Abstract
Tumor microenvironment-targeted therapies are emerging as promising treatment options for different cancer types. Tumor-associated macrophages and microglia (TAMs) represent an abundant nonmalignant cell type in brain metastases and have been proposed to modulate metastatic colonization and outgrowth. Here we demonstrate that targeting TAMs at distinct stages of the metastatic cascade using an inhibitor of colony-stimulating factor 1 receptor (CSF1R), BLZ945, in murine breast-to-brain metastasis models leads to antitumor responses in prevention and intervention preclinical trials. However, in established brain metastases, compensatory CSF2Rb–STAT5-mediated pro-inflammatory TAM activation blunted the ultimate efficacy of CSF1R inhibition by inducing neuroinflammation gene signatures in association with wound repair responses that fostered tumor recurrence. Consequently, blockade of CSF1R combined with inhibition of STAT5 signaling via AC4-130 led to sustained tumor control, a normalization of microglial activation states and amelioration of neuronal damage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Sequencing data reported in this study have been deposited to the Gene Expression Omnibus under accession code GSE133887. RNA-seq data of cell populations from nontumor-bearing brains, healthy donors and breast-to-brain metastases (previously published14) were downloaded from the ‘Brain TIME’ website (https://joycelab.shinyapps.io/braintime/). Transcripts per million were estimated using the GDC.h38 GENCODE v.22 annotation (https://api.gdc.cancer.gov/data/25aa497c-e615-4cb7-8751-71f744f9691f).
Source data for Figs. 1–8 and Extended Data Figs. 1–10 are provided as Source Data files. All other data supporting the findings of this study are available from the corresponding authors on reasonable request. Source data are provided with this paper.
Code availability
Custom scripts for analyzing RNA-seq data and vesicle quantification are available upon request.
References
Suh, J. H. et al. Current approaches to the management of brain metastases. Nat. Rev. Clin. Oncol. 17, 279–299 (2020).
Tabouret, E. et al. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 32, 4655–4662 (2012).
Valiente, M. et al. The evolving landscape of brain metastasis. Trends Cancer 4, 176–196 (2018).
Kotecki, N., Lefranc, F., Devriendt, D. & Awada, A. Therapy of breast cancer brain metastases: challenges, emerging treatments and perspectives. Ther. Adv. Med. Oncol. 10, 1–10 (2018).
Owonikoko, T. K. et al. Current approaches to the treatment of metastatic brain tumours. Nat. Rev. Clin. Oncol. 11, 203–222 (2014).
Lim, M., Xia, Y., Bettegowda, C. & Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 15, 422–442 (2018).
Caponnetto, S. et al. Cancer immunotherapy in patients with brain metastases. Cancer Immunol. Immunother. 67, 703–711 (2018).
Long, G. V. et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 19, 672–681 (2018).
Sevenich, L. Turning ‘cold’ into ‘hot’ tumors-opportunities and challenges for radio-immunotherapy against primary and metastatic brain cancers. Front. Oncol. 9, 163 (2019).
Tawbi, H. A. et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N. Engl. J. Med. 379, 722–730 (2018).
Aldape, K. et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 8, 509–520 (2019).
Quail, D. F. & Joyce, J. A. The microenvironmental landscape of brain tumors. Cancer Cell 31, 326–341 (2017).
Bowman, R. L. et al. Macrophage ontogeny underlies differences in tumor-specific education in brain malignancies. Cell Rep. 17, 2445–2459 (2016).
Klemm, F. et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell 181, 1643–1660 (2020).
Sevenich, L. et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat. Cell Biol. 16, 876–888 (2014).
Friebel, E. et al. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell 181, 1626–1642 (2020).
Schulz, M. et al. Cellular and molecular changes of brain metastases-associated myeloid cells during disease progression and therapeutic response. iScience 23, 101178 (2020).
Qian, B. Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011).
Bejarano, L., Jordao, M. J. C. & Joyce, J. A. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 11, 933–959 (2021).
Guldner, I. H. et al. CNS-native myeloid cells drive immune suppression in the brain metastatic niche through Cxcl10. Cell 183, 1234–1248 (2020).
Patsialou, A. et al. Invasion of human breast cancer cells in vivo requires both paracrine and autocrine loops involving the colony-stimulating factor-1 receptor. Cancer Res. 69, 9498–9506 (2009).
