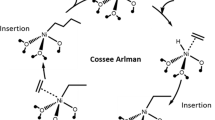
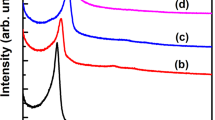
Abstract
Wet-kneaded silica–magnesia is a benchmark catalyst for the one-step ethanol-to-butadiene Lebedev process. Magnesium silicates, formed during wet kneading, have been proposed as the active sites for butadiene formation, and their properties are mainly explained in terms of the ratio of acid and base sites. However, their mechanism of formation and reactivity have not yet been fully established. Here we show that magnesium silicates are formed by the dissolution of Si and Mg subunits from their precursors, initiated by the alkaline pH of the wet-kneading medium, followed by cross-deposition on the precursor surfaces. Using two individual model systems (Mg/SiO2 and Si/MgO), we demonstrate that the location of the magnesium silicates (that is, Mg on SiO2 or Si on MgO) governs not only their chemical nature, but also the configuration of adsorbed ethanol and resulting selectivity. By using an NMR approach together with probe molecules, we demonstrate that acid and basic sites in close atomic proximity (~5 Å) promote butadiene formation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this article are available in the paper and its Supplementary Information or from the corresponding author on reasonable request.
Change history
11 September 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41929-023-01042-y
References
Bruijnincx, P. C. A. & Weckhuysen, B. M. Shale gas revolution: an opportunity for the production of biobased chemicals? Angew. Chem. Int. Ed. 52, 11980–11987 (2013).
Angelici, C., Weckhuysen, B. M. & Bruijnincx, P. C. A. Chemocatalytic conversion of ethanol into butadiene and other bulk chemicals. ChemSusChem 6, 1595–1614 (2013).
Makshina, E. V. et al. Review of old chemistry and new catalytic advances in the on-purpose synthesis of butadiene. Chem. Soc. Rev. 43, 7917–7953 (2014).
Pomalaza, G., Arango Ponton, P., Capron, M. & Dumeignil, F. Ethanol-to-butadiene: the reaction and its catalysts. Catal. Sci. Technol. 10, 4860–4911 (2020).
Bin Samsudin, I., Zhang, H., Jaenicke, S. & Chuah, G. K. Recent advances in catalysts for the conversion of ethanol to butadiene. Chem. Asian J. 15, 4199–4214 (2020).
Jones, M. D. Catalytic transformation of ethanol into 1,3-butadiene. Chem. Cent. J. 8, 53 (2014).
Pomalaza, G., Capron, M., Ordomsky, V. & Dumeignil, F. Recent breakthroughs in the conversion of ethanol to butadiene. Catalysts 6, 203 (2016).
Dussol, D., Cadran, N., Laloue, N., Renaudot, L. & Schweitzer, J.-M. M. New insights of butadiene production from ethanol: elucidation of concurrent reaction pathways and kinetic study. Chem. Eng. J. 391, 123586 (2020).
Li, F. et al. Cooperative CO2-to-ethanol conversion via enriched intermediates at molecule–metal catalyst interfaces. Nat. Catal. 3, 75–82 (2020).
Wang, X. et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 5, 478–486 (2020).
Wang, Y. et al. Direct conversion of CO2 to ethanol boosted by intimacy-sensitive multifunctional catalysts. ACS Catal. 11, 11742–11753 (2021).
Cabello González, G. M. et al. Ethanol conversion into 1,3-butadiene over a mixed Hf-Zn catalyst: effect of reaction conditions and water content in ethanol. Fuel Process. Technol. 193, 263–272 (2019).
Angelici, C., Velthoen, M. E. Z., Weckhuysen, B. M. & Bruijnincx, P. C. A. Influence of acid–base properties on the Lebedev ethanol-to-butadiene process catalyzed by SiO2–MgO materials. Catal. Sci. Technol. 5, 2869–2879 (2015).
Natta, G. & Rigamonti, R. Studio roentgenografico e chimico dei catalizzatori usati per la produzione del butadiene dall’alcool. Chim. Ind. 29, 239–243 (1947).
Kvisle, S., Aguero, A. & Sneeden, R. P. A. Transformation of ethanol into 1,3-butadiene over magnesium oxide/silica catalysts. Appl. Catal. 43, 117–131 (1988).
Janssens, W. et al. Ternary Ag/MgO-SiO2 catalysts for the conversion of ethanol into butadiene. ChemSusChem 8, 994–1008 (2015).
