeline (Steps 23–24). The image analysis procedure allows segmentation of the 3D data such that voxels representing vessels are assigned value 1 and all other voxels are assigned value 0. Furthermore, this binary representation is converted into a vessel graph (Fig. 4) that facilitates quantitative analysis of all network parameters, optionally separated for capillaries and noncapillaries, including vascular volume fraction (Figs. 5–7, Extended Data Figs. 3,4, Supplementary Figs. 2–5); branch point density and degree (Fig. 8, Extended Data Figs. 5, 6, Supplementary Figs. 6, 9–11); segment density, diameter, length, volume and tortuosity (Figs. 9, 10, Supplementary Fig. 7); extravascular distance (Fig. 11 and Supplementary Fig. 8); and vessel directionality (Fig. 12 and Supplementary Figs. 12–16) (Steps 25–49). Finally, the data extracted using image analysis procedures are evaluated statistically (Step 50).
Experimental animals
Although we have used C57/BL6 WT and Nogo-A−/− mice at the ages of P10 and P60, the present protocol is applicable to mice of any type, either sex, and at any postnatal and adult stage of development. To illustrate the potency of this technique in detecting the effects of different molecules, proteins and drugs on 3D vessel morphology, we quantified the difference in 3D vessel morphology between healthy P10 and P60 mice as well as wild-type and Nogo-A−/− mice of either age. Importantly, as genetic or other experimental manipulations such as intraperitoneal antibody or tamoxifen injections were described to affect the growth and, thus, the weight of animals140, it is crucial to weigh the animals prior to resin perfusion. Although genetic modifications are not necessarily reflected to the same extent in all organs, similar weights and ages were chosen as parameters to ensure comparable developmental stages of mice between the different study groups. This is especially true for mouse pups at the early postnatal stage because the vasculature develops relatively fast at that stage37. Therefore, it is important to monitor the developmental stage of the mice (including age and weight of the animals) carefully. Since various genetic modifications were shown to alter the overall postnatal development, matching animal weight is important to enable comparative vessel structure analysis153. Moreover, littermate controls should always be used where available.
Power calculations based on the expected or biologically relevant difference between the groups should be made prior to the experiments. In principle, more pronounced differences between two test groups require a smaller number of animals to detect the effect and vice versa. Incorporation of appropriate controls, for instance, brains from postnatal wild-type littermates, is essential for analyses of angiogenesis and vascular network architecture in genetically modified mice during postnatal CNS development as well as in adulthood. For all quantitative analysis in the different brain regions (Figs. 5–12, Extended Data Figs. 5, 6, Supplementary Figs. 2–16), we used 4–6 postnatal (P10) and 8–12 adult (P60) animals, respectively. All animal procedures must be carried out in accordance with the guidelines outlined by the institutional and local review committees for animal experiments. All the animal experiments in this study were approved by the Veterinary Office of the Canton of Zurich (license number 173/2010).
Vascular corrosion casts: resin PU4ii infusion
The resin we use, PU4ii (vasQtec), is a polyurethane-based casting resin with superior physical and imaging characteristics, timely polymerization, minimal shrinking and low viscosity persisting up to 25 min using a 30% (wt/vol) dilution with ethylmethylketone (EMK), which guarantees perfusion into the finest capillaries100 (Step 8). These casts are highly elastic while retaining their original structure to facilitate post-casting tissue dissection and pruning100.
The adapted perfusion protocol had to be optimized for postnatal animals to fulfill two requirements: (i) to perfectly fill the vessels with PU4ii and (ii) to simultaneously preserve the mechanical vessel integrity in order to avoid rupture of the more immature vessels (Extended Data Fig. 2 and Supplementary Fig. 1).
Therefore, mice (P10 or P60) are first intracardially perfused through the left ventricle with 10–20 ml ACSF containing 25,000 U/L heparin, followed by 4% (wt/vol) PFA in PBS, and then by resin PU4ii (vasQtec), at 90–100 mmHg (4 ml/min) for postnatal animals and 100–120 mmHg (8 ml/min) for adult mice (Steps 1–8). This adapted infusion pressure for P10 mice as compared with adult animals ensured mechanical stability, preventing vessel rupture while at the same time ensuring adequate perfusion of the blood vessel network.
Resin curing, maceration/decalcification and brain dissection
After resin curing of the perfused mouse at room temperature (RT, 18–23 °C) for at least 24 h (embedded in a diaper), the tissue is macerated in 7.5% (wt/vol) KOH at 50 °C for 24 h followed by decalcification of the surrounding bone structures with 5% (vol/vol) formic acid, also at 50 °C for 24 h (Fig. 2k,l and Extended Data Fig. 1e,f) (Steps 9–12). The cerebral vasculature is then dissected from the remaining extracranial vessels (Fig. 2k–n and Extended Data Fig. 1g,h) (Step 13). Subsequently, the cerebral vascular corrosion casts are washed thoroughly in distilled water and dried by lyophilization (Steps 14–15).
Imaging and quantification: SEM
For qualitative control of the vascular corrosion casts, we inspect the perfused blood vessels of the entire mouse body including the whole brain by visual observation and conventional light microscopy (Extended Data Fig. 2). Then, a subset of the casts is mounted on stubs and sputter-coated with gold104 for quality control SEM imaging (SEM; Hitachi S4000)104,132 (Step 15). Imaging of resin-perfused, dissected and osmicated P10 and P60, WT and Nogo-A−/− brain tissue samples reveals a dense vascular network in the superficial cortex with well-defined vessel structures of all sizes (Extended Data Fig. 2). Moreover, the entire brain vasculature is uniformly filled, and the vessel lumen was molded with its finest details (Extended Data Fig. 2).
In addition, larger blood vessels show oval endothelial nuclei imprints on the resin cast surface, a quality feature also observed in vascular corrosion casts of adult mice demonstrating the perfect molding of the vascular lumen, suggesting complete filling (Extended Data Fig. 2)105. The fine capillary network is well visible and devoid of vessel interruptions in the selected P10 and P60 brain cortices (Extended Data Fig. 2). Taken together, all these morphological blood vessel features indicate that the vascular corrosion casts are of excellent quality.
Typical signs of insufficient vascular corrosion casting consist of irregular shapes combined with rounded abrupt ends of larger vessels, lacking endothelial nuclei imprints (Supplementary Fig. 1). A possible interpretation is that the casting material is pushing against air introduced during the perfusion by gaseous solutions, and trapped in vessels interrupting the normal flow154,155. This increased pressure in the preceding vessel segment distorts the vessel surface. Moreover, absence of imprints of endothelial cell nuclei further indicates insufficient casting (Supplementary Fig. 1).
Desktop µCT imaging and ROI selection
Three-dimensional images of whole-brain samples are acquired using a desktop μCT system (μCT 40, Scanco Medical). Details can be found in Steps 21–24 of the Procedure. The images are evaluated visually, and desired ROIs in various anatomical regions of the brain are chosen. To ensure precise navigation within the postnatal mouse brain parenchyma, we refer to the Allen Developing Mouse Brain Atlas (http://developingmouse.brain-map.org/docs/Overview.pdf) or, alternatively, to any other mouse brain atlas. To find the same ROIs in the SRµCT imaging (see below), the coordinates of the ROIs are determined relative to well-distinguishable details in the sample. For this purpose, we employ two metallic pins in the sample holder rod. When scanning the entire vascular corrosion cast, ROI selection can be omitted.
SRμCT
We acquired high-resolution (pixel size 0.73 µm) SRµCT images of the selected ROIs at the TOMCAT beamline of the Paul Scherrer Institute (Switzerland). The ROIs are located using the steel pins in the sample holder rod as described above. X-ray projection images are acquired with monochromatic X-rays. Paganin phase retrieval57,156 is performed before tomographic reconstruction using the Gridrec algorithm57,157 (more details can be found in Steps 23–24 of the Procedure).
Additionally, high-resolution images of selected whole-brain casts are acquired using a similar setup as compared with the ROI-based approach, now with 0.65 µm pixel size. Here, the field of view of a single tomographic image is too small to cover the whole brain, and thus we use mosaic imaging mode where multiple smaller, partially overlapping, images are acquired. The reconstructions are then stitched into a single large image representing the whole brain vasculature158. The SRμCT images obtained in this protocol have an SNR >10 (and typically in the range 20–30), and therefore they seemed to be well suited for computational reconstruction of the vascular network (Supplementary Video 2).
Alternatives for broader accessibility
In this protocol, we propose to use a desktop μCT scanner to acquire a low-resolution overview of the whole brain sample after which we use a SRμCT imaging approach to study the selected ROIs at a high resolution in more detail (Fig. 4a–l). Alternatively, both the low-resolution ROI selection and the high-resolution imaging of the selected ROIs (Fig. 4a–l) can be performed using SRμCT techniques without the use of a desktop μCT scanner, for instance in the case of well-accessible SRµCT scanners and to avoid relocalization in different CT machines. As another alternative, all the imaging steps in this protocol can also be performed with a high-resolution desktop μCT scanner only (machines from manufacturers such as Scanco, Bruker, Zeiss, Phoenix and Nikon can be used) without the use and need of an often less accessible SRμCT scanner, and if the number of samples is relatively small as scanning times are higher with desktop μCT scanners.
Computational reconstruction: vessel segmentation
The vessels in the SRμCT reconstructions are segmented to yield a binary image where the foreground represents vessels and background represents the structures in between them (Fig. 4m–p). The high SNR allows us to use simple Otsu thresholding57,159, where all pixels with a value above a specific, algorithmically determined threshold are classified as vessels (Fig. 4o). In the Otsu method, the threshold is chosen such that it minimizes the intensity variance of pixels inside both groups. The threshold value does not seem to be very sensitive to the selection of the thresholding method, provided that the selected method is suitable for thresholding images with bivariate histograms. For example, other previously described methods160,161,162 typically give threshold values that are within a 10% range of the value given by the Otsu method (Step 25).
The thresholding step is followed by morphological closing using a spherical structuring element (radius 0.75 µm) in order to eliminate small gaps in the vessels. A few regions primarily located in the largest vessels contained small, isolated background regions (i.e., holes or bubbles) that were eliminated by merging all isolated background regions to the foreground (Steps 26–27).
Computational reconstruction: vessel skeletonization
To analyze the individual vessel branches, we convert the segmented image into a graph representing the vessel network. To this end, the segmented vessels are first reduced into a one-pixel-wide skeleton that approximated the centerline of each vessel branch57. This is done by iterative thinning of the structures until only lines are left57,163 (Fig. 4o,p).
The skeleton is traced into a graph structure, where each graph node corresponds to an intersection point between three or more skeleton branches, and the branches are represented by edges between the nodes. For each edge, the locations of the skeleton pixels that form the corresponding branch are stored. Additionally, for each stored location, the distance to the nearest nonvessel pixel is calculated in order to determine the (local) radius of the vessel57,164 (Steps 28–30).
An anchored convolution approach is used to smooth the pixel-accurate representation of the vessel branches for more accurate length measurements and visualizations57,165. In the anchored convolution, the locations of the points forming the branch are convolved with a Gaussian kernel, but the points are not allowed to move more than a specific distance from their original locations. As a result, small deviations in the curve will smooth out, but larger ones remain. In our case, the standard deviation of the smoothing Gaussian was 1.125 µm, and the maximum distance a point was allowed to move was 0.5 µm. The endpoints of each branch were not allowed to move at all (Step 31).
Finally, spurious branches originating from the ill-conditioned nature of the skeletonization procedure and from remaining imaging noise were removed using a simple heuristic. Therefore, all branches with at least one free end and a length of less than twice the radius at the non-free end are removed. Additionally, isolated branches <5 µm in length are erased, as these corresponded to small, spurious foreground regions not representing vessels (Steps 32–36).
The image analysis is done using the freely available pi2 software (version 3.0) (https://github.com/arttumiettinen/pi2) and Python 3.6.
Computational reconstruction: global morphometry and local topological vascular network analysis
Global morphometry and local topological vascular network analysis are performed for each high-resolution image in collaboration with Nemtics (http://www.nemtics.com) and in accordance with our previous work39. Global morphometry analysis is based on the vessel segmentation map. It considers the characteristics of the vascular structure on a per-voxel level, and allows the vascular parameters vascular volume fraction and extravascular distance to be assessed.
In contrast to global morphometry analysis, using local topological analysis of the 3D vascular network, individual per-vessel parameters—e.g., branch point density and degree and vessel/segment density, diameter, length, tortuosity and directionality—can be evaluated and calculated in addition to the vascular volume fraction.
The nodes in the previously created graph are interpreted as branch points and the edges in between nodes as vessel segments. Branch points are junctions between at least three vessel segments where the number of vessel segments joining in one branch point is called branch point degree (which equals at least 3). The branch points and all segments were categorized into capillary (diameter <7 µm), noncapillary (diameter ≥7 µm) and global (without distinction by diameter), with global being the union of capillaries and noncapillaries (Step 37).
We define the branch point diameter as the diameter of the thickest joining segment, allowing the discrimination between capillary and noncapillary branch points. The branch point density per volume is defined as the number of branch points per cubic millimeter (Fig. 8 and Extended Data Figs. 5, 6, Supplementary Figs. 6, 9–11).
The number of branch points per degree is counted and separated for capillaries, noncapillaries and global branch points in order to plot branch point degree and branch point diameter statistics (Supplementary Figs. 6, 9–11). The values per branch point degree are then divided by network volume to obtain branch point density statistics (Fig. 8c,f–h, Extended Data Figs. 5, 6, Supplementary Figs. 6, 9–11) (Step 38).