Pyonteck, S. M. et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19, 1264–1272 (2013).
Quail, D. F. et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science 352, aad3018 (2016).
Yan, D. et al. Inhibition of colony stimulating factor-1 receptor abrogates microenvironment-mediated therapeutic resistance in gliomas. Oncogene 36, 6049–6058 (2017).
Qiao, S., Qian, Y., Xu, G., Luo, Q. & Zhang, Z. Long-term characterization of activated microglia/macrophages facilitating the development of experimental brain metastasis through intravital microscopic imaging. J. Neuroinflammation 16, 4 (2019).
Andreou, K. E. et al. Anti-inflammatory microglia/macrophages as a potential therapeutic target in brain metastasis. Front. Oncol. 7, 251 (2017).
Valiente, M. et al. Brain metastasis cell lines panel: a public resource of organotropic cell lines. Cancer Res. 80, 4314–4323 (2020).
Goldmann, T. et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 17, 797–805 (2016).
Akkari, L. et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci. Transl. Med. 12, eaaw7843 (2020).
Muller, S. et al. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol. 18, 234 (2017).
Vareslija, D. et al. Transcriptome characterization of matched primary breast and brain metastatic tumors to detect novel actionable targets. J. Natl Cancer Inst. 111, 388–398 (2019).
Oishi, K. et al. Identity of neocortical layer 4 neurons is specified through correct positioning into the cortex. eLife 5, e10907 (2016).
Bury, F. J. et al. Xenopus BTBD6 and its Drosophila homologue lute are required for neuronal development. Dev. Dyn. 237, 3352–3360 (2008).
Hama, T. et al. Identification and molecular cloning of a novel brain-specific receptor protein that binds to brain injury-derived neurotrophic peptide: possible role for neuronal survival. J. Biol. Chem. 276, 31929–31935 (2001).
Ehrlich, A. T. et al. Expression map of 78 brain-expressed mouse orphan GPCRs provides a translational resource for neuropsychiatric research. Commun. Biol. 1, 102 (2018).
Zeng, Q. et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531 (2019).
Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Rempe, R. G., Hartz, A. M. S. & Bauer, B. Matrix metalloproteinases in the brain and blood–brain barrier: versatile breakers and makers. J. Cereb. Blood Flow Metab. 36, 1481–1507 (2016).
Re, F. et al. Granulocyte-macrophage colony-stimulating factor induces an expression program in neonatal microglia that primes them for antigen presentation. J. Immunol. 169, 2264–2273 (2002).
Wingelhofer, B. et al. Pharmacologic inhibition of STAT5 in acute myeloid leukemia. Leukemia 32, 1135–1146 (2018).
Chitu, V. et al. Microglial homeostasis requires balanced CSF-1/CSF-2 receptor signaling. Cell Rep. 30, 3004–3019 (2020).
Sharma, P. et al. The next decade of immune checkpoint therapy. Cancer Discov. 11, 838–857 (2021).
Chongsathidkiet, P. et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat. Med. 24, 1459–1468 (2018).
Peranzoni, E. et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc. Natl Acad. Sci. USA 115, E4041–E4050 (2018).
Simoni, Y. et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018).
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Salvagno, C. et al. Therapeutic targeting of macrophages enhances chemotherapy efficacy by unleashing type I interferon response. Nat. Cell Biol. 21, 511–521 (2019).
Becher, B., Spath, S. & Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 17, 49–59 (2017).
Wingrove, E. et al. Transcriptomic hallmarks of tumor plasticity and stromal interactions in brain metastasis. Cell Rep. 27, 1277–1292 (2019).
Neman, J. et al. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc. Natl Acad. Sci. USA 111, 984–989 (2014).
Schnepp, P. M. et al. GAD1 upregulation programs aggressive features of cancer cell metabolism in the brain metastatic microenvironment. Cancer Res. 77, 2844–2856 (2017).
Beckmann, N. et al. Brain region-specific enhancement of remyelination and prevention of demyelination by the CSF1R kinase inhibitor BLZ945. Acta Neuropathol. Commun. 6, 9 (2018).
Lloyd, A. F. et al. Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nat. Neurosci. 22, 1046–1052 (2019).