Chung, S.-H. et al. Role of magnesium silicates in wet-kneaded silica–magnesia catalysts for the Lebedev ethanol-to-butadiene process. ACS Catal. 6, 4034–4045 (2016).
Angelici, C., Velthoen, M. E. Z. Z., Weckhuysen, B. M. & Bruijnincx, P. C. A. Effect of preparation method and CuO promotion in the conversion of ethanol into 1,3-butadiene over SiO2–MgO catalysts. ChemSusChem 7, 2505–2515 (2014).
Li, S. et al. Morphological control of inverted MgO-SiO2 composite catalysts for efficient conversion of ethanol to 1,3-butadiene. Appl. Catal. A 577, 1–9 (2019).
Huang, X., Men, Y., Wang, J., An, W. & Wang, Y. Highly active and selective binary MgO–SiO2 catalysts for the production of 1,3-butadiene from ethanol. Catal. Sci. Technol. 7, 168–180 (2017).
Chung, S. et al. The importance of thermal treatment on wet-kneaded silica–magnesia catalyst and Lebedev ethanol-to-butadiene process. Nanomaterials 11, 579 (2021).
Yi, H. et al. A novel method for surface wettability modification of talc through thermal treatment. Appl. Clay Sci. 176, 21–28 (2019).
Lewandowski, M. et al. Investigations into the conversion of ethanol to 1,3-butadiene using MgO:SiO2 supported catalysts. Catal. Commun. 49, 25–28 (2014).
Taifan, W. E., Bučko, T. & Baltrusaitis, J. Catalytic conversion of ethanol to 1,3-butadiene on MgO: a comprehensive mechanism elucidation using DFT calculations. J. Catal. 346, 78–91 (2017).
Taifan, W. E. & Baltrusaitis, J. In situ spectroscopic insights on the molecular structure of the MgO/SiO2 catalytic active sites during ethanol conversion to 1,3-butadiene. J. Phys. Chem. C. 122, 20894–20906 (2018).
Zhang, T., Cheeseman, C. R. & Vandeperre, L. J. Development of low pH cement systems forming magnesium silicate hydrate (M-S-H). Cem. Concr. Res. 41, 439–442 (2011).
Tonelli, M. et al. Structural characterization of magnesium silicate hydrate: towards the design of eco-sustainable cements. Dalton Trans. 45, 3294–3304 (2016).
Nied, D., Enemark-Rasmussen, K., L’Hopital, E., Skibsted, J. & Lothenbach, B. Properties of magnesium silicate hydrates (M-S-H). Cem. Concr. Res. 79, 323–332 (2016).
Cole, W. F. A crystalline hydrated magnesium silicate formed in the breakdown of a concrete sea-wall. Nature 171, 354–355 (1953).
Roosz, C. et al. Crystal structure of magnesium silicate hydrates (M-S-H): the relation with 2:1 Mg–Si phyllosilicates. Cem. Concr. Res. 73, 228–237 (2015).
Sindorf, D. & Maciel, G. Cross-polarization magic-angle-spinning silicon-29 nuclear magnetic resonance study of silica gel using trimethylsilane bonding as a probe of surface geometry and reactivity. J. Phys. Chem. 86, 5208–5219 (1982).
Leonardelli, S., Facchini, L., Fretigny, C., Tougne, P. & Legrand, A. P. Silicon-29 NMR study of silica. J. Am. Chem. Soc. 114, 6412–6418 (1992).
Kobayashi, T., Perras, F. A., Slowing, I. I., Sadow, A. D. & Pruski, M. Dynamic nuclear polarization solid-state NMR in heterogeneous catalysis research. ACS Catal. 5, 7055–7062 (2015).
Magi, M., Lippmaa, E., Samoson, A., Engelhardt, G. & Grimmer, A. R. Solid-state high-resolution silicon-29 chemical shifts in silicates. J. Phys. Chem. 88, 1518–1522 (1984).
Moravetski, V., Hill, J.-R., Eichler, U., Cheetham, A. K. & Sauer, J. 29Si NMR chemical shifts of silicate species: ab initio study of environment and structure effects. J. Am. Chem. Soc. 118, 13015–13020 (1996).
Pustovgar, E. et al. Understanding silicate hydration from quantitative analyses of hydrating tricalcium silicates. Nat. Commun. 7, 10952 (2016).
Szabó, B. et al. MgO−SiO2 catalysts for the ethanol to butadiene reaction: the effect of Lewis acid promoters. ChemCatChem 12, 5686–5696 (2020).
