Abstract
The effective utilization of natural variation has become essential in addressing the challenges that climate change and population growth pose to global food security. Currently adopted protracted approaches to introgress exotic alleles into elite cultivars need substantial transformation. Here, through a strategic three-way crossing scheme among diverse exotics and the best historical elites (exotic/elite1//elite2), 2,867 pre-breeding lines were developed, genotyped and screened for multiple agronomic traits in four mega-environments. A meta-genome-wide association study, selective sweeps and haplotype-block-based analyses unveiled selection footprints in the genomes of pre-breeding lines as well as exotic-specific associations with agronomic traits. A simulation with a neutrality assumption demonstrated that many pre-breeding lines had significant exotic contributions despite substantial selection bias towards elite genomes. National breeding programmes worldwide have adopted 95 lines for germplasm enhancement, and 7 additional lines are being advanced in varietal release trials. This study presents a great leap forwards in the mobilization of GenBank variation to the breeding pipelines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data used in the present study are provided in the supplementary files and on GitHub at https://github.com/ajighly/Seeds-of-Discovery_PBL. Source data are provided with this paper.
Code availability
All codes used in the present study can be found at https://github.com/ajighly/Seeds-of-Discovery_PBL.
References
Haudry, A. et al. Grinding up wheat: a massive loss of nucleotide diversity since domestication. Mol. Biol. Evol. 24, 1506–1517 (2007).
Joukhadar, R., Daetwyler, H. D., Bansal, U. K., Gendall, A. R. & Hayden, M. J. Genetic diversity, population structure and ancestral origin of Australian wheat. Front. Plant Sci. 8, 2115 (2017).
Jighly, A. et al. Population‐dependent reproducible deviation from natural bread wheat genome in synthetic hexaploid wheat. Plant J. 100, 801–812 (2019).
Jighly, A., Joukhadar, R., Singh, S. & Ogbonnaya, F. C. Decomposing additive genetic variance revealed novel insights into trait evolution in synthetic hexaploid wheat. Front. Genet. 9, 27 (2018).
Liu, J. et al. Genome-wide variation patterns between landraces and cultivars uncover divergent selection during modern wheat breeding. Theor. Appl. Genet. 132, 2509–2523 (2019).
Singh, S. et al. GWAS revealed a novel resistance locus on chromosome 4D for the quarantine disease Karnal bunt in diverse wheat pre-breeding germplasm. Sci. Rep. 10, 5999 (2020).
Singh, S. et al. Harnessing genetic potential of wheat germplasm banks through impact-oriented-prebreeding for future food and nutritional security. Sci. Rep. 8, 12527 (2018).
Qian, L. et al. Exploring and harnessing haplotype diversity to improve yield stability in crops. Front. Plant Sci. 8, 1534 (2017).
Singh, S. et al. GWAS to identify genetic loci for resistance to yellow rust in wheat pre-breeding lines derived from diverse exotic crosses. Front. Plant Sci. 10, 1390 (2019).
Shokat, S., Sehgal, D., Vikram, P., Liu, F. & Singh, S. Molecular markers associated with agro-physiological traits under terminal drought conditions in bread wheat. Int. J. Mol. Sci. 21, 3156 (2020).
Bolormaa, S. et al. A multi-trait, meta-analysis for detecting pleiotropic polymorphisms for stature, fatness and reproduction in beef cattle. PLoS Genet. 10, e1004198 (2014).
Acuña‐Galindo, M. A., Mason, R. E., Subramanian, N. K. & Hays, D. B. Meta-analysis of wheat QTL regions associated with adaptation to drought and heat stress. Crop Sci. 55, 477–492 (2015).
Battenfield, S. D. et al. Breeding-assisted genomics: applying meta-GWAS for milling and baking quality in CIMMYT wheat breeding program. PLoS ONE 13, e0204757 (2018).
Afzal, F. et al. Genome-wide analyses reveal footprints of divergent selection and drought adaptive traits in synthetic-derived wheats. G3 9, 1957–1973 (2019).
Joukhadar, R., Daetwyler, H. D., Gendall, A. R. & Hayden, M. J. Artificial selection causes significant linkage disequilibrium among multiple unlinked genes in Australian wheat. Evol. Appl. 12, 1610–1625 (2019).
Bouwman, A. C. et al. Meta-analysis of genome-wide association studies for cattle stature identifies common genes that regulate body size in mammals. Nat. Genet. 50, 362–367 (2018).
Wright, S. The relation of livestock breeding to theories of evolution. J. Anim. Sci. 46, 1192–1200 (1978).
Ogbonnaya, F. C. et al. Synthetic hexaploids: harnessing species of the primary gene pool for wheat improvement. Plant Breed. Rev. 37, 35–122 (2013).