All segments are classified into either capillary or noncapillary, counted and divided by network volume to plot segment density statistics (Fig. 9d–f and Supplementary Fig. 7a–c). Segment diameter is obtained as the average value of local vessel diameter over all the points defining the segment (Fig. 9g–j and Supplementary Fig. 7d–f). Segment length is calculated as the sum of lengths of line segments between points defining the vessel segment (Fig. 9k–n and Supplementary Fig. 7g–i) (Steps 40–44). Tortuosity is calculated as the ratio between the real length of a segment and the shortest distance between the segment’s endpoints (Fig. 10a–d and Supplementary Fig. 7j–l) (Step 45). To calculate segment volume, each of the line segments is assumed to represent a cylinder whose diameter is taken to be the average vessel diameter at its endpoints, and the sum of all the cylinder volumes gives the segment volume (Fig. 10e–h and Supplementary Fig. 7m–o).
To determine extravascular distance, the Euclidean distance transform of the background in the segmented image is calculated. The distance transformation gives the distance to the nearest vessel for each background point (Fig. 11). Binning the distance values into a histogram gives an estimate of the extravascular distance distribution (Fig. 11b,i–k and Supplementary Fig. 8) (Step 46).
The volume fraction of global segments is determined as the ratio between the count of vessel voxels and the total count of voxels in the segmented image (Figs. 5–7 and Supplementary Figs. 2–5). This value is then divided into capillary and noncapillary volume fractions in proportion to the total volumes of vessels in these two vessel groups (Fig. 7f–h). In the example demonstrated in this protocol, quantitative analysis of the relative angles of the vascular segments emanating from a certain branch point was performed for both the developing P10 and mature P60 stages (Fig. 12d–f and Supplementary Fig. 12c–e,h–j). Three-dimensional renderings of the computationally reconstructed vessel networks were obtained, color-coded by (i) the average vessel radius (Fig. 5e–g), (ii) the division into capillary and noncapillary vessels (Fig. 7d,e and Supplementary Figs. 3a,b and 4a,b), and (iii) vessel directionality (Fig. 12b,c and Supplementary Figs. 12a,b,f,g and 13–16) (Steps 47–49).
Computational reconstruction: statistical analysis
For the ROI-based approach, the mean of the parameters obtained from the animals in the different groups (e.g., P10 WT, P10 Nogo-A−/−, P60 WT and P60 Nogo-A−/−) are compared using an appropriate t-test (we used the Welch t-test, which is an adoption of the Student’s t-test to better cope with unequal sample distribution variance).
Histograms and boxplots were used to visualize the data (Figs. 5–13, Extended Data Figs. 5 and 6 and Supplementary Figs. 2–12, 17–21) (Step 50), with whiskers spanning at most 1.5 times the interquartile range (the height of the boxes) above and below the 25% and 75% quartiles. Whiskers are drawn at the highest/lowest data value if it is inside the 1.5 interquartile range. In compliance with the standard166, the open dot represents the mean and the horizontal line represents the median166. We used Matplotlib (https://matplotlib.org) and NumPy (https://numpy.org) for statistical testing and figure plotting. For all quantitative analysis in the different brain regions (Figs. 5–12, Extended Data Figs. 5, 6, Supplementary Figs. 2–12), we used 4–6 postnatal (P10) and 8–12 adult (P60) animals. On average, we analyzed about three ROIs per brain region.
In addition to the ROI-based approach and in order to visualize the variability of the measured quantities in a single brain specimen, we use whole-brain images acquired for one P10 WT and one P60 WT sample. The whole-brain images were manually registered to the Allen Mouse Brain Atlas and divided into regions of the same size as the ROIs used earlier. The vascular network in the cortex, hippocampus, superior colliculus, corpus callosum, hippocampal subregions (i.e., CA1, CA3 and dentate gyrus), basal ganglia, thalamus, hypothalamus, olfactory bulb, cerebellum, pons and medulla were analyzed using the same approach (Fig. 13 and Supplementary Figs. 17–21). The anatomical regions are divided into 5–126 parts depending on the size of that region and quantified similarly to the ROI-based approach. The means of the parameters obtained from each animal in both groups (n = 1 for P10 WT, n = 1 for P60 WT) were compared using the Welch t-test. We used histograms and boxplot to visualize the data (Fig. 13 and Supplementary Figs. 17–21), as well as statistical testing and figure plotting undertaken as described for the ROI-based approach above (Step 50).
Materials
Animals
-
10-d-old and 2-month-old mice (we used wild type and Nogo-A−/− C57BL/6, male or female) (Charles River Laboratories)
Caution
Institutional and governmental ethics regulations concerning animal use must be followed. All the animal experiments herein were performed in accordance with governmental, institutional (University of Zurich) and ARRIVE guidelines, and had been approved by the Veterinary Office of the Canton of Zurich (license number 173/2010).
Reagents
Caution
Some of the chemical substances mentioned below are extremely harmful if inhaled and swallowed, and/or are irritating to the eyes, respiratory system and skin. They may cause sensitization of skin upon contact. Therefore, in principle, wear protective goggles, clothing and gloves as appropriate. Use the chemicals in a fume hood. Carefully read the safety data sheets of all chemicals used in this protocol.
-
Pentobarbital solution 1.0 mg/ml in methanol (Sigma Aldrich, cat. no. P-010)
-
NaCl, 0.9% (wt/vol) in distilled water, 250 ml (BBraun, cat. no. 3570110)
-
Heparin sodium salt from porcine, grade 1A (Sigma Aldrich, cat. no. H3393-25KU)
-
PFA (Sigma-Aldrich, cat. no. P6148)
-
PU-4ii resin, hardener, color, fluorescence (www.vasqtec.com)
-
EMK EMPLURA (Merck, cat. no. 106014)
-
XTEND-IT, dry gas blanket (Kaupo, cat. no. 09291-010-000012)
-
KOH, 1 kg (Merck, cat. no. 1050331000)
-
Formic acid, 99% techn. (Fisher Scientific, cat. no. 270480250)
-
Osmium tetroxide solution, 2% (wt/vol) in water, 5 ml (Sigma Aldrich, cat. no. 20816-12-0)
Caution
OsO4 is very toxic. Work under a fume hood and use protection gloves, glasses and laboratory coat. Properly dispose of the OsO4 waste.
-
PBS, 0.1 M, or PBS tablets (Sigma-Aldrich, cat. no. P4417)
-
Mounting medium (Dako, cat. no. 53023)
-
Instant adhesive, cyanoacrylate (Kisling, Ergo, cat. no. 5889)
-
Distilled water (Millipore, Milli-Q synthesis A10)
-
DAPI (4′,6-diamidino-2-phenylindole, dihydrochloride) nucleic acid stain (Invitrogen) (excitation/emission maxima 358/461 nm) staining for cell nuclei
-
FluoroMyelin Red (Invitrogen) (excitation/emission maxima: 558/654 nm) staining for myelin
Equipment
-
Tabletop balance (e.g., ED Precision balance, Sartorius, cat. no. 14558792)
-
Plastic foil
-
Paper towels
-
Green protection diapers (Polymed Medical Center)
-
Gloves (M/L) Nitril (Sigma-Aldrich, cat. no. Z543500/Z543519)
-
Syringes, 1 ml (BD Luer-Lok, cat. no. 309628)
-
Syringes, 50 ml (Fisher Scientific, cat. no. 13-689-8)
-
Omnifix Luer-lock syringes, 20 ml (BBraun, cat. no. 4616200V)
-
Needles (0.5 × 16 mm/25G) (Gima, cat. no. 23760)
-
Butterfly needles 23G and 25G (needle: 20 mm, 0.6/0.4 mm; tube: 300 mm, 0.36 ml) (Ospedalia AG)
-
Infusion pump (Harvard Apparatus Pump 11 Elite, Instech Laboratories, cat. no. 70-4504)
-
Dissecting instruments: gross/fine dissection scissors (Aichele Medico AG)
-
Dissection tray with corkboard (Shandon, Thermo Fisher Scientific)
-
Pinning needles, 1.2 × 40 mm/18G (BBraun)
-
Beaker cups, 120 ml/160 ml (Semadeni, cat. no. 2294 or 4438)
-
Pipette with pipette tips, 10 ml (Soccorex, cat. no. 312.10)
-
Wheaton scintillation vials, 20 ml (Fisher Scientific, cat. no. 03-340-25N)
-
Vacuum pump (Laboport N820, KNF Group)
-
Lyophilization machine, benchtop freeze dry system (Labconco)
-
SEM specimen stubs (Agar Scientific, cat. no. AGG301)
-
Light microscope (Axiolab 5, Zeiss). We use conventional light microscopes for quality control of vascular corrosion casts
-
Fluorescence microscope (Olympus, BX-UCB)
-
Scanning electron microscope (Hitachi S4000), see ‘Equipment setup’
-
Gold sputter/coater device (Agar Scientific)
-
Desktop μCT (μCT 40, Scanco Medical), see ‘Equipment setup’. Similar instruments from other manufacturers can be used as well
-
SRμCT. We used the TOMCAT beamline of the Swiss Light Source; see ‘Equipment setup’. Similar experimental setups are available in most synchrotron facilities, and imaging settings should be selected according to the available instrumentation
Reagent setup
Formaldehyde solution (4% (wt/vol) in 0.1 M PBS)
Under a chemical fume hood, prepare 0.1 M phosphate buffer (pH 7.4) by adding 19 ml 0.2 M NaH2PO4 × H2O (27.58 g/l) to 81ml Na2HPO4 (28.38 g/l). Next, add 8 g of PFA powder to 100 ml distilled water (Aqua Millipore). Dissolve the PFA powder by heating the solution (containing a clean magnetic stir bar) to 60 °C on a magnetic stirrer. To facilitate dissolving the PFA powder, add few drops of 5 or 10 M NaOH when mixing starts. After PFA powder dissolves completely, filter the PFA solution using filter paper. To dilute the 8% (wt/vol) formaldehyde solution to the final concentration of 4% (wt/vol) PFA in 0.1 M phosphate buffer, add 100 ml of 0.2 M phosphate buffer. When the solution has cooled down to RT, check the pH and adjust to pH 7.4 if necessary. Aliquots can be stored at −20 °C for up to 1 year35,140; however, we recommend freshly preparing the 4% (wt/vol) PFA solution for every experiment. Thaw the aliquots in a warm water bath (35–40 °C) and cool them to 4 °C on ice before use.
ACSF (with 24 KU heparin sodium salt)
Under a chemical fume hood, mix NaCl (7.6 g/l), NaHCO3 (2.02 g/l), glucose, MgSO4 × 7H2O (0.37 g/l), KCl (0.26 g/l), NaH2PO4 × H2O (0.17 g/l) (water-free 0.15 g/l) and CaCl2 × 2H2O (0.29 g/l) (must be added last). Add 25 KU heparin sodium salt per liter. If you prepare it 1 d in advance, store the solution at 4 °C. It should be stored for no longer than 4 weeks167. Before use, warm up to 37 °C in a water bath.
Resin (PU4ii)
Dilute 5–10 mg of pigment into 3 g of EMK. Then add the EMK/pigment mixture to 5 g of PU4ii, and mix in a glass vial using a vortex mixer. The hardener (0.8 g) should only be added to the resin mixture after infusion of the formaldehyde fixation solution. Mix this final resin mixture using a vortex mixer, briefly evacuated in a standard vacuum chamber to remove remaining air bubbles, and then transfer into a 10 ml syringe. The hardener has the shortest shelf life and should be covered with the dry gas (XTEND-IT) (see reagent list) after each use. Use the resin mixture immediately after preparation.
Equipment setup
Dissection board
A dissection board placed into a tray is used for the perfusion steps. The board should be slightly tilted to collect the efflux after cardiac infusion.
Scanning electron microscope
We assess the quality of random samples of the corrosion casts before μCT imaging using Hitachi S4000 SEM with backscattering detector. Multiple resolutions and acceleration voltages are used until clear views of the perfused vessels can be attained. The imaging parameters and exact type of SEM are not critical as the images are used only for visual evaluation and prescreening to eliminate any insufficiently perfused samples.
Desktop μCT system
We use a cone-beam microtomograph to create 3D overview images of the whole brain samples and facilitate selection of suitable regions for high-resolution imaging. A Scanco Medical μCT 40 device with a nominal isotropic voxel size of 16 µm is used in this protocol. The imaging settings are set to suitable values as recommended by the device manufacturer (e.g., for the system we used, a resolution of <8 μm 10% modulation transfer function and a 3–72 µm nominal isotropic voxel size with an image format of 512 × 512 to 4,096 × 4,096 pixels could be selected). The exact settings are not as critical as the images are not used for quantitative analysis.
SRμCT
We acquired high-resolution tomographic images of selected ROIs at the TOMCAT beamline of the Swiss Light Source. The imaging system was operated at 17.5 keV X-ray energy with a sample–detector distance of 30 mm. For each ROI, 1,001 projection images were acquired with 0.73 µm pixel size and 2 s exposure time. Paganin phase retrieval was used on the projection data, followed by reconstruction with the gridrec algorithm.
Procedure
Perfusion with resin PU4ii (day 1)
Timing ~5–10 min per mouse
-
1
After weighing, anesthetize mice using 100 mg/kg pentobarbital intraperitoneally. Check proper anesthetic depth before proceeding (if using the tail-pinch method, the mouse must not move anymore). Fix both the postnatal (P10) and adult (P60) animals onto a dissection board (Fig. 2c–j and Extended Data Fig. 1).
Critical step
It is crucial to weigh the animals prior to resin perfusion as developmental stages are only comparable between the different study groups for mice with similar weights (and ages).