Walling, S. A., Kinoshita, H., Bernal, S. A., Collier, N. C. & Provis, J. L. Structure and properties of binder gels formed in the system Mg(OH)2–SiO2–H2O for immobilisation of Magnox sludge. Dalton Trans. 44, 8126–8137 (2015).
Bernard, E. et al. Characterization of magnesium silicate hydrate (M-S-H). Cem. Concr. Res. 116, 309–330 (2019).
Salomão, R., Arruda, C. C. & Antunes, M. L. P. Synthesis, dehydroxylation and sintering of porous Mg(OH)2-MgO clusters: evolution of microstructure and physical properties. Interceram. Int. Ceram. Rev. 69, 52–62 (2020).
van Aken, P. A. & Langenhorst, F. Nanocrystalline, porous periclase aggregates as product of brucite dehydration. Eur. J. Mineral. 13, 329–341 (2001).
Gomez-Villalba, L. S., Sierra-Fernandez, A., Rabanal, M. E. & Fort, R. TEM-HRTEM study on the dehydration process of nanostructured Mg–Ca hydroxide into Mg–Ca oxide. Ceram. Int. 42, 9455–9466 (2016).
Rollinson, H. & Adetunji, J. in Encyclopedia of Geochemistry: A Comprehensive Reference Source on the Chemistry of the Earth (ed White, W. M.) 738–743 (Springer, 2018); https://doi.org/10.1007/978-3-319-39312-4_340
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 32, 751–767 (1976).
Green, J. Calcination of precipitated Mg(OH)2 to active MgO in the production of refractory and chemical grade MgO. J. Mater. Sci. 18, 637–651 (1983).
Litasov, K. D. & Ohtani, E. in Advances in High-Pressure Mineralogy Vol. 421 (ed Ohtani, E.) 115–156 (Geological Society of America, 2007).
Lippmaa, E., Mägi, M., Samoson, A., Engelhardt, G. & Grimmer, A. R. Structural studies of silicates by solid-state high-resolution 29Si NMR. J. Am. Chem. Soc. 102, 4889–4893 (1980).
MacKenzie, K. J. D., Bradley, S., Hanna, J. V. & Smith, M. E. Magnesium analogues of aluminosilicate inorganic polymers (geopolymers) from magnesium minerals. J. Mater. Sci. 48, 1787–1793 (2013).
Chabrol, K. et al. Functionalization of synthetic talc-like phyllosilicates by alkoxyorganosilane grafting. J. Mater. Chem. 20, 9695–9706 (2010).
Mackenzie, K. J. D. & Meinhold, R. H. Thermal reactions of chrysotile revisited: a 29Si and 25Mg MAS NMR study. Am. Mineral. 79, 43–50 (1994).
Stebbins, J. F., Smyth, J. R., Panero, W. R. & Frost, D. J. Forsterite, hydrous and anhydrous wadsleyite and ringwoodite (Mg2SiO4): 29Si NMR results for chemical shift anisotropy, spin-lattice relaxation, and mechanism of hydration. Am. Mineral. 94, 905–915 (2009).
Temuujin, J., Okada, K. & MacKenzie, K. J. D. Role of water in the mechanochemical reactions of MgO–SiO2 systems. J. Solid State Chem. 138, 169–177 (1998).
d’Espinose de la Caillerie, J.-B., Kermarec, M. & Clause, O. 29Si NMR observation of an amorphous magnesium silicate formed during impregnation of silica with Mg(II) in aqueous solution. J. Phys. Chem. 99, 17273–17281 (1995).
Hartman, J. S. & Millard, R. L. Gel synthesis of magnesium silicates: a 29Si magic angle spinning NMR study. Phys. Chem. Miner. 17, 1–8 (1990).
Benhelal, E. et al. Insights into chemical stability of Mg-silicates and silica in aqueous systems using 25Mg and 29Si solid-state MAS NMR spectroscopy: applications for CO2 capture and utilisation. Chem. Eng. J. 420, 127656 (2021).
Freitas, J. C. C. & Smith, M. E. Recent advances in solid-state 25Mg NMR spectroscopy. Annu. Rep. NMR Spectrosc. 75, 25–114 (2012).
Pallister, P. J., Moudrakovski, I. L. & Ripmeester, J. A. Mg-25 ultra-high field solid state NMR spectroscopy and first principles calculations of magnesium compounds. Phys. Chem. Chem. Phys. 11, 11487–11500 (2009).