He, F. et al. Exome sequencing highlights the role of wild-relative introgression in shaping the adaptive landscape of the wheat genome. Nat. Genet. 51, 896–904 (2019).
Ellis, R. et al. Phenotype/genotype associations for yield and salt tolerance in a barley mapping population segregating for two dwarfing genes. J. Exp. Bot. 53, 1163–1176 (2002).
Lopes, M., Dreisigacker, S., Peña, R., Sukumaran, S. & Reynolds, M. P. Genetic characterization of the Wheat Association Mapping Initiative (WAMI) panel for dissection of complex traits in spring wheat. Theor. Appl. Genet. 128, 453–464 (2015).
Jiang, Q. et al. The wheat (T. aestivum) sucrose synthase 2 gene (TaSus2) active in endosperm development is associated with yield traits. Funct. Integr. Genomics 11, 49–61 (2011).
Ma, D., Yan, J., He, Z., Wu, L. & Xia, X. Characterization of a cell wall invertase gene TaCwi-A1 on common wheat chromosome 2A and development of functional markers. Mol. Breed. 29, 43–52 (2012).
Qin, L. et al. Homologous haplotypes, expression, genetic effects and geographic distribution of the wheat yield gene TaGW2. BMC Plant Biol. 14, 107 (2014).
Jiang, Y. et al. A yield-associated gene TaCWI, in wheat: its function, selection and evolution in global breeding revealed by haplotype analysis. Theor. Appl. Genet. 128, 131–143 (2015).
Wei, B. et al. Dreb1 genes in wheat (Triticum aestivum L.): development of functional markers and gene mapping based on SNPs. Mol. Breed. 23, 13–22 (2009).
Li, Q., Chen, X., Wang, M. & Jing, J. Yr45, a new wheat gene for stripe rust resistance on the long arm of chromosome 3D. Theor. Appl. Genet. 122, 189–197 (2011).
Mason, R. E., Hays, D. B., Mondal, S., Ibrahim, A. M. & Basnet, B. R. QTL for yield, yield components and canopy temperature depression in wheat under late sown field conditions. Euphytica 194, 243–259 (2013).
Fan, M. S. et al. Evidence of decreasing mineral density in wheat grain over the last 160 years. J. Trace Elem. Med. Biol. 22, 315–324 (2008).
Srinivasa, J. et al. Zinc and iron concentration QTL mapped in a Triticum spelta × T. aestivum cross. Theor. Appl. Genet. 127, 1643–1651 (2014).
Sehgal, D. et al. Exploring and mobilizing the gene bank biodiversity for wheat improvement. PLoS ONE 10, e0132112 (2015).
Vikram, P. et al. Unlocking the genetic diversity of Creole wheats. Sci. Rep. 6, 23092 (2016).
Hao, Y. et al. Characterization of a major QTL for adult plant resistance to stripe rust in US soft red winter wheat. Theor. Appl. Genet. 123, 1401–1411 (2011).
Peterson, R. F., Campbell, A. & Hannah, A. A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can. J. Res. 26, 496–500 (1948).
Saari, E. & Prescott, J. Scale for appraising the foliar intensity of wheat diseases. Plant Dis. Rep. 59, 377–380 (1975).
Paltridge, N. G. et al. Energy-dispersive X-ray fluorescence spectrometry as a tool for zinc, iron and selenium analysis in whole grain wheat. Plant Soil 361, 261–269 (2012).
de León, D. G. Laboratory Protocols: CIMMYT Applied Molecular Genetics Laboratory (CIMMYT, 1994).
Frutos, E., Galindo, M. P. & Leiva, V. An interactive biplot implementation in R for modeling genotype-by-environment interaction. Stoch. Environ. Res. Risk Assess. 28, 1629–1641 (2014).
Yan, W., Hunt, L., Sheng, Q. & Szlavnics, Z. Cultivar evaluation and mega‐environment investigation based on the GGE biplot. Crop Sci. 40, 597–605 (2000).
Yan, W. GGEbiplot—a Windows application for graphical analysis of multienvironment trial data and other types of two‐way data. Agron. J. 93, 1111–1118 (2001).
Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655–1664 (2009).
Pagani, L. et al. Genomic analyses inform on migration events during the peopling of Eurasia. Nature 538, 238–242 (2016).
Gabriel, S. B. et al. The structure of haplotype blocks in the human genome. Science 296, 2225–2229 (2002).
Jighly, A. et al. Insights into population genetics and evolution of polyploids and their ancestors. Mol. Ecol. Resour. 18, 1157–1172 (2018).