Caution
All animal experiments must be performed in accordance with governmental, institutional and ARRIVE guidelines. The procedure we describe here was approved by the Cantonal Veterinary Department of Zurich (license number 173/2010).
-
2
Test with the tail-pinch method if the mouse is properly anesthetized, and turn it on its back.
-
3
For the surgical preparation, lift the skin with forceps and cut perpendicular to expose the sternum (Fig. 2g,d,g,h and Extended Data Fig. 1a).
-
4
Next, lift the sternum and make lateral cuts through the rib cage to expose the chest cavity. Pull back the cage flap, and fix using a pin/needle. Carefully remove the pericardium using rounded forceps (Fig. 2d,h and Extended Data Fig. 1b).
-
5
Lift the heart slightly using forceps, and then insert butterfly needle (23G butterfly needle for adult mice, needle: 20 mm, 0.6/0.4 mm; tube: 300 mm, 0.36 ml, and 25G butterfly needle for P10 mice) into the left ventricle (Fig. 2e,i and Extended Data Fig. 1c). Note the angle of insertion of ~30 °C in the sagittal plane (Fig. 2e).
Critical step
Do not insert the needle too deep to avoid damage of the atrioventricular valve or puncture of the heart basis. The inserted perfusion needle should be glued to the heart using instant glue to prevent the needle from sliding out during perfusion and leakage of the perfusion fluids. Only a small drop is required.
-
6
Open the right atrium with a pair of sharp and pointed scissors. Immediately start the perfusion with 20 ml (for adult animals) and 5 ml (for P10 animals) ACSF containing 25,000 U/L heparin through the left ventricle, then stop the pump, block the tube with a hemostat and exchange the syringe.
Critical step
Before reconnecting the syringe, make sure that all air in the tube is completely removed.
Critical step
For P10 animals, adapt the amount of ACSF containing 25,000 U/L heparin according to the animal age.
-
7
Start perfusion with 4% (wt/vol) PFA (in PBS). Immediate muscle fasciculation indicates good quality of fixation.
-
8
Add the hardener (0.8 g) to resin PU4ii solution (prepared by diluting 5–10 mg of pigment into 3 g of EMK, adding the EMK/pigment mixture to 5g of PU4ii; see ‘Reagent setup’), and mix using a vortex mixer (Fig. 2b,f,j and Supplementary Video 1). Briefly evacuate in a standard vacuum chamber to remove remaining air bubbles, and then transfer into a 10 ml syringe. When perfusing the resin solution, tilt the rear of the perfusion pump upwards to prevent occasional air bubbles leaving the syringe. The tray should be tilted to avoid a mess due to the efflux from the atrium.
Critical step
Resin curing (days 2–3)
Timing ~1 d
-
9
Keep animals for at least 24 h at RT without manipulation. The resin curing time does not differ between P10 and P60 animals.
Critical step
Do not manipulate the sample during the 24 h. Otherwise, this may influence the curing and may lead to deformations in the vascular corrosion cast.
-
10
(Optional) For simultaneous visualization and analysis of both the blood vessel structures and the surrounding soft tissue as well as for covisualization of vascular and, e.g., perivascular cell markers, take selected resin-perfused brains. Make thick, 100–150 µm sections with a vibratome, and then stain them with virtually any protein of interest (in our case, DAPI for cell nuclei and FluoroMyelin Red (Invitrogen) for myelin). Image the sections with fluorescence light microscopy (Olympus, BX-UCB) (Extended Data Fig. 7). Proceed to the next step to macerate the remaining brains.
Tissue maceration of soft tissue (days 2–3)
Timing ~1 d
-
11
Transfer the animal into 300–400 ml (for P60 animals) 7.5% (wt/vol) KOH, and incubate at 50 °C for at least 24 h. Place only one animal into the solution at one time, and make sure the entire body is covered.
Decalcification of bony structures (days 3–4)
Timing ~1 d
-
12
Incubate the animal in 300–400 ml (for P60 animals) 5% (vol/vol) formic acid at 50 °C for at least 24 h. Place only one animal into the solution at one time, and make sure the entire body is covered.
Brain dissection (day 4)
Timing ~5 min per cast
-
13
Free the cerebral vasculature from remaining extracranial vessels using fine scissors and forceps (Fig. 2k–n). Optionally, the extracranial vessels can also be further processed and analyzed by following a similar procedure to the one described here, depending on the vessels/organs of interest.
Wash in distilled water (day 4)
Timing ~5 min per cast
-
14
Wash the brain vascular corrosion cast in distilled water. Place only one brain at a time into 15 ml of distilled water.
Critical step
Use plenty of distilled water to eliminate all residues of tissue and dissected pieces of the extracranial vessels.
Quality control using SEM (day 4)
Timing ~1 h per cast
-
15
For routine SEM, mount a small random sample of the brain casts on stubs and sputter-coat them with gold.
Osmification with osmium tetroxide (OsO4) of brain vascular corrosion cast (day 4)
Timing ~24 h
-
16
Transfer the brain vascular corrosion casts not used for SEM into 2% (wt/vol) OsO4 in 5 ml water at RT and incubate for 24 h to increase absorption contrast, and reduce SNR during the SRμCT imaging.
Caution
OsO4 is very toxic. Work under a fume hood, and use protection gloves, glasses and laboratory coat. Properly dispose of the OsO4 waste.
Wash in distilled water (days 4–5)
Timing ~5 min per cast
-
17
Wash the brain vascular corrosion cast thoroughly in distilled water. Place only one brain at a time into 15 ml of distilled water.
Critical step
Use plenty of distilled water to eliminate all residues.
Lyophilization by freeze-drying (days 4–5)
Timing ~ 24 h per cast
-
18
Dry the brain vascular corrosion casts by freeze-drying in a lyophilization machine overnight at −54 °C in a vacuum.
Mounting on plexiglas stubs (days 5–6)
Timing ~5 min per cast
-
19
Mount each brain resin cast using cyanoacrylate glue on plexiglas stubs (size of the stubs is dependent on the µCT setup and brand) equipped with stainless steel pins for sample repositioning in the μCT.
Pause point
The brain vascular corrosion casts can be stored at RT (for years) before continuing with the imaging steps of the protocol.
SEM (days 5–6)
Timing ~15 min per cast
-
20
Evaluate all corrosion casts by light microscopy, and then analyze the subset of corrosion casts selected and prepared in Step 15 with SEM as quality control. Corrosion casts are suitable for further analysis if a dense vascular network without holes or irregularities is present and there are endothelial cell nuclei imprints on large vessel casts (Extended Data Fig. 2 and Supplementary Fig. 1).
Desktop μCT imaging (day 6)
Timing ~15 min per cast
-
21
Acquire 3D images of whole-brain samples not used for SEM using a desktop µCT system by selecting the various imaging parameters according to manufacturer’s instructions (or optionally using a SRμCT scanner; see ‘Alternatives for broader accessibility’ under ‘Experimental design’). We use a µCT 40, Scanco Medical setup, operated at 50 kVp and 160 µA with a nominal isotropic voxel size of 16 µm. Per scan, we acquire 1,000 projection images with an integration time of 200 ms and an additional three times frame averaging for improved SNR. The exact settings depend on the desktop μCT system used.
Selection of the ROIs (day 6)
Timing ~15 min per cast
Critical
This step is not needed if the entire brain cast is scanned in high resolution in Steps 23–24.
-
22
Reconstruct, segment and import tomographic images into custom-made or existing ROI picker software (here, we used the Imaging Processing Language of Scanco Medical, http://www.scanco.ch/; for details, we refer to ref. 168). Place one to three ROIs of 1 mm³ per animal and brain region in the cerebral cortex, hippocampus, superior colliculus and brain stem (Fig. 4a–l). Then, calculate relative coordinates of ROIs with respect to the two-sample holder pins, and save them for subsequent reorientation and measurement at the synchrotron beamline.
High-resolution SRμCT imaging of selected ROIs or the entire brain vascular corrosion cast (days 6–7)
Timing ~30 min per cast (~24 h for scanning one entire brain vascular corrosion cast)
-
23
Acquire high-resolution 3D images of selected ROIs or the whole brain using the tomography setup established for the SRμCT system being used. We use a previously described local tomography setup detailed in refs. 39,99,103. Briefly, the system we use operates at 17.5 keV with a sample–detector distance of 30 mm for distinct edge enhancement, and an optical magnification resulting in an isotropic voxel size of 0.73 µm. Each measurement includes 2,000 projections acquired at an integration time of 0.1 s each. Phase retrieval is performed with the Paganin method and data reconstructed using the gridrec algorithm. The net volume of the final images is 2,560 × 2,560 × 2,160 voxels, corresponding to 5.5 mm3 of tissue volume.
-
24
When the entire brain vascular corrosion cast is scanned in high resolution (as compared with the high-resolution scanning of preselected ROIs), the mosaic images should be stitched together. To construct an artifact-free image mosaic from the subimages, we use a nonrigid stitching approach, according to ref. 158. This image mosaic can then be registered to the Allen Mouse Brain Atlas (http://developingmouse.brain-map.org/docs/Overview.pdf) or, alternatively, to any other mouse brain atlas and divided into segments. We divide the anatomical structures of interest into segments with the exact same dimensions as the previously used ROIs (in the ROI-based approach), in order to proceed with a similar analysis process.
Computational 3D reconstruction of the 3D vascular network—vessel segmentation (days 8–9)
Timing ~15 min per ROI (~10 h for one whole brain scan, depending on the technical settings)
-
25
If the reconstructed CT images are noisy, apply bilateral filtering to improve the SNR. This step is not required if the SNR is high. To separate vessels from the background in the reconstructed CT images, use the Otsu thresholding method159 (Fig. 4m–p).
-
26
Erase small gaps and holes in vessels by performing morphological closing with a spherical structuring element (with a suggested radius of one to three voxels).
-
27
Perform a hole filling operation that merges isolated background regions into the foreground.
-
28
Calculate the distance map of the segmented vessels, where each vessel voxel is assigned the distance to the nearest background pixel.
-
29
Calculate the skeleton of the segmented vessels, where each vessel is reduced into a one-voxel wide centerline (Fig. 4o).
-
30
Trace the centerlines into a graph where graph nodes represent bifurcation points and graph edges represent vessels. For each edge, store a list of locations of voxels that form the edge. Store also the distance map value at each edge voxel as it describes the local radius of the vessel at that pixel (Fig. 4o).
-
31
Use anchored convolution to smooth the voxel list of each edge (suggested sigma 1.125 µm, maximal displacement 0.5 µm). Do not allow any displacement in the ends of the edge.
-
32
Calculate the length of each edge by summing distances between consecutive smoothed edge voxels.
-
33
Remove all isolated branches whose length is small (suggested threshold length 5 µm).
-
34
Remove all branches that have one free end and that satisfy L < 2 max (ri,r2) where L is the length of the branch and \({\mathrm{r}}_i\) are the radii at the endpoints of the branch.
-
35
Remove all nodes that have exactly two edges connected to them (these might have been created in Step 34), and combine the two branches into one.
-
36
Remove all nodes that have no edges connected to them.
Global vascular network morphometry and local vascular network topological analysis (day 10)
Timing ~variable (depending on the parameters to analyze)
-
37
Categorize vessels and branch points into capillary (diameter <7 µm), noncapillary (diameter ≥7 µm) and global (without distinction by diameter). Global is the union of capillaries and noncapillaries.
-
38
Calculate the degree of each branch point. Count the number of branch points per degree, for capillary, noncapillary and global branch points. Use this to plot branch point degree statistics (Supplementary Figs. 9–11).
-
39
Take the values per branchpoint degree (from Step 38), and divide them by network volume. Notice that the tomographic image that the graph is based on is usually cylindrical and the volume of the cylinder should be used. Use this to plot branch point density statistics (Fig. 8c,f–h and Extended Data Figs. 5, 6). The branch point density per volume is defined as the number of branch points per cubic millimeter.
-
40
Use the branch point diameter (from Step 37), classified into global, capillary and noncapillary, to plot branch point diameter statistics (Fig. 8c and Extended Data Figs. 5b and 6b).
-
41
Classify segments into global, capillary and noncapillary. Count all occurrences per classification. Divide by network volume to get the segment density. Use this to plot segment density statistics (Fig. 8c,f–h, Extended Data Figs. 5, 6, Supplementary Figs. 7a–c, 9–11).
-
42
Take segment diameter and length per classification (from Step 41) to plot segment diameter and length statistics (Fig. 9c,g–n and Supplementary Fig. 7d–i).
-
43
Use the values from Step 42, and relate length to diameter to plot their relation (Fig. 9c).
-
44
Use the values from Step 42, and calculate the volume of the segment as the sum of volumes of cylinders corresponding to line segments in that segment. Use this to plot segment volume statistics (Fig. 10e–h and Supplementary Fig. 7m–o).
-
45
Calculate vessel tortuosity of each vessel segment as the ratio between the effective length/distance between the endpoints of a vessel segment and the shortest length/distance between those vessel segment endpoints. Use this to plot the vessel tortuosity statistics (Fig. 10a–d and Supplementary Fig. 7j–l).
-
46
Use the image containing the segmented vessels, and calculate the Euclidean distance transform of nonvessel regions. This creates a volume containing the distance to the closest vessel for each nonvessel voxel. Create a histogram of the distance values to estimate the extravascular distance distribution (Fig. 11b,i–k and Supplementary Fig. 5).
-
47
Count the number of background and vessel voxels. Calculate vascular volume fraction (volume fraction of all vessels) as the ratio between number of vessel voxels and total number of voxels (Figs. 5h–j,7f–h and Supplementary Figs. 2–5).