Blanc, F., Middlemiss, D. S., Gan, Z. & Grey, C. P. Defects in doped LaGaO3 anionic conductors: linking NMR spectral features, local environments, and defect thermodynamics. J. Am. Chem. Soc. 133, 17662–17672 (2011).
Jäger, C., Kunath, G., Losso, P. & Scheler, G. Determination of distributions of the quadrupole interaction in amorphous solids by 27Al satellite transition spectroscopy. Solid State Nucl. Magn. Reson. 2, 73–82 (1993).
Hatakeyama, M., Nemoto, T., Kanehashi, K. & Saito, K. Natural abundance solid-state 25Mg MQMAS NMR studies on inorganic solids at a high magnetic field of 16.4 T. Chem. Lett. 34, 864–865 (2005).
Hayashi, Y. et al. Experimental and computational studies of the roles of MgO and Zn in talc for the selective formation of 1,3-butadiene in the conversion of ethanol. Phys. Chem. Chem. Phys. 18, 25191–25209 (2016).
Chieregato, A. et al. On the chemistry of ethanol on basic oxides: revising mechanisms and intermediates in the Lebedev and Guerbet reactions. ChemSusChem 8, 377–388 (2015).
Sushkevich, V. L. & Ivanova, I. I. Mechanistic study of ethanol conversion into butadiene over silver promoted zirconia catalysts. Appl. Catal. B 215, 36–49 (2017).
Gruver, V., Sun, A. & Fripiat, J. J. Catalytic properties of aluminated sepiolite in ethanol conversion. Catal. Lett. 34, 359–364 (1995).
Velasquez Ochoa, J., Malmusi, A., Recchi, C. & Cavani, F. Understanding the role of gallium as a promoter of magnesium silicate catalysts for the conversion of ethanol into butadiene. ChemCatChem 9, 2128–2135 (2017).
Taifan, W. E., Yan, G. X. & Baltrusaitis, J. Surface chemistry of MgO/SiO2 catalyst during the ethanol catalytic conversion to 1,3-butadiene: in-situ DRIFTS and DFT study. Catal. Sci. Technol. 7, 4648–4668 (2017).
Taifan, W. E. Catalytic Transformation of Ethanol to 1,3-Butadiene over MgO/SiO2 Catalyst. PhD thesis, Lehigh University (2018).
Klein, A., Keisers, K. & Palkovits, R. Formation of 1,3-butadiene from ethanol in a two-step process using modified zeolite-β catalysts. Appl. Catal. A 514, 192–202 (2016).
Szabó, B. et al. Conversion of ethanol to butadiene over mesoporous In2O3-promoted MgO-SiO2 catalysts. Mol. Catal. 491, 110984 (2020).
Karkanas, P., Bar-Yosef, O., Goldberg, P. & Weiner, S. Diagenesis in prehistoric caves: the use of minerals that form in situ to assess the completeness of the archaeological record. J. Archaeol. Sci. 27, 915–929 (2000).
Lide, D. R. & Haynes, W. M. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data: 2009–2010 (ed Lide, D. R.) 90th edn (CRC Press, 2009).
Iler, R. K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry of Silica (Wiley, 1979).
Ayral, A. et al. Silica membranes—basic principles. Period. Polytech. Chem. Eng. 50, 67–79 (2006).
Kim, H. N. & Lee, S. K. Atomic structure and dehydration mechanism of amorphous silica: insights from 29Si and 1H solid-state MAS NMR study of SiO2 nanoparticles. Geochim. Cosmochim. Acta 120, 39–64 (2013).
Alba, M. D., Becerro, A. I., Castro, M. A. & Perdigón, A. C. High-resolution 1H MAS NMR spectra of 2∶1 phyllosilicates. Chem. Commun. 37–38 (2000).
Schnell, I. & Spiess, H. W. High-resolution 1H NMR spectroscopy in the solid state: very fast sample rotation and multiple-quantum coherences. J. Magn. Reson. 151, 153–227 (2001).
Yarulina, I. et al. Structure–performance descriptors and the role of Lewis acidity in the methanol-to-propylene process. Nat. Chem. 10, 804–812 (2018).
Ochoa, J. V. et al. An analysis of the chemical, physical and reactivity features of MgO–SiO2 catalysts for butadiene synthesis with the Lebedev process. Green Chem. 18, 1653–1663 (2016).
Singh, M., Zhou, N., Paul, D. K. & Klabunde, K. J. IR spectral evidence of aldol condensation: acetaldehyde adsorption over TiO2 surface. J. Catal. 260, 371–379 (2008).