Lee, S. H. & Van der Werf, J. H. MTG2: an efficient algorithm for multivariate linear mixed model analysis based on genomic information. Bioinformatics 32, 1420–1422 (2016).
Yang, J. et al. Common SNPs explain a large proportion of the heritability for human height. Nat. Genet. 42, 565–569 (2010).
Lee, S. H. et al. Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat. Genet. 44, 247–250 (2012).
Zhou, X. & Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 44, 821–824 (2012).
Zhou, X. & Stephens, M. Efficient multivariate linear mixed model algorithms for genome-wide association studies. Nat. Methods 11, 407–409 (2014).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Schwarz, G. Estimating the dimension of a model. Ann. Stat. 6, 461–464 (1978).
VanRaden, P. M. Efficient methods to compute genomic predictions. J. Dairy Sci. 91, 4414–4423 (2008).
Voight, B. F., Kudaravalli, S., Wen, X. & Pritchard, J. K. A map of recent positive selection in the human genome. PLoS Biol. 4, e72 (2006).
Nielsen, R., Hellmann, I., Hubisz, M., Bustamante, C. & Clark, A. G. Recent and ongoing selection in the human genome. Nat. Rev. Genet. 8, 857–868 (2007).
Acknowledgements
We acknowledge the Seeds of Discovery project of CIMMYT and the directors of the Genetic Resources Program, as well as the Global Wheat Program, for their valuable support and encouragement of the team. We acknowledge the financial support received from the Secretariat of Agriculture and Rural Development. We thank R. Singh, M. Ellis and T. Payne, who provided seeds of elite and exotic germplasm lines. We also thank Punjab Agricultural University, Ludhiana; ICAR-IIWBR, Karnal; and CSKHP Palampur, India, for providing valuable support in conducting phenotypic evaluation of the pre-breeding germplasm. We also thank IFS, Sweden, for grant number C-5897-I for phenotyping at the Nuclear Institute for Agriculture and Biology. We acknowledge the direct and indirect support of researchers and non-scientific staff. We thank C. J. Vander Jagt (Agriculture Victoria, Australia) for editing the manuscript.
Author information
Authors and Affiliations
Contributions
Sukhwinder Singh, P.V. and P.W. conceived and designed the experiments. Sukhwinder Singh, A.J. and D.S. planned the structure of the paper. A.S., S.K.S., K.A.L., Sanjay Singh, M.A.R.A., D.B., A.K.B., N.P., Sukhwinder Singh and P.V. conducted the traits evaluation. C.P.S., P.V., N.P. and Sukhwinder Singh did the genotyping. J.B., Sukhwinder Singh, N.P. and P.V. conducted the experimental design and field data analysis. A.J. carried out the GGE, genetic correlation and simulation analyses. R.J. conducted the exotic contribution, meta-GWAS and selective sweep analyses. D.S. and Sukhwinder Singh conducted the haplotype detection and haplotype-based GWAS. Sukhwinder Singh, A.J., D.S., J.B. and R.J. interpreted the results. Sukhwinder Singh, A.J., D.S., R.J., J.B., A.S., S.K.S., N.S.B. and H.K.C. prepared the manuscript. All authors read and approved the final version of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Food thanks Xuehui Huang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9.
Supplementary Tables
Supplementary Tables 1–20.
Source data
Source Data Fig. 1
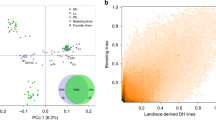
Statistical analysis. Sheet ‘LD’ contains the LD decay results for empirical and simulated PBLs with Syn and LR backgrounds used for Fig. 1a. Sheet ‘Contribution’ contains the percentage of the exotic parent contribution to each PBL used for Fig. 1b,c.
Source Data Fig. 2
Statistical analysis (−log10(P) for SNPs) for SL, PH, YLD and Fe as well as iHS scores.
Rights and permissions
About this article
Cite this article
Singh, S., Jighly, A., Sehgal, D. et al. Direct introgression of untapped diversity into elite wheat lines. Nat Food 2, 819–827 (2021). https://doi.org/10.1038/s43016-021-00380-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43016-021-00380-z
This article is cited by
-
Genomic wide association study and selective sweep analysis identify genes associated with improved yield under drought in Turkish winter wheat germplasm
Scientific Reports (2024)
-
Exotic alleles contribute to heat tolerance in wheat under field conditions
Communications Biology (2023)
-
Skipping irrigation at pre- and post-anthesis stages influences grain yield and starch contents of bread wheat derived from synthetic or landraces
Cereal Research Communications (2023)
-
Analysis of historical selection in winter wheat
Theoretical and Applied Genetics (2022)
-
Genomics-informed prebreeding unlocks the diversity in genebanks for wheat improvement
Nature Genetics (2022)