-
48
Calculate the sum of all segment volumes for global, capillary and noncapillary segments. Divide the capillary volume by the global volume. Use this value to scale the volume fraction calculated in Step 47 to get the approximate capillary volume fraction. Do the same for noncapillaries. Use these values to plot the capillary and noncapillary vascular volume fraction statistics (Fig. 7f–h and Supplementary Figs. 2–5).
-
49
Derive the vessel directionality by attributing every vessel to its main direction (x, y, z) in the 3D space within the selected ROI. For each line of each segment, normalize the vector that represents the line. To quantify the directions of vessels emanating from each branchpoint, calculate the relative angles between all segments that join at a branch point. This allows for a quantitative representation of how orthogonal (90°) or straight (0° and 180° ranges) vessels go in and out of branchpoints. This normalized direction (x, y, z) is directly mapped to ‘redness’ (r), ‘blueness’ (b) and ‘greenness’ (g) (r,g,b), and then scaled by 1.75 to get a better color saturation and clamped to [0,1]. Render the computational 3D reconstructions with color mapped to (1) directionality, (2) vessel radius and (3) classification (capillary or noncapillary) of each vessel (Fig. 12 and Supplementary Figs. 12–16).
Statistical analysis (day 11)
Timing ~variable (depending on the parameters to analyze).
-
50
Compare the mean of the parameters of each ROI obtained from the different sample groups using an appropriate test. We used the Welch t-test (statistically significant differences are defined as P values < 0.05). Use Matplotlib (https://matplotlib.org) and NumPy (https://numpy.org) or comparable software to visualize the data with histograms and boxplots.
Critical step
In our example for the whole-brain scan method, the mean of the parameters obtained from one animal in the different groups (P10 WT, P60 WT) were also compared using the Welch t-test. Histograms and boxplot to visualize the data (Fig. 13 and Supplementary Figs. 17–21), as well as statistical testing and figure plotting, were performed in the exact same manner as described for the ROI-based method.
Troubleshooting
Troubleshooting advice can be found in Table 1.
Timing
-
Steps 1–8, animal perfusion with resin PU4ii (day 1): ~5–10 min per mouse
-
Step 9, resin curing (days 2–3): 1 d
-
Step 10, immunofluorescence staining (optional step, days 2–3): 2 d
-
Step 11, tissue maceration of soft tissue (days 2–3): 1 d
-
Step 12, decalcification of bony structures (days 3–4): 1 d
-
Step 13, brain dissection (day 4): ~5 min per cast
-
Step 14, wash in distilled water (day 4): ~5 min per cast
-
Step 15, gold-sputtering for SEM (quality control) (day 4): ~1 h per cast
-
Step 16, osmification with osmium tetroxide (day 4): ~24 h
-
Step 17, wash in distilled water (days 4–5): ~5 min per cast
-
Step 18, lyophilization by freeze drying (days 4–5): 24 h per cast
-
Step 19, mounting on plexiglas stubs (days 5–6): ~5 min per cast
-
Step 20, SEM imaging (quality control) (days 5–6): ~15 min per cast
-
Step 21, desktop μCT imaging (day 6): ~15 min per cast
-
Step 22, selection of the ROIs (day 6): ~15 min per cast
-
Steps 23 and 24, high-resolution SRμCT imaging of selected ROIs or the whole brain (days 6–7): ~30 min per cast (~10–24 h for one whole brain scan, depending on the technical settings)
-
Steps 25–36 (days 8–9), computational reconstruction of the 3D vascular network—vessel segmentation: ~15 min per ROI (~10 h for one whole brain scan, depending on the technical settings)
-
Steps 37–49 (day 10), global vascular network morphometry and local vascular network topological analysis: ~variable (depending on the parameters to analyze)
-
Step 50 (day 11), statistical analysis: ~variable (depending on the parameters to analyze)
Anticipated results
With the protocol described here, perfused blood vessels of the total vascular network can be quantitatively assessed in any desired mouse brain region, starting from the postnatal (at least as early as P10) to the adult stage by analyzing various vessel parameters such as vascular volume fraction; branch point density and degree; segment density, diameter, length, tortuosity, volume and directionality; and the extravascular distance. Here we present examples of the types of data that can be obtained using this protocol. The vascular volume fraction, the vessel segment and branch point density in various brain regions including cortex, hippocampus and superior colliculus are significantly increased in the adult (P60) as compared with the postnatal (P10) mouse brain (Figs. 5h–j, 7a–c,f–h, 8f–h and Extended Data Figs. 5e–g, 6e–g), indicating active sprouting angiogenesis and vascular network formation from the developing postnatal to the mature adult stage of mouse brain development.
Application of the existing protocol to the postnatal mouse brain vasculature
The 3D vasculature of mice during postnatal brain development and at the adult stage is visible in the polyurethane resin PU4ii-perfused P10 WT and P60 WT mice (Extended Data Fig. 2). Light microscopy and SEM of brain tissue samples from resin-perfused P10 and P60 WT mice reveals a dense vascular network in the superficial cortex with well-defined vessel structures of all sizes (Extended Data Fig. 2). During resin perfusion, endothelial cell nuclei are exposed to the passing resin material, resulting in imprints of blood vessels on the cast surface (Extended Data Fig. 2f), which were observed in vascular corrosion casts of both P10 and P60 mice, demonstrating the perfect molding of the vascular lumen105. Moreover, the fine capillary network was well defined and visible and devoid of vessel interruptions in both the P10 and the P60 brain cortices (Extended Data Fig. 2). All these morphological features are in line with the characteristics of the normal cerebral vasculature in postnatal adult mice that we and others have previously published39,99,104. The raw images of the SRμCT of the vascular corrosion casts, which were the basis for subsequent computer-aided 3D reconstruction (Fig. 5a), showed very high quality (Figs. 4m,n and 5a–d and Supplementary Video 2). These typical results further confirm that the vascular corrosion casting technique is an excellent method to visualize the 3D vessel network architecture in the brains of both P60 and also developing P10 mice. These results demonstrate that, by making the minor adaptations discussed here, vascular corrosion casting can be applied in very young and small mice at the stage of postnatal brain development at which vascular sprouting is highly active2,3,24,37,38,74.
Functional, perfused blood vessels that constitute the 3D brain vascular network can be best visualized with a marker that is perfused into the circulation of an intact mouse. We therefore used polyurethane resin PU4ii as this labels the inner vascular lumen of actively, in vivo perfused blood vessels (Figs. 2k–n and 3). Resin PU4ii-perfused blood vessels and, in consequence, the entire 3D brain vascular network can be imaged and computationally analyzed in both postnatal and adult mouse brains (Figs. 5–14), allowing the development of the vascular network to be compared between two developmental stages.
As an additional validation step, vascular corrosion casting can be combined with immunofluorescent or other stainings, which may ease navigation within the vascular network, and allows direct correlation with molecules or cell structures of interest (Extended Data Fig. 7), e.g., perivascular cells of the neurovascular unit such as pericytes, astrocytes or neurons. We recommend using additional labeling with other markers such as DAPI to label cell nuclei when using markers for any other cell or protein of interest (Extended Data Fig. 7).
Hierarchical 3D imaging of the postnatal and adult mouse brain vasculature: global vascular network morphometry and local vascular network topology
Perfused blood vessels were hierarchically imaged by SRµCT and subsequently computationally analyzed and quantified by an image analysis pipeline (Figs. 4m–p, 5a–d and 6a–d). Various vessel parameters (see above) of PU4ii resin-perfused vessels, as well as the extravascular distance, can be quantified using computational analysis consisting of either global vascular network morphometry analysis or local topological vascular network analysis39,140,159,163,164,165.
Global vascular network morphometry
Global vascular network morphometry allows quantification of properties of each dataset without considering the form and shape properties of individual vascular structures, generating an abstract quantification of the data (Figs. 5 and 7–9). The typical results we present here demonstrated that, in the P10 WT animals, between 1.67% in the cortex, 1.93% in the superior colliculus and 2.14% in the hippocampus of the total volume of the postnatal mouse brain tissue was occupied by perfused vessel structures, and this percentage was between 2.58% in the cortex, 2.81% in the superior colliculus and 2.97% in the hippocampus of the total volume of the adult mouse brain tissue. These data are in accordance with previous findings by our28,39,103 and other groups22,25. In the P60 WT mouse brain, the vascular volume fraction was significantly increased as compared with P10 WT animals in the cortex (P60 WT versus P10 WT (mean ± SD; applies also for all subsequent values): 2.58 ± 0.77% versus 1.67 ± 0.45%, P = 0.0032), hippocampus (P60 WT versus P10 WT: 2.97 ± 0.70% versus 2.14 ± 0.22%, P = 0.019) and superior colliculus (P60 WT versus P10 WT: 2.81 ± 1.15% versus 1.93 ± 0.26%, P = 0.048) (Fig. 5h–j). The increase of the vascular volume fraction of perfused vessels between the two developmental stages P10 and P60 was between 39% and 54% (+54% in cortex, +39% in hippocampus, +45% in superior colliculus), in line with what we observed previously using stereological methods38. This demonstrates that postnatal and adult mouse brain vasculatures can be compared using this protocol at the network level.
To illustrate the sensitivity and broad applicability of the proposed method, we used a knockout mouse model of the well-known antiangiogenic molecule Nogo-A (Nogo-A−/−)28,38,39,169,170,171, at both the developing P10 as well as at the P60 stages. Interestingly, the increase in vascular volume fraction in the P10 Nogo-A−/− as compared with the P10 WT was similar to the comparison between the P60 WT and the P10 WT mice in all brain regions, whereas these effects were no longer visible between P60 Nogo-A−/− and P60 WT mice (Supplementary Fig. 5), indicating (i) a hypervascularization/accelerated vascularization in the Nogo-A−/− mice during postnatal brain development, and (ii) that these effects are compensated/strongly attenuated in the adulthood38, in agreement with what we previously showed38,39. Given the redundancy of mechanisms acting on the vascular system, effects of genetic deletion of angiogenic/antiangiogenic molecules on angiogenesis and the vasculature network that are observed during embryonic or postnatal development are often compensated at the adult stage, and this is also the case for the Nogo-A−/− mouse38,39 (Supplementary Figs. 5–8).
Local vascular network topology
In contrast to global vascular morphometry, local vascular network topology analyzes each individual vascular structure and bifurcation point separately, thereby allowing quantification of the form and shape of the vascular network (Fig. 6a–d). The vessel-specific properties of each individual vascular structure (= each vessel segment) and each bifurcation point within the dataset thereby affects the quantification and analysis of the 3D vascular network, and local topological analysis of the 3D vascular network allows the characterization of individual per-vessel parameters such as segment density, diameter, length, tortuosity, and volume, and branch point density and degree (Figs. 6–10). Importantly, local topological analysis allows differentiation between capillary (<7 µm in diameter) and noncapillary vessels (≥7 µm in diameter) of the highly interwoven hierarchical 3D vessel/capillary network (Figs. 6 and 7)21,172.
For quantitative assessment of angiogenic features such as branch point density and degree; segment density, diameter, length, tortuosity and volume; and vascular branch points, we therefore referred to local topological vessel analysis provided by a computational image analysis pipeline (Figs. 6–10, Procedure Steps 24–48). Examples of the quantification of the aforementioned vessel parameters in different regions of the postnatal and adult mouse brains are shown in Figs. 5h–j, 7a–c,f–h, 8c,f–h, 9c–n and 10a–h and Extended Data Figs. 5b,e–g and 6b,e–g. The absolute values for the vascular volume fraction are in accordance with previous findings by our group using immunofluorescent staining and stereological analysis28,38 and with studies using similar99,103 or different 3D methods22,173.
Distinction of capillaries and noncapillaries
Based on the distinction between capillaries and noncapillaries, there is a very important significant increase (P < 0.001) in the density of capillary blood vessels (as revealed by the vascular volume fraction) between the developing postnatal and the mature adult mouse brain vascular network (Fig. 5h and Supplementary Figs. 2–5), whereas the noncapillary blood vessels showed only a nonsignificant increase between these two time points, in various brain regions (Fig. 7g and Supplementary Figs. 2–5). Local vascular network topology revealed that the 3D vascular volume fraction in the P60 WT cortex was increased by 54% as compared with the P10 WT cortex (Fig. 7f), and by 39% and 45% in the hippocampus (Supplementary Fig. 3c) and superior colliculus (Supplementary Fig. 4c), respectively, and these values were all similar to the fold change calculated by global vascular morphometry (Fig. 5h,i,j). Deeper insight into the 3D vascular network architecture is provided by addressing specific properties of each individual vessel structure, for instance by addressing the vessel diameter (Figs. 5–7).
Accordingly, of the total vessel tissue volume (= vascular volume fraction) of 1.67% in the P10 cortex, 1.24% (74.2% of the total vessel volume) was occupied by noncapillary vessels (Fig. 7g) and 0.43% (25.8% of the total vessel volume) by capillaries (Fig. 7h). Values for the hippocampus and superior colliculus can be found in Supplementary Figs. 3 and 4.