Moteki, T. & Flaherty, D. W. Mechanistic insight to C–C bond formation and predictive models for cascade reactions among alcohols on Ca- and Sr-hydroxyapatites. ACS Catal. 6, 4170–4183 (2016).
Alabugin, I. V., Bresch, S. & dos Passos Gomes, G. Orbital hybridization: a key electronic factor in control of structure and reactivity. J. Phys. Org. Chem. 28, 147–162 (2015).
Ochoa, J. V., Malmusi, A., Recchi, C. & Cavani, F. Understanding the role of gallium as a promoter of magnesium silicate catalysts for the conversion of ethanol into butadiene. ChemCatChem 9, 2128–2135 (2017).
Tsuchida, T. et al. Reaction of ethanol over hydroxyapatite affected by Ca/P ratio of catalyst. J. Catal. 259, 183–189 (2008).
Díez, V. K., Apesteguía, C. R. & Di Cosimo, J. I. Acid–base properties and active site requirements for elimination reactions on alkali-promoted MgO catalysts. Catal. Today 63, 53–62 (2000).
Di Cosimo, J. I., Dı́ez, V. K., Xu, M., Iglesia, E. & Apesteguı́a, C. R. Structure and surface and catalytic properties of Mg-Al basic oxides. J. Catal. 178, 499–510 (1998).
Kozlowski, J. T. & Davis, R. J. Heterogeneous catalysts for the Guerbet coupling of alcohols. ACS Catal. 3, 1588–1600 (2013).
Gines, M. J. L. & Iglesia, E. Bifunctional condensation reactions of alcohols on basic oxides modified by copper and potassium. J. Catal. 176, 155–172 (1998).
Birky, T. W., Kozlowski, J. T. & Davis, R. J. Isotopic transient analysis of the ethanol coupling reaction over magnesia. J. Catal. 298, 130–137 (2013).
Abdulrazzaq, H. T., Rahmani Chokanlu, A., Frederick, B. G. & Schwartz, T. J. Reaction kinetics analysis of ethanol dehydrogenation catalyzed by MgO–SiO2. ACS Catal. 10, 6318–6331 (2020).
Qi, L. et al. Ethanol conversion to butadiene over isolated zinc and yttrium sites grafted onto dealuminated beta zeolite. J. Am. Chem. Soc. 142, 14674–14687 (2020).
Idriss, H., Diagne, C., Hindermann, J. P., Kiennemann, A. & Barteau, M. A. Reactions of acetaldehyde on CeO2 and CeO2-supported catalysts. J. Catal. 155, 219–237 (1995).
Guil, J. M., Homs, N., Llorca, J. & Ramírez de la Piscina, P. Microcalorimetric and infrared studies of ethanol and acetaldehyde adsorption to investigate the ethanol steam reforming on supported cobalt catalysts. J. Phys. Chem. B 109, 10813–10819 (2005).
Chavez Diaz, C. D., Locatelli, S. & Gonzo, E. E. Acetaldehyde adsorption on HZSM-5 studied by infrared spectroscopy. Zeolites 12, 851–857 (1992).
Yan, T. et al. Mechanistic insights into one-step catalytic conversion of ethanol to butadiene over bifunctional Zn–Y/beta zeolite. ACS Catal. 8, 2760–2773 (2018).
Hemelsoet, K. et al. Experimental and theoretical IR study of methanol and ethanol conversion over H-SAPO-34. Catal. Today 177, 12–24 (2011).
Aronson, M. T., Gorte, R. J. & Farneth, W. E. An infrared spectroscopy study of simple alcohols adsorbed on H-ZSM-5. J. Catal. 105, 455–468 (1987).
Sushkevich, V. L., Ivanova, I. I., Ordomsky, V. V. & Taarning, E. Design of a metal-promoted oxide catalyst for the selective synthesis of butadiene from ethanol. ChemSusChem 7, 2527–2536 (2014).
Fan, D., Dong, X., Yu, Y. & Zhang, M. A DFT study on the aldol condensation reaction on MgO in the process of ethanol to 1,3-butadiene: understanding the structure–activity relationship. Phys. Chem. Chem. Phys. 19, 25671–25682 (2017).
Dong, X., Liu, C., Fan, D., Yu, Y. & Zhang, M. Insight into the effect of promoters (M = Cu, Ag, Zn, Zr) on aldol condensation reaction based on MgO surface in the process of ethanol to 1,3-butadiene: a comparative DFT study. Appl. Surf. Sci. 481, 576–587 (2019).