Increase in capillary density from the postnatal to the adult stage
Adult (P60) WT animals showed an increased absolute frequency of capillaries as compared with P10 WT animals throughout the different brain regions, whereas the absolute frequency of bigger vessels was very similar between the two developmental stages (Fig. 7f–h). Computational 3D analysis revealed that the increased vascular volume fraction in the cortices, hippocampi and superior colliculi of P60 WT animals was mainly due to a significant increase in the vascular volume fraction at the capillary level (cortex: P60 WT versus P10 WT: 1.16 ± 0.37% versus 0.43 ± 0.22, P = \(5.8 \times 10^{ - 6}\)) whereas a slight increase of the vascular volume fraction of larger vessels (cortex: noncapillaries, P60 WT versus P10 WT: 1.42 ± 0.56% versus 1.24 ± 0.37%, P = 0.41) did not reach statistical significance (Fig. 7f–h; see Supplementary Figs. 3 and 4 for hippocampus and superior colliculus). Quantitatively, the vascular volume fraction was increased by 169% for capillaries and by 14% for noncapillaries in the cortex (Fig. 7f–h; see Supplementary Figs. 3 and 4 for hippocampus and superior colliculus). Taken together, these results indicate that the increased vascular volume fraction observed from the postnatal to the adult stage is mainly due to additionally formed capillaries while the number of larger noncapillaries remains relatively unaffected.
Increase of vascular branching of capillary microvessels from the postnatal to the adult stage
The observed increased vascular volume fraction may be due to increased vessel branching and concomitant newly formed vessel segments or caused by an increased vessel diameter, by an increased vessel length, or a combination of these factors. To illustrate that this protocol allows the origin of the additionally formed vessels to be addressed, the number of vascular branch points in the cortices of P10 WT and P60 WT animals referring to local topological analysis was determined (Fig. 8). Vessel branch points are characterized by their degree103, defined as the number of vessels adjacent to a vessel branch point, usually ranging from degree 3 to degree 5 (Fig. 8b). Accordingly, vascular branch points were defined as points where three (branch point degree 3), four (branch point degree 4) or five (branch point degree 5) vessel segments converge (Fig. 8b)28,39. For discrimination between ‘capillary branch points’ and ‘noncapillary branch points’, the branch point diameter was considered to be that of the thickest vessel adjacent to a given branch point (Fig. 8b). Accordingly, if the thickest vessel adjacent to a given branch point was <7 μm, the branch point was classified as a ‘capillary branch point’, whereas if the thickest vessel adjacent to a given branch point was ≥7 μm, the branch point was classified as a ‘noncapillary branch point’. Adult (P60) WT animals showed a pronounced increase of branch point density (of all degrees) at the capillary level (P60 WT versus P10 WT: 13,994.1 ± 7,395.6mm−3 versus 1,929.1 ± 819.1 mm−3, P = \(5.3 \times 10^{ - 5}\)) and a slighter increase of the number of branch points for noncapillaries (P60 WT versus P10 WT: 2,959.9 ± 1,559.7 mm−3 versus 1,993.3 ± 683.7 mm−3, P = 0.10) as compared with the P10 WT mice in the cortex, as well as in the hippocampus and superior colliculus (Fig. 8c,f–h and Extended Data Fig. 5b,e–g). Computational reconstructions revealed an increased number of vessel branch points in cortices, hippocampus and superior colliculi of P60 WT animals as compared with P10 WT mice (Fig. 8d,e and Extended Data Figs. 5c,d and 6c,d). Quantitatively, the density of branch points for all vessels in the cortex was significantly—by 332%, 230% and 435%, respectively—increased (cortex: P60 WT versus P10 WT: 16,954.0 ± 8,329.6 mm−3 versus 3,922.4 ± 1,376.9 mm−3, P = \(9.6 \times 10^{ - 5}\)) in adult (P60) WT as compared with postnatal (P10) WT animals (Fig. 8d,f–h; see Extended Data Figs. 5e–g and 6e–g for hippocampus and superior colliculus), predominantly due to a more than sevenfold increased (+625%) number of branch points in the capillary bed in the cortex (cortex: P60 WT versus P10 WT: 13,994.1 ± 7,395.6mm−3 versus 1,929.1 ± 819.1mm−3, P =\(5.3 \times 10^{ - 5}\)) (Fig. 8d,f–h; see Extended Data Figs. 5e–g and 6e–g for hippocampus and superior colliculus). Interestingly, in the adult animals, this was mainly due to an increased number of vessel branch points of degree 4 for all vessel sizes (cortex: +839% for all vessels, P60 WT versus P10 WT: 1,769.4 ± 1,047.0 mm−3 versus 188.4 ± 62.2 mm−3, P = 0.00015, +182% for noncapillaries, and +1,345% for capillaries) (Supplementary Fig. 9h–j; see also Supplementary Figs. 9h–j and 10h–j for hippocampus and superior colliculus), while showing a highly significant increase also for vessel branch points of degree 3 for all vessel sizes (cortex: +304% for all vessels, +42% for noncapillaries and +578% for capillaries) (Supplementary Fig. 9e–g; see also Supplementary Figs. 9e–g and 10e–g for hippocampus and superior colliculus), indicating both formation of new vessel branch points (degree 3) and further branching of existing degree 3 branch points (degree 4). Vessel branch points of degree 5 were not detected in either study group (Supplementary Figs. 7–9). Moreover, the mean vessel branch point degree for the different vessel sizes showed an overall increase between the two developmental stages (cortex: +2% for all vessels, +1% for noncapillaries and +2% for capillaries; hippocampus: +2% for all vessels, +2% for noncapillaries and +2% for capillaries; superior colliculus: +3% for all vessels, +2% for noncapillaries and +3% for capillaries) (Supplementary Figs. 7–9), indicating that branch point degree increases during physiological vascular brain development. Together, these data reveal that the enhanced vascular volume density observed from the postnatal to the adult stage can be mainly explained by an increased number of vascular branch points mainly at the level of capillaries.
Interestingly, we observed a significant increase (+39%** for all vessels) for the vascular volume fraction (again mainly at the level of capillaries, +71%**) when comparing data on the cortex of P10 Nogo-A−/− with P10 WT mice, and these effects were reminiscent of the comparison between P10 WT and P60 WT mice mentioned above (Supplementary Fig. 5). At the adult stage, however, the effect of genetic deletion of Nogo-A did not show significant differences in the brain vasculature between the WT and Nogo-A−/− groups, suggesting that the inhibitory effect of Nogo-A on sprouting angiogenesis is compensated by the redundancy of mechanisms acting on the vascular system during these later stages of cerebrovascular development38.
Both the effects of the developmental stage (e.g., adult P60 versus developing P10) as well as the effect of deletion of the Nogo-A gene at the postnatal (but not adult) stage were consistent throughout various brain regions (Figs. 5–12, Extended Data Figs. 5, 6, Supplementary Figs. 2–16).
Morphological changes (segment density, diameter, length, tortuosity and extravascular distance) in the 3D vascular network from the postnatal to the adult stage
In addition to these measured values, this protocol allows the quantification of parameters such as the average segment density (Fig. 9d–f and Supplementary Fig. 7a–c), diameter (Fig. 9g–j and Supplementary Fig. 7d–f), length (Fig. 9k–n and Supplementary Fig. 7g–i), tortuosity (Fig. 10a–d and Supplementary Fig. 7j–l) and volume (Fig. 10e–h and Supplementary Fig. 7m–o), and the average maximal perfusion distance (Fig. 11 and Supplementary Fig. 8). Given the high angiogenic activity in the postnatal rodent brain28,37, the precise values of the aforementioned parameters will obviously depend on the exact developmental stage of the mouse.
The functional consequences of the increased vascular volume fraction in the P60 (and the Nogo-A knockout animals), can be addressed by determining the extravascular distance—defined as the shortest distance of any given extravascular voxel in the tissue to the nearest vessel structure (Fig. 11 and Supplementary Fig. 8)—which measures the diffusion distance. The extravascular distance in P10 WT cortices was 31.9 µm on average and, thus, similar to our previous work39, whereas the P10 WT hippocampus and P10 WT superior colliculus showed lower average values of 18.4 µm and 19.2 µm, respectively (Fig. 11i–k and Supplementary Fig. 8). Overall, the extravascular distance was markedly reduced in P60 WT mice as compared with P10 WT cortices, hippocampi and superior colliculi (Fig. 11i–k and Supplementary Fig. 8). Accordingly, histogram distribution analysis revealed a marked shift towards smaller extravascular distances in the P60 animals (Fig. 11i–k and Supplementary Fig. 8), which is a consequence of the higher vascular volume fractions observed in the P60 as compared with the P10 WT animals. Quantitative analysis showed a highly significant reduction of the extravascular distance in P60 WT animals to almost half (−45%) as compared with P10 WT mice in the cortex, (P60 WT versus P10 WT: 17.42 ± 6.52 μm versus 31.94 ± 10.63 μm, P =\(1.6 \times 10^{ - 5}\), Fig. 11i), with similar findings in the hippocampus (Fig. 11j) and superior colliculus (Fig. 11k). Although we are aware that the extravascular distance reflects the distance from any given extravascular voxel within the tissue to the nearest vascular structure, this absolute value of 17.42 μm (P60 WT) is comparable to a study by Tsai et al.22, which, using automated histological imaging and analysis tools simultaneously mapping the locations of all neuronal and nonneuronal nuclei and centerlines of the entire vasculature within slabs of mice adult mice neocortex, found that each individual neuron is no more than 15 μm away from the closest microcapillary, thereby ensuring proper oxygen delivery22.
In line with the findings presented above, the extravascular distance was also reduced in the P10 Nogo-A−/− animals as compared with the P10 WT animals in the different regions, and the effects observed were similar to the comparison between P60 WT mice and P10 WT mice (Supplementary Fig. 8).
Together, these data suggest that, as the 3D vascular network undergoes significant changes (e.g., increased vascular volume fraction and increased vessel branching) from the developing P10 to the P60 mature stage, these result in a markedly lowered vessel spacing and diffusion distance throughout various brain regions.
Vessel directionality as a novel parameter in the postnatal and adult mouse brain
Further, this protocol illustrates the ability to derive the parameter vessel directionality by determining for every vessel segment its main angle to the x-, y- and z-axis in the 3D space within the selected ROIs (Fig. 12 and Supplementary Figs. 12–16) and observed different patterns of vessel directionality throughout the different brain regions examined. Most notably, in the cortex, the superficial PNVP extended in the x- and y-directions, whereas the INVP exhibited a radial sprouting pattern into the brain parenchyma along the z-axis, perpendicular to the PNVP (Fig. 12b,c), in accordance with literature28,33,126. The distinction between noncapillaries and capillaries revealed that the PNVP and INVP were mainly composed of noncapillary vessels (Supplementary Figs. 12–15), and that noncapillaries also formed the base of vessel sprouts emanating from the INVP (and to a lesser extent from the PNVP) mainly in the x- and y-directions (Supplementary Figs. 12–15). Capillaries, on the other hand, vascularized the cortical CNS parenchyma via sprouting from noncapillaries and extended equally in all directions (x, y, z), thereby ensuring adequate microvascularization and perfusion of the brain (Supplementary Figs. 12–15). At the adult stage, these patterns of vessel directionality observed at the postnatal developing stage were confirmed and further strengthened (Fig. 12c and Supplementary Figs. 12–15). Region-dependent variations and region-specific properties in vessel directionality were observed in the hippocampus and superior colliculus (Supplementary Fig. 12).
Quantitative analysis of the relative angles of the vascular segments emanating from a certain branch point revealed that the capillaries in the cortex, hippocampus and superior colliculus derived at angles between around 75° and 150° at both the developing P10 and mature P60 stages (Fig. 12d–f and Supplementary Fig. 12c–e,h–j). The noncapillaries, however, showed two main angles of orientation, namely ~90° and at ~170° in three brain regions examined (Fig. 12d–f and Supplementary Fig. 12c–e,h–j). Interestingly, these two main angles of orientation were more pronounced at the adult P60 as compared with the P10 stage (Fig. 12d–f and Supplementary Fig. 12c–e,h–j), indicating some sort of preferred vessel orientation of the larger vessels (noncapillaries) that is established throughout vascular brain development. Strikingly, these results were again very similar in the three brain regions, suggesting a more general underlying concept of vessel directionality/orientation. Even though we do not fully understand these data at present, one might think of these preferred orientations of noncapillaries as predefined vascular ‘routes’ into specific brain structures that then terminate into the capillary beds, which are more randomly orientated. Comparison of the quantification of individual vascular parameters obtained by scanning the entire mouse brain in high resolution with that of the two-step ROI-based approach during which the ROIs first have to be placed on a lower-resolution overview μCT scan with subsequent high-resolution SRμCT scanning of selected ROIs revealed very similar results (Fig. 13 and Supplementary Figs. 17–21).
The exact values might vary in different experimental settings and thus correlate with the angiogenic activity of the specificity of investigated tissue76. Nevertheless, we feel that the selected parameters, obtained by our improved protocol, might be helpful readouts for better understanding angiogenesis in various biological settings.
A powerful novel tool to image and analyze the postnatal and adult mouse brain vasculature
Concluding, 3D computational reconstructions of corrosion casts are a powerful tool to investigate any biologically relevant question regarding angiogenesis (genetic mechanisms in physiology and pathophysiology as well as the effect of drugs) because they allow quantification of the morphological features of entire vascular networks. This protocol of vascular corrosion casting techniques combined with SEM, with or without SRµCT imaging, and computational network analysis, can be used to demonstrate quantitatively the morphological changes occurring in the mouse brain between birth and adult stage of vascular brain development. We anticipate that, following appropriate validation, this method could be used to visualize and analyze 3D vascular networks in additional mouse organs.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data Availability
The raw data underlying all graphical figures are provided in individual Excel (comma-separated value (‘.csv’)) files via Figshare (https://doi.org/10.6084/m9.figshare.13483119). For each panel of every figure, a .csv file is provided with the linked figure noted within both the file and the file name.
References
Carmeliet, P. & Jain, R. K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 (2011).
Potente, M., Gerhardt, H. & Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887 (2011).
Wälchli, T. et al. Wiring the vascular network with neural cues: a CNS perspective. Neuron 87, 271–296 (2015).
Jain, R. K. & Carmeliet, P. SnapShot: tumor angiogenesis. Cell 149, 1408–1408.e1401 (2012).
Herbert, S. P. & Stainier, D. Y. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol. 12, 551–564 (2011).
Quaegebeur, A., Lange, C. & Carmeliet, P. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron 71, 406–424 (2011).
Burri, P. H., Hlushchuk, R. & Djonov, V. Intussusceptive angiogenesis: Its emergence, its characteristics, and its significance. Dev. Dyn. 231, 474–488 (2004).
Makanya, A. N., Hlushchuk, R. & Djonov, V. G. Intussusceptive angiogenesis and its role in vascular morphogenesis, patterning, and remodeling. Angiogenesis 12, 113–123 (2009).
Garcia-Gomez, P. & Valiente, M. Vascular co-option in brain metastasis. Angiogenesis https://doi.org/10.1007/s10456-019-09693-x (2019).
Kuczynski, E. A., Vermeulen, P. B., Pezzella, F., Kerbel, R. S. & Reynolds, A. R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 16, 469–493 (2019).
Angara, K., Borin, T. F. & Arbab, A. S. Vascular mimicry: a novel neovascularization mechanism driving anti-angiogenic therapy (AAT) resistance in glioblastoma. Transl. Oncol. 10, 650–660 (2017).
Fernández-Cortés, M., Delgado-Bellido, D. & Oliver, F. J. Vasculogenic mimicry: become an endothelial cell “but not so much”. Front. Oncol. 9, 803–803 (2019).
Ricci-Vitiani, L. et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 468, 824–828 (2010).
Wang, R. et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 468, 829–833 (2010).
Cheng, L. et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 153, 139–152 (2013).
Zhou, W. et al. Targeting glioma stem cell-derived pericytes disrupts the blood-tumor barrier and improves chemotherapeutic efficacy. Cell Stem Cell 21, 591–603.e594 (2017).
Coelho-Santos, V. & Shih, A. Y. Postnatal development of cerebrovascular structure and the neurogliovascular unit. Wiley Interdiscip. Rev. Dev. Biol. 9, e363–e363 (2020).
Mink, J. W., Blumenschine, R. J. & Adams, D. B. Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 241, R203–R212 (1981).
Begley, D. J. & Brightman, M. W. Structural and functional aspects of the blood-brain barrier. Prog. Drug Res. 61, 39–78 (2003).
Zlokovic, B. V. & Apuzzo, M. L. Strategies to circumvent vascular barriers of the central nervous system. Neurosurgery 43, 877–878 (1998).
Cassot, F., Lauwers, F., Lorthois, S., Puwanarajah, P. & Duvernoy, H. Scaling laws for branching vessels of human cerebral cortex. Microcirculation 16, 331–344 (2009).
Tsai, P. S. et al. Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels. J. Neurosci. 29, 14553–14570 (2009).
Blinder, P. et al. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat. Neurosci. 16, 889–897 (2013).
Shih, A. Y. et al. Robust and fragile aspects of cortical blood flow in relation to the underlying angioarchitecture. Microcirculation 22, 204–218 (2015).
Harb, R., Whiteus, C., Freitas, C. & Grutzendler, J. In vivo imaging of cerebral microvascular plasticity from birth to death. J. Cereb. Blood Flow. Metab. 33, 146–156 (2013).
Iadecola, C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96, 17–42 (2017).
Vallon, M., Chang, J., Zhang, H. & Kuo, C. J. Developmental and pathological angiogenesis in the central nervous system. Cell. Mol. Life Sci. 71, 3489–3506 (2014).
Wälchli, T. et al. Quantitative assessment of angiogenesis, perfused blood vessels and endothelial tip cells in the postnatal mouse brain. Nat. Protoc. 10, 53–74 (2015).
Weller, R. O., Sharp, M. M., Christodoulides, M., Carare, R. O. & Møllgård, K. The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS. Acta Neuropathol. 135, 363–385 (2018).
Hogan, K. A., Ambler, C. A., Chapman, D. L. & Bautch, V. L. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development 131, 1503–1513 (2004).
Paredes, I., Himmels, P. & Ruiz de Almodovar, C. Neurovascular communication during CNS development. Dev. Cell 45, 10–32 (2018).
Mancuso, M. R., Kuhnert, F. & Kuo, C. J. Developmental angiogenesis of the central nervous system. Lymphat. Res. Biol. 6, 173–180 (2008).
Fantin, A., Vieira, J. M., Plein, A., Maden, C. H. & Ruhrberg, C. The embryonic mouse hindbrain as a qualitative and quantitative model for studying the molecular and cellular mechanisms of angiogenesis. Nat. Protoc. 8, 418–429 (2013).
Vasudevan, A., Long, J. E., Crandall, J. E., Rubenstein, J. L. & Bhide, P. G. Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat. Neurosci. 11, 429–439 (2008).
Fantin, A. et al. NRP1 acts cell autonomously in endothelium to promote tip cell function during sprouting angiogenesis. Blood 121, 2352–2362 (2013).
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010).
Zeller, K., Vogel, J. & Kuschinsky, W. Postnatal distribution of Glut1 glucose transporter and relative capillary density in blood-brain barrier structures and circumventricular organs during development. Brain Res. Dev. Brain Res. 91, 200–208 (1996).
Wälchli, T. et al. Nogo-A is a negative regulator of CNS angiogenesis. Proc. Natl Acad. Sci. USA 110, E1943–E1952 (2013).
Wälchli, T. et al. Nogo-A regulates vascular network architecture in the postnatal brain. J. Cereb. Blood Flow. Metab. 37, 614–631 (2017).
Whiteus, C., Freitas, C. & Grutzendler, J. Perturbed neural activity disrupts cerebral angiogenesis during a postnatal critical period. Nature 505, 407–411 (2014).
Sweeney, M. D., Ayyadurai, S. & Zlokovic, B. V. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 19, 771–783 (2016).
Muoio, V., Persson, P. B. & Sendeski, M. M. The neurovascular unit—concept review. Acta Physiol. 210, 790–798 (2014).
Eichmann, A. & Thomas, J. L. Molecular parallels between neural and vascular development. Cold Spring Harb. Perspect. Med. 3, a006551 (2013).
Yu, P. et al. FGF-dependent metabolic control of vascular development. Nature 545, 224–228 (2017).
Li, X., Sun, X. & Carmeliet, P. Hallmarks of endothelial cell metabolism in health and disease. Cell Metab. 30, 414–433 (2019).
Andrae, J., Gallini, R. & Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 22, 1276–1312 (2008).
Jih, Y. J. et al. Distinct regulation of genes by bFGF and VEGF-A in endothelial cells. Angiogenesis 4, 313–321 (2001).
Dong, J. et al. Glioma stem cells involved in tumor tissue remodeling in a xenograft model. J. Neurosurg. 113, 249–260 (2010).
Lawler, J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J. Cell Mol. Med. 6, 1–12 (2002).
Lawler, P. R. & Lawler, J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb. Perspect. Med. 2, a006627 (2012).
O’Reilly, M. S. et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88, 277–285 (1997).
Dhanabal, M. et al. Angioarrestin: an antiangiogenic protein with tumor-inhibiting properties. Cancer Res. 62, 3834–3841 (2002).
Lu, X. et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature 432, 179–186 (2004).
Sakurai, A., Doçi, C. L. & Gutkind, J. S. Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer. Cell Res. 22, 23–32 (2012).
Eilken, H. M. & Adams, R. H. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell Biol. 22, 617–625 (2010).
Strilic, B. et al. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev. Cell 17, 505–515 (2009).
Pitulescu, M. E. et al. Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nat. Cell Biol. 19, 915–927 (2017).
Lammert, E. & Axnick, J. Vascular lumen formation. Cold Spring Harb. Perspect. Med. 2, a006619 (2012).
Tung, J. J., Tattersall, I. W. & Kitajewski, J. Tips, stalks, tubes: Notch-mediated cell fate determination and mechanisms of tubulogenesis during angiogenesis. Cold Spring Harb. Perspect. Med. 2, a006601 (2012).
Wacker, A. & Gerhardt, H. Endothelial development taking shape. Curr. Opin. Cell Biol. 23, 676–685 (2011).
Mazzone, M. et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136, 839–851 (2009).
Dela Paz, N. G., Melchior, B. & Frangos, J. A. Shear stress induces Gα(q/11) activation independently of G protein-coupled receptor activation in endothelial cells. Am. J. Physiol. Cell Physiol. 312, C428–C437 (2017).
Takuwa, N. et al. Tumor-suppressive sphingosine-1-phosphate receptor-2 counteracting tumor-promoting sphingosine-1-phosphate receptor-1 and sphingosine kinase 1—Jekyll Hidden behind Hyde. Am. J. Cancer Res. 1, 460–481 (2011).
Mendelson, K., Evans, T. & Hla, T. Sphingosine 1-phosphate signalling. Development 141, 5–9 (2014).
Gaengel, K. et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev. Cell 23, 587–599 (2012).
Jin, Z. G. et al. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ. Res. 93, 354–363 (2003).
Nigro, P., Abe, J. & Berk, B. C. Flow shear stress and atherosclerosis: a matter of site specificity. Antioxid. Redox Signal 15, 1405–1414 (2011).
Baeyens, N. et al. Defective fluid shear stress mechanotransduction mediates hereditary hemorrhagic telangiectasia. J. Cell Biol. 214, 807–816 (2016).
Franco, C. A. et al. Non-canonical Wnt signalling modulates the endothelial shear stress flow sensor in vascular remodelling. eLife 5, e07727–e07727 (2016).
Pries, A. R. & Secomb, T. W. Modeling structural adaptation of microcirculation. Microcirculation 15, 753–764 (2008).
Pries, A. R., Secomb, T. W. & Gaehtgens, P. Structural adaptation and stability of microvascular networks: theory and simulations. Am. J. Physiol. 275, H349–H360 (1998).
Hjelmeland, A. B., Lathia, J. D., Sathornsumetee, S. & Rich, J. N. Twisted tango: brain tumor neurovascular interactions. Nat. Neurosci. 14, 1375–1381 (2011).
Carmeliet, P., De Smet, F., Loges, S. & Mazzone, M. Branching morphogenesis and antiangiogenesis candidates: tip cells lead the way. Nat. Rev. Clin. Oncol. 6, 315–326 (2009).
Frahm, K. A., Nash, C. P. & Tobet, S. A. Endocan immunoreactivity in the mouse brain: method for identifying nonfunctional blood vessels. J. Immunol. Methods 398–399, 27–32 (2013).
Geudens, I. & Gerhardt, H. Coordinating cell behaviour during blood vessel formation. Development 138, 4569–4583 (2011).
Blanco, R. & Gerhardt, H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med. 3, a006569 (2013).
Phng, L. K. & Gerhardt, H. Angiogenesis: a team effort coordinated by notch. Dev. Cell 16, 196–208 (2009).
Noguera-Troise, I. et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444, 1032–1037 (2006).
Thurston, G., Noguera-Troise, I. & Yancopoulos, G. D. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat. Rev. Cancer 7, 327–331 (2007).
Yan, M. et al. Chronic DLL4 blockade induces vascular neoplasms. Nature 463, E6–E7 (2010).
Lobov, I. B. et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc. Natl Acad. Sci. USA. 104, 3219–3224 (2007).
Ridgway, J. et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 444, 1083–1087 (2006).
Gest, T. R. & Carron, M. A. Embryonic origin of the caudal mesenteric artery in the mouse. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 271, 192–201 (2003).
Adamson, S. L. et al. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev. Biol. 250, 358–373 (2002).
Iwagaki, T., Suzuki, T. & Nakashima, T. Development and regression of cochlear blood vessels in fetal and newborn mice. Hear. Res. 145, 75–81 (2000).
Carraro, M. & Harrison, R. V. Degeneration of stria vascularis in age-related hearing loss; a corrosion cast study in a mouse model. Acta Otolaryngol. 136, 385–390 (2016).
Carraro, M., Park, A. H. & Harrison, R. V. Partial corrosion casting to assess cochlear vasculature in mouse models of presbycusis and CMV infection. Hear. Res. 332, 95–103 (2016).
Carraro, M. et al. Cytomegalovirus (CMV) infection causes degeneration of cochlear vasculature and hearing loss in a mouse model. J. Assoc. Res. Otolaryngol. 18, 263–273 (2017).
Hossler, F. E., Lametschwandtner, A., Kao, R. & Finsterbusch, F. Microvascular architecture of mouse urinary bladder described with vascular corrosion casting, light microscopy, SEM, and TEM. Microsc. Microanal. 19, 1428–1435 (2013).
Peão, M. N., Aguas, A. P., de Sá, C. M. & Grande, N. R. Neoformation of blood vessels in association with rat lung fibrosis induced by bleomycin. Anat. Rec. 238, 57–67 (1994).
Peáo, M. N., Aguas, A. P., de Sá, C. M. & Grande, N. R. Identification of vascular sphincters at the junction between alveolar capillaries and pulmonary venules of the mouse lung. Anat. Rec. 241, 383–390 (1995).
Ackermann, M. et al. Effects of nintedanib on the microvascular architecture in a lung fibrosis model. Angiogenesis 20, 359–372 (2017).
Gibney, B. C. et al. Structural and functional evidence for the scaffolding effect of alveolar blood vessels. Exp. Lung Res. 43, 337–346 (2017).
Dahl, E. The fine structure of intracerebral vessels. Z. Zellforsch. Mikrosk. Anat. 145, 577–586 (1973).