Sudarsanam, P. et al. Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chem. Soc. Rev. 47, 8349–8402 (2018).
Deimund, M. A. et al. Effect of heteroatom concentration in SSZ-13 on the methanol-to-olefins reaction. ACS Catal. 6, 542–550 (2016).
Gallego, E. M. et al. Making nanosized CHA zeolites with controlled Al distribution for optimizing methanol-to-olefin performance. Chem. Eur. J. 24, 14631–14635 (2018).
Di Iorio, J. R., Nimlos, C. T. & Gounder, R. Introducing catalytic diversity into single-site chabazite zeolites of fixed composition via synthetic control of active site proximity. ACS Catal. 7, 6663–6674 (2017).
Bernauer, M. et al. Proton proximity—new key parameter controlling adsorption, desorption and activity in propene oligomerization over H-ZSM-5 zeolites. J. Catal. 344, 157–172 (2016).
Tabor, E., Bernauer, M., Wichterlová, B. & Dedecek, J. Enhancement of propene oligomerization and aromatization by proximate protons in zeolites: FTIR study of the reaction pathway in ZSM-5. Catal. Sci. Technol. 9, 4262–4275 (2019).
Gates-Rector, S. & Blanton, T. The Powder Diffraction File: a quality materials characterization database. Powder Diffr. 34, 352–360 (2019).
Černý, R., Valvoda, V. & Chládek, M. Empirical texture corrections for asymmetric diffraction and inclined textures. J. Appl. Crystallogr. 28, 247–253 (1995).
Rouquerol, J., Llewellyn, P. & Rouquerol, F. in Studies in Surface Science and Catalysis Vol. 160 (ed Llewellyn, P. L.) 49–56 (Elsevier, 2007).
Dutta Chowdhury, A., Yarulina, I., Abou-Hamad, E., Gurinov, A. & Gascon, J. Surface enhanced dynamic nuclear polarization solid-state NMR spectroscopy sheds light on Brønsted–Lewis acid synergy during the zeolite catalyzed methanol-to-hydrocarbon process. Chem. Sci. 10, 8946–8954 (2019).
Amoureux, J. P., Fernandez, C. & Steuernagel, S. Z filtering in MQMAS NMR. J. Magn. Reson. A 123, 116–118 (1996).
Larsen, F. H., Skibsted, J., Jakobsen, H. J. & Nielsen, N. C. Solid-state QCPMG NMR of low-γ quadrupolar metal nuclei in natural abundance. J. Am. Chem. Soc. 122, 7080–7086 (2000).
Massiot, D. et al. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 40, 70–76 (2002).
Acknowledgements
The authors are grateful for financial support from King Abdullah University of Science and Technology. We thank J. Vittenet and E. Kaliyamoorthy for ICP-OES analysis, S. Lopatin for TEM–EDX measurements, C. Canlas for technical support in recording solid-state NMR spectra, Y. Yuan for in situ PXRD measurements and K. Eichele (Universität Tübingen) for WSolids1 software support. The preliminary experiments in this research were performed within the framework of the CatchBio programme. B.M.W. and P.C.A.B. acknowledge the support of the Smart Mix Program of the Netherlands Ministry of Economic Affairs, the Netherlands Ministry of Education, Culture and Science, and NWO (Middelgroot programme, grant no. 700.58.102).
Author information
Authors and Affiliations
Contributions
S.-H.C. and J.R.-M. conceived the research and directed the project. S.-H.C. designed the experiments and analysed the data. T.L. and A.R. collected and processed the DRIFTS–MS spectra and catalytic activity test results, respectively. T.S., S.K., I.M., G.S., S.T., A.D., E.A.-H., X.T. and P.L. characterized the prepared catalysts under the guidance of S.-H.C., J.G. and J.R.-M. B.S. conducted preliminary experiments for the catalyst synthesis under the supervision of B.M.W. and P.C.A.B. S.-H.C. and J.R.-M. co-wrote the paper. All authors discussed the results and commented on different versions of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Attila Domján and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–27, Tables 1–4, Notes 1–6 and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chung, SH., Li, T., Shoinkhorova, T. et al. Origin of active sites on silica–magnesia catalysts and control of reactive environment in the one-step ethanol-to-butadiene process. Nat Catal 6, 363–376 (2023). https://doi.org/10.1038/s41929-023-00945-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-00945-0
This article is cited by
-
Preparation of porous silica microspheres using silica nanoparticles with different morphologies and their properties as catalyst carriers
Journal of Porous Materials (2024)