Levesque, M. J., Cornhill, J. F. & Nerem, R. M. Vascular casting. A new method for the study of the arterial endothelium. Atherosclerosis 34, 457–467 (1979).
Duvernoy, H. M., Delon, S. & Vannson, J. L. Cortical blood vessels of the human brain. Brain Res. Bull. 7, 519–579 (1981).
Reina-De La Torre, F., Rodriguez-Baeza, A. & Sahuquillo-Barris, J. Morphological characteristics and distribution pattern of the arterial vessels in human cerebral cortex: a scanning electron microscope study. Anat. Rec. 251, 87–96 (1998).
Zagórska-Swiezy, K., Litwin, J. A., Gorczyca, J., Pityński, K. & Miodoński, A. J. Arterial supply and venous drainage of the choroid plexus of the human lateral ventricle in the prenatal period as revealed by vascular corrosion casts and SEM. Folia Morphol. (Warsz.) 67, 209–213 (2008).
Heinzer, S. et al. Hierarchical microimaging for multiscale analysis of large vascular networks. Neuroimage 32, 626–636 (2006).
Krucker, T., Lang, A. & Meyer, E. P. New polyurethane-based material for vascular corrosion casting with improved physical and imaging characteristics. Microsc. Res. Tech. 69, 138–147 (2006).
Sangiorgi, S. et al. Arterial and microvascular supply of cerebral hemispheres in the nude mouse revealed using corrosion casting and scanning electron microscopy. J. Anat. 232, 739–746 (2018).
Quintana, D. D. et al. The cerebral angiome: high resolution MicroCT imaging of the whole brain cerebrovasculature in female and male mice. NeuroImage 202, 116109 (2019).
Heinzer, S. et al. Novel three-dimensional analysis tool for vascular trees indicates complete micro-networks, not single capillaries, as the angiogenic endpoint in mice overexpressing human VEGF(165) in the brain. NeuroImage 39, 1549–1558 (2008).
Krucker, T., Schuler, A., Meyer, E. P., Staufenbiel, M. & Beckmann, N. Magnetic resonance angiography and vascular corrosion casting as tools in biomedical research: application to transgenic mice modeling Alzheimer’s disease. Neurol. Res. 26, 507–516 (2004).
Meyer, E. P., Ulmann-Schuler, A., Staufenbiel, M. & Krucker, T. Altered morphology and 3D architecture of brain vasculature in a mouse model for Alzheimer’s disease. Proc. Natl Acad. Sci. USA. 105, 3587–3592 (2008).
Walker, E. J., Shen, F., Young, W. L. & Su, H. Cerebrovascular casting of the adult mouse for 3D imaging and morphological analysis. J. Vis. Exp. https://doi.org/10.3791/2958 (2011).
Walker, E. J. et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann. Neurol. 69, 954–962 (2011).
Risser, L. et al. From homogeneous to fractal normal and tumorous microvascular networks in the brain. J. Cereb. Blood Flow. Metab. 27, 293–303 (2007).
Sangiorgi, S. et al. Early-stage microvascular alterations of a new model of controlled cortical traumatic brain injury: 3D morphological analysis using scanning electron microscopy and corrosion casting. J. Neurosurg. 118, 763 (2013).
Ohtake, M., Morino, S., Kaidoh, T. & Inoué, T. Three-dimensional structural changes in cerebral microvessels after transient focal cerebral ischemia in rats: scanning electron microscopic study of corrosion casts. Neuropathology 24, 219–227 (2004).
Rodríguez-Baeza, A., Reina-de la Torre, F., Poca, A., Martí, M. & Garnacho, A. Morphological features in human cortical brain microvessels after head injury: a three-dimensional and immunocytochemical study. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 273, 583–593 (2003).
Zhang, F., Wen, Y. & Guo, X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum. Mol. Genet. 23, R40–R46 (2014).
Gerhardt, H. et al. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev. Dyn. 231, 503–509 (2004).
Gerhardt, H. et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163–1177 (2003).
Provost, J. et al. 3-D ultrafast Doppler imaging applied to the noninvasive mapping of blood vessels in vivo. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 62, 1467–1472 (2015).
Demene, C. et al. 4D microvascular imaging based on ultrafast Doppler tomography. NeuroImage 127, 472–483 (2016).
Pathak, A. P., Kim, E., Zhang, J. & Jones, M. V. Three-dimensional imaging of the mouse neurovasculature with magnetic resonance microscopy. PLoS One 6, e22643–e22643 (2011).
Hartung, M. P., Grist, T. M. & François, C. J. Magnetic resonance angiography: current status and future directions. J. Cardiovasc. Magn. Reson. 13, 19–19 (2011).
Kelch, I. D. et al. Organ-wide 3D-imaging and topological analysis of the continuous microvascular network in a murine lymph node. Sci. Rep. 5, 16534 (2015).
Marien, K. M. et al. Development and validation of a histological method to measure microvessel density in whole-slide images of cancer tissue. PLoS One 11, e0161496–e0161496 (2016).
Bukenya, F., Nerissa, C., Serres, S., Pardon, M. C. & Bai, L. An automated method for segmentation and quantification of blood vessels in histology images. Microvasc. Res. 128, 103928 (2020).
Oren, R. et al. Whole organ blood and lymphatic vessels imaging (WOBLI). Sci. Rep. 8, 1412–1412 (2018).
Kennel, P., Teyssedre, L., Colombelli, J. & Plouraboué, F. Toward quantitative three-dimensional microvascular networks segmentation with multiview light-sheet fluorescence microscopy. J. Biomed. Opt. 23, 1–14 (2018).
Di Giovanna, A. P. et al. Tailored sample mounting for light-sheet fluorescence microscopy of clarified specimens by polydimethylsiloxane casting. Front. Neuroanat. 13, 35–35 (2019).
Lugo-Hernandez, E. et al. 3D visualization and quantification of microvessels in the whole ischemic mouse brain using solvent-based clearing and light sheet microscopy. J. Cereb. Blood Flow. Metab. 37, 3355–3367 (2017).
Todorov, M. I. et al. Machine learning analysis of whole mouse brain vasculature. Nat. Methods 17, 442–449 (2020).
Kirst, C. et al. Mapping the fine-scale organization and plasticity of the brain vasculature. Cell 180, 780–795.e725 (2020).
Susaki, E. A. & Ueda, H. R. Whole-body and whole-organ clearing and imaging techniques with single-cell resolution: toward organism-level systems biology in mammals. Cell Chem. Biol. 23, 137–157 (2016).
Zagorchev, L. et al. Micro computed tomography for vascular exploration. J. Angiogenes. Res. 2, 7 (2010).
Blery, P. et al. Vascular imaging with contrast agent in hard and soft tissues using microcomputed-tomography. J. Microsc. 262, 40–49 (2016).
Starosolski, Z. et al. Ultra high-resolution in vivo computed tomography imaging of mouse cerebrovasculature using a long circulating blood pool contrast agent. Sci. Rep. 5, 10178 (2015).
Beckmann, N. et al. Age-dependent cerebrovascular abnormalities and blood flow disturbances in APP23 mice modeling Alzheimer’s disease. J. Neurosci. 23, 8453–8459 (2003).
Gore, A. V., Monzo, K., Cha, Y. R., Pan, W. & Weinstein, B. M. Vascular development in the zebrafish. Cold Spring Harb. Perspect. Med. 2, a006684 (2012).
Dorr, A., Sled, J. G. & Kabani, N. Three-dimensional cerebral vasculature of the CBA mouse brain: a magnetic resonance imaging and micro computed tomography study. NeuroImage 35, 1409–1423 (2007).
Figueiredo, G., Boll, H., Kramer, M., Groden, C. & Brockmann, M. A. In vivo X-ray digital subtraction and CT angiography of the murine cerebrovasculature using an intra-arterial route of contrast injection. Am. J. Neuroradiol. 33, 1702 (2012).
Ben-Zvi, A. et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509, 507–511 (2014).
Vanlandewijck, M. et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475–480 (2018).
Kalucka, J. et al. Single-cell transcriptome atlas of murine endothelial cells. Cell 180, 764–779.e720 (2020).
Goveia, J. et al. An integrated gene expression landscape profiling approach to identify lung tumor endothelial cell heterogeneity and angiogenic candidates. Cancer Cell 37, 21–36.e13 (2020).
Pitulescu, M. E., Schmidt, I., Benedito, R. & Adams, R. H. Inducible gene targeting in the neonatal vasculature and analysis of retinal angiogenesis in mice. Nat. Protoc. 5, 1518–1534 (2010).
Sawamiphak, S., Ritter, M. & Acker-Palmer, A. Preparation of retinal explant cultures to study ex vivo tip endothelial cell responses. Nat. Protoc. 5, 1659–1665 (2010).
Willner, M. et al. Quantitative three-dimensional imaging of lipid, protein, and water contents via X-ray phase-contrast tomography. PLoS One 11, e0151889 (2016).
Moyon, D., Pardanaud, L., Yuan, L., Bréant, C. & Eichmann, A. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development 128, 3359–3370 (2001).
Villa, N. et al. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech. Dev. 108, 161–164 (2001).
Seki, T., Yun, J. & Oh, S. P. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ. Res. 93, 682–689 (2003).
Cui, X. et al. Venous endothelial marker COUP-TFII regulates the distinct pathologic potentials of adult arteries and veins. Sci. Rep. 5, 16193 (2015).
You, L. R. et al. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435, 98–104 (2005).
Wang, H. U., Chen, Z. F. & Anderson, D. J. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93, 741–753 (1998).
Malcontenti-Wilson, C., Chan, L., Nikfarjam, M., Muralidharan, V. & Christophi, C. Vascular targeting agent Oxi4503 inhibits tumor growth in a colorectal liver metastases model. J. Gastroenterol. Hepatol. 23, e96–e104 (2008).
Kaidoh, T., Yasugi, T. & Uehara, Y. The microvasculature of the 7,12-dimethylbenz(a)anthracene (DMBA)-induced rat mammary tumour. Virchows Arch. A 418, 111–117 (1991).
Chakhoyan, A. et al. Validation of vessel size imaging (VSI) in high-grade human gliomas using magnetic resonance imaging, image-guided biopsies, and quantitative immunohistochemistry. Sci. Rep. 9, 2846 (2019).
Burrell, J. S. et al. MRI measurements of vessel calibre in tumour xenografts: Comparison with vascular corrosion casting. Microvasc. Res. 84, 323–329 (2012).
O’Connor, J. P. et al. Quantifying antivascular effects of monoclonal antibodies to vascular endothelial growth factor: insights from imaging. Clin. Cancer Res. 15, 6674–6682 (2009).
Miyake, T., Murakami, T. & Ohtsuka, A. Incomplete vascular casting for a scanning electron microscope study of the microcirculatory patterns in the rat pancreas. Arch. Histol. Cytol. 55, 397–406 (1992).
Christoffersonm, R. H. & Nilsson, B. O. Microvascular corrosion casting with analysis in the scanning electron microscope. Scanning 10, 43–63 (1988).
Paganin, D., Mayo, S. C., Gureyev, T. E., Miller, P. R. & Wilkins, S. W. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Microsc. 206, 33–40 (2002).
Marone, F. & Stampanoni, M. Regridding reconstruction algorithm for real-time tomographic imaging. J. Synchrotron Radiat. 19, 1029–1037 (2012).
Miettinen, A., Oikonomidis, I. V., Bonnin, A. & Stampanoni, M. NRStitcher: non-rigid stitching of terapixel-scale volumetric images. Bioinformatics 35, 5290–5297 (2019).
Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 9, 62–66 (1979).
Huang, L.-K. & Wang, M.-J. J. Image thresholding by minimizing the measures of fuzziness. Pattern Recogn. 28, 41–51 (1995).
Prewitt, J. M. S. & Mendelsohn, M. L. The analysis of cell images. Ann. NY Acad. Sci. 128, 1035–1053 (1966).
Li, C. H. & Tam, P. K. S. An iterative algorithm for minimum cross entropy thresholding. Pattern Recogn. Lett. 19, 771–776 (1998).
Lee, T. C., Kashyap, R. L. & Chu, C. N. Building skeleton models via 3-D medial surface axis thinning algorithms. CVGIP Graph. Models Image Process. 56, 462–478 (1994).
Calvin, R., Maurer, J., Qi, R. & Raghavan, V. A linear time algorithm for computing exact euclidean distance transforms of binary images in arbitrary dimensions. IEEE Trans. Pattern Anal. Mach. Intell. 25, 265–270 (2003).
Suhadolnik, A., Petrišič, J. & Kosel, F. An anchored discrete convolution algorithm for measuring length in digital images. Measurement 42, 1112–1117 (2009).
Tukey, J. W. Exploratory Data Analysis (Addison-Wesley, 1977).
ACSF for LSPS. Cold Spring Harbor Protocols https://doi.org/10.1101/pdb.rec071944 (2012).
Heinzer, S. et al. Computer-based analysis of microvascular alterations in a mouse model for Alzheimer’s disease. Proc. SPIE 6511, 65114 (2007).
Rust, R. et al. Nogo-A targeted therapy promotes vascular repair and functional recovery following stroke. Proc. Natl Acad. Sci. 116, 14270 (2019).
Joly, S., Dejda, A., Rodriguez, L., Sapieha, P. & Pernet, V. Nogo-A inhibits vascular regeneration in ischemic retinopathy. Glia 66, 2079–2093 (2018).
Seiler, S., Di Santo, S. & Widmer, H. R. Nogo-A neutralization improves graft function in a rat model of Parkinson’s disease. Front. Cell Neurosci. 10, 87 (2016).
Weber, B., Keller, A. L., Reichold, J. & Logothetis, N. K. The microvascular system of the striate and extrastriate visual cortex of the macaque. Cereb. Cortex 18, 2318–2330 (2008).
Fenrich, K. K., Zhao, E. Y., Wei, Y., Garg, A. & Rose, P. K. Isolating specific cell and tissue compartments from 3D images for quantitative regional distribution analysis using novel computer algorithms. J. Neurosci. Methods 226, 42–56 (2014).
Acknowledgements
We thank K. De Bock, M. Schwab, M. Ghobrial and J. Zhang for help with the animal perfusions, S. Eichelbaum for help with the computational analysis and 3D visualizations (http://www.nemtics.com), and N. Chu Ji for help with the illustrations. T.W. was supported by the OPO Foundation, the Swiss Cancer Research foundation (KFS-3880-02-2016-R, KFS-4758-02-2019-R), the Stiftung zur Krebsbekämpfung, the Kurt und Senta Herrmann Foundation, Forschungskredit of the University of Zurich, the Zurich Cancer League, the Theodor und Ida Herzog Egli Foundation, the Novartis Foundation for Medical-Biological Research, and the HOPE Foundation. P.C. was supported by long-term structural Methusalem funding by the Flemish Government (14/08), and a European Research Council (ERC) Advanced Research Grant (EU-ERC269073). J.V. was supported by the Swiss National Science Foundation (no. 310000 120321/1). All the animal experiments were conducted in J.V.’s laboratory and were approved by the Veterinary Office of the Canton of Zurich.
Author information
Authors and Affiliations
Contributions
T.W. had the idea for the study, designed the experiments, wrote the manuscript and made the figures. T.W., A.U.S., E.P.M. and C.H. conducted the experiments. T.W., J.V., A.M. and J.B. analyzed the data. T.W. wrote the manuscript, and J.B. helped editing the manuscript and figures. R.W. helped with the animal experiments and gave critical inputs to the manuscript. A.M., A.U.S., T.K., K.D.B., P.C., J.V. and M.S. gave critical inputs to the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
T.K. is an employee of the Novartis Institutes for BioMedical Research, Inc., and E.P.M is co-founder of vasQtec.
Additional information
Peer review information Nature Protocols thanks Hongbin Lu, Guoyuan Yang and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Heinzer, S. et al. Neuroimage 39, 1549–1558 (2008): https://doi.org/10.1016/j.neuroimage.2007.10.054
Meyer, E. P. et al. Proc. Natl Acad. Sci. USA 105, 3587–3592 (2008): https://doi.org/10.1073/pnas.0709788105
Wälchli, T. et al. J. Cereb. Blood Flow Metab. 37, 614–631 (2017): https://doi.org/10.1177/0271678X16675182
Extended data
Extended Data Fig. 1 Intracardial resin perfusion and brain dissection for adult (P60) animals.
The main steps of resin perfusion and brain dissection are shown as photographs for the perfusion and brain dissection of adult (P60) mice. a-c Fixation (a), surgical opening (b,c) and site of needle insertion (c) for intracardial perfusion (via the left ventricle) with 10–20 ml ACSF containing 25,000 U/L Heparin, followed by 4% PFA in PBS, and then by the polymer resin PU4ii (vasQtec, Zürich, Switzerland), all infused at the same rate (8 ml/min and 100-120 mmHg) in an adult (P60) mouse (see also Supplementary Video 1). d Successful perfusion is indicated by a bluish aspect of the animal, which can be easiest observed at the paws (white arrow) or the snout (white arrowhead). e,f Resin cast of a P60 animal after soft tissue maceration, and before (e) and after (f) decalcification of bone structures. f-h The cerebral vasculature is sharply dissected from the extracranial vessels resulting in the isolated brain vascular corrosion cast of the P60 mouse brain (g,h). Scale bars, 10 mm (e-h).
Extended Data Fig. 2 Validation of vascular corrosion casting in postnatal (P10) and adult (P60) mice using light microscopy (LM) and SEM.
a-f Quality control of the vascular corrosion casts of P10 and P60 WT mice by visual inspection by LM (a), and by SEM (b-f). a Light microscopy image of the entire brain vasculature of the P60 WT mice (gold sputtered for SEM). b SEM image of the entire brain vascular corrosion cast illustrating the dense vascular network including blood vessels of all sizes with recognizable vascular anatomy. c-f The entire brain was uniformly filled with resin and the lumen of the vessels imprinted in the casting material with the finest details. e The capillary network in the cortex is well defined and devoid of interruptions. f Note the elongated cellular and nuclear imprints on the large vessel that are also typical for good-quality vascular corrosion casts in adult mice105. Scale bars, 4 mm (a); 4 mm (b); 50 μm (c); 50 μm (d), 100 μm (e), 100 μm (f). a,d-f reproduced with permission from ref. 39. b reproduced with permission from ref. 100.
Extended Data Fig. 3 Global vascular network morphometry: increased vascular volume fraction in various regions of the adult (P60) Nogo-A-/- versus postnatal (P10) Nogo-A-/- mouse brain.
Genetic deletion of Nogo-A leads to increased vascular volume fraction in various regions of the postnatal mouse brain. a-c Computational 3D reconstructions of μCT scans of vascular networks of P10 and P60 Nogo-A-/- mice displayed with color-coded vessel thickness. The increased vessel density in the Nogo-A-/- animals, as compared to the WT animals (Fig. 5) in the cortices (a), hippocampi (b) and superior colliculi (c) is obvious. Color bar indicates vessel radius. The boxed areas are enlarged at right. (n = 11 for P10 Nogo-A-/-; n = 13 for P60 Nogo-A-/- animals; and in average three ROIs per animal and brain region were used). Scale bars: 100 µm (a-c overviews); 50 µm (a-c zooms).
Extended Data Fig. 4 Local vascular network topology: increased vascular volume fraction of the adult (P60) Nogo-A-/- versus postnatal (P10) Nogo-A-/- mouse brain mainly found at the capillary level.
Genetic deletion of Nogo-A leads to increased vascular volume fraction in various regions of the P10 mouse brain. a Computational 3D reconstruction of μCT images of vascular networks of P10 and P60 Nogo-A-/- mice separated for noncapillaries (magenta) and capillaries (green). The increased density of capillaries (green) in the P60 and P10 Nogo-A-/- samples is evident when compared to the WT animals (Fig. 7), noncapillaries (magenta) appear largely unchanged. The boxed areas are enlarged at right (a-c). Scale bars: 100 mm (a-c, overviews); 50 mm (a-c, zooms).
Extended Data Fig. 5 Local vascular network topology: visualization and quantification of vascular branch point diameter and vascular branch point density in the hippocampus of the adult (P60) WT versus the postnatal (P10) WT mouse brain.
The vascular branch point diameter and vascular branch point density are significantly increased in the P60 as compared to the P10 mouse brain hippocampus. a Scheme depicting the definition of vascular branch points (see Fig. 8b for details) b Histogram showing the distribution of branch point diameter plotted against the branch point density in P10 WT and P60 WT animals. P60 WT animals show an increased branch point density as compared to P10 WT (bin width = 0.38 μm; number of bins = 40). Black dashed line marks the separation between capillaries (< 7 μm) and non-capillaries (≥7 μm), as defined in Fig. 6. c,d Computational 3D reconstructions of μCT images of vascular networks in the hippocampi of P10 WT and P60 WT mice with visualizations of the vessel branch points displayed as dots, separated for noncapillaries (magenta) and capillaries (green). The higher density of branch points in the P60 WT hippocampi especially at the capillary level (green) is obvious, a slight increase of branch point density can be observed at the noncapillary level (magenta). The boxed areas are enlarged at right. e–g Quantitative analysis of the branch point density for all vessels (e), noncapillaries (f), and capillaries (g) in P10 WT and P60 WT hippocampi by local morphometry analysis. The significant increase of the branch point density for all vessels in the P60 WT animals (e) was mainly due to a significant increase at the level of capillaries (g) and in part due to a significant increase at the level of noncapillaries (f). In average, n = 2-7 animals were used for the hippocampi and in average three ROIs per animal and brain region were used. All data are shown as mean distributions where the open dot represents the mean. Boxplots indicate the 25% to 75% quartiles of the data. The shaded blue and red areas indicate the SD. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars: 100 µm (c-d overviews); 50 µm (c-d zooms).
Extended Data Fig. 6 Local vascular network topology: visualization and quantification of vascular branch point diameter and vascular branch point density in the superior colliculus of the adult (P60) WT versus the postnatal (P10) WT mouse brain.
The vascular branch point diameter and vascular branch point density are significantly increased in the P60 as compared to the P10 mouse brain superior colliculus. a Scheme depicting the definition of degree 3 and 4 vascular branch point (see Fig. 8b for details). b Histogram showing the distribution of branch point diameter plotted against the branch point density in P10 WT and P60 WT animals. P60 WT animals show an increased branch point density as compared to P10 WT mice mainly at the capillary level (bin width = 0.38; number of bins = 40). Black dashed line marks the separation between capillaries (< 7 μm) and noncapillaries (≥7 μm), as defined in Fig. 6. c,d Computational 3D reconstructions of μCT images of vascular networks of the superior colliculi of P10 WT and P60 WT mice with visualizations of the vessel branch points displayed as dots, separately for noncapillaries (magenta) and capillaries (green). The higher density of branch points in the P60 WT superior colliculi especially at the capillary level (green) is obvious, a slight increase of branch point density can be observed at the noncapillary level (magenta). The boxed areas are enlarged at right. e–g Quantitative analysis of the branch point density for all vessels (e), noncapillaries (f), and capillaries (g) in P10 WT and P60 WT superior colliculi by local morphometry analysis. The significant increase of the branch point density for all vessels in the P60 WT animals (e) was mainly due to a significant increase at the level of capillaries (g) and in part due to a significant increase at the level of noncapillaries (f). In average, n = 2-4 animals were used for the superior colliculus; and in average three ROIs per animal and brain region were used. All data are shown as mean distributions where the open dot represents the mean. Boxplots indicate the 25% to 75% quartiles of the data. The shaded blue and red areas indicate the SD. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars: 100 μm (c,d, overviews); 50 μm (c,d, zooms).
Extended Data Fig. 7 Resin PU4ii vascular corrosion casting combined with immunofluorescence.
Visualization by fluorescence light microscopy of the brain vasculature after the addition of fluorescent pigments to the PU4ii resin solution in the P60 mouse brains with preserved/non-macerated soft tissue (a-f), co-labeled with red fluorescent myelin stainings (g-j). Overviews (a,b,d,e) and coronal cortical sections (c,f) of P60 mouse brains with yellow/green (a,b,c) and UV-blue (d,e,f) fluorescent dyes (vasQtec) added to the PU4ii resin showing the brain vasculature; overviews: dorsal overviews (a,d), ventral overviews (b,e), coronal sections (c,f). The selection of the brain regions analyzed here has been done on coronal sections in the structures selected using the Allen Mouse Brain Atlas. c,f,g-j Fluorescence light microscopy overviews of multiple brain regions in 150 μm coronal sections of a P60 mouse brain perfused with either yellow/green- (c,h-j) or UV-blue (f,g) fluorescent pigments added to the PU4ii resin solution, showing the cortical layers and part of the hippocampus (c,f). Fluorescence light microscopy of coronal sections in which the preserved/non-macerated soft tissue was labeled with a red fluorescent myelin stain (FluoroMyelin Red, Molecular Probes Inc) to visualize the corpus callosum and other highly myelinated brain structures (red). Scale bars, 1 mm (a,b,d,e); 500 μm (c,d,g-j). Excitation/emission of the yellow/green pigments at 450 nm/550 nm, excitation/emission of the UV-blue fluorescent pigment at 375 nm/430 nm.
Supplementary information
Supplementary Information
Supplementary Figs. 1–21.
Supplementary Video 1
Intracardial resin perfusion of a postnatal (P10) wildtype mouse. This video shows the intracardial resin perfusion of a postnatal (P10) wildtype mouse pub. All steps are precisely explained in the Procedure.
Supplementary Video 2
Three-dimensional animation of a reconstructed SRμCT scan of the entire vascular brain corrosion cast of an adult (P60) wildtype mouse. This animation shows a reconstructed SRμCT of an entire brain vascular corrosion cast of an adult (P60) wildtype animal. Five anatomical regions (cortex, hippocampus, superior colliculus, thalamus and cerebellum) were zoomed into with high resolution. Artificial coloring illustrates the vessel radius and the division between capillaries (vessel diameter <7 μm) and noncapillaries (vessel diameter ≥7 μm).
Rights and permissions
About this article
Cite this article
Wälchli, T., Bisschop, J., Miettinen, A. et al. Hierarchical imaging and computational analysis of three-dimensional vascular network architecture in the entire postnatal and adult mouse brain. Nat Protoc 16, 4564–4610 (2021). https://doi.org/10.1038/s41596-021-00587-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00587-1
This article is cited by
-
Shaping the brain vasculature in development and disease in the single-cell era
Nature Reviews Neuroscience (2023)
-
Towards virtual histology with X-ray grating interferometry
Scientific Reports (2023)
-
Measurements of cerebral microvascular blood flow, oxygenation, and morphology in a mouse model of whole-brain irradiation-induced cognitive impairment by two-photon microscopy and optical coherence tomography: evidence for microvascular injury in the cerebral white matter
GeroScience (2023)
-
A multiscale X-ray phase-contrast tomography dataset of a whole human left lung
Scientific Data (2022)
-
Microvascular imaging of the unstained human superior colliculus using synchrotron-radiation phase-contrast microtomography
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.