Harter, P. N. et al. Distribution and prognostic relevance of tumor-infiltrating lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain metastases. Oncotarget 6, 40836–40849 (2015).
Jung, S. et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell Biol. 20, 4106–4114 (2000).
Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31, 1116–1128 (2006).
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Ponomarev, V. et al. A novel triple-modality reporter gene for whole-body fluorescent, bioluminescent, and nuclear noninvasive imaging. Eur. J. Nucl. Med. Mol. Imaging 31, 740–751 (2004).
Shree, T. et al. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 25, 2465–2479 (2011).
Dzyubenko, E., Rozenberg, A., Hermann, D. M. & Faissner, A. Colocalization of synapse marker proteins evaluated by STED-microscopy reveals patterns of neuronal synapse distribution in vitro. J. Neurosci. Methods 273, 149–159 (2016).
Rueden, C. T. et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinf. 18, 529 (2017).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Sergushichev, A. A. An algorithm for fast preranked gene set enrichment analysis using cumulative statistic calculation. Preprint at bioRxiv https://doi.org/10.1101/060012 (2016).
Hanzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinf. 14, 7 (2013).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis. (Springer-Verlag, 2016).
R-core-Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2018).
Acknowledgements
We thank X. Chen, M. Quick, K. Simpson, P. Dinse, P. Gebhardt and E. De Iaco for excellent technical support and members of the Joyce laboratory and the GSH for insightful discussion. We are grateful to Novartis for providing BLZ945 and M. Wiesmann, Novartis, for critical input and discussion. We thank the GSH, Goethe University and MSKCC Core Facilities for Molecular Cytology for technical assistance, in particular M. Basoglu for electron microscopy and the Core Facility for Microscopy at the Institute of Molecular Biology Mainz for access to the Imaris software. Research in the laboratory of J.A.J. for this project was provided in part by the Breast Cancer Research Foundation (to J.A.J.), Ludwig Institute for Cancer Research (to J.A.J.), University of Lausanne (to J.A.J.), Cancer Research UK (to J.A.J.) and the Swiss Cancer Research Foundation (KFS-3390-08-2016 to J.A.J.) and research fellowships from the German Research Foundation (KL2491/1-1 to F.K. and SE2234/1-1 to L.S.), Fondation Medic (to F.K.) and Metastasis Research Center at MSKCC (to L.S.). Research in the laboratory of L.S. is supported by institutional funds from the GSH jointly funded by the German Federal Ministry of Health and the Ministry of Higher Education, Research and the Arts of the State of Hesse and grants from the LOEWE Center Frankfurt Cancer Institute (to L.S.), the German Cancer Consortium (partner site Frankfurt/Mainz (to L.S.)), the German Cancer Aid (Max-Eder Junior Group Leader Program 70111752 to L.S.), the German Research Foundation (SE2234/3-1 to L.S.), the Beug Foundation for Metastasis Research and the Bodo Sponholz Foundation. Scientific illustrations were created using BioRender.com.
Author information
Authors and Affiliations
Contributions
F.K., J.A.J. and L.S. designed experiments. F.K., A.M., A.S.B., T.A., A.S., M.S., K.N., R.R.M., M.G., B.T.E., R.L.B., P.S.Z., J.Z. and L.S. performed experiments and analyzed data. F.K. performed computational analysis. M.E.H., R.T.D., P.N.H. and K.P. provided patient samples. F.K., J.A.J. and L.S. wrote the manuscript. All authors edited or commented on the manuscript. J.A.J. and L.S. conceived and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Cancer thanks Brunie Felding and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
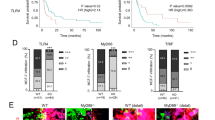
Extended Data Fig. 1 CSF1R expression in human BrM and experimental BrM models.
a, Schematic overview of the cell marker combination used to histologically distinguish TAM populations in patient BrM. b, Representative images of single channel and merged immunofluorescence (IF) images of CD45, CD68/ P2RY12, CD49D and CSF1R stainings used to identify CSF1R expression in different cell populations. Scale bar, 100 µm. c,d, Quantification of Csf1r/CSF1R expression by qRT-PCR in (c) mouse (n99LN-BrM = 6, nTS1 = 7, nTS2 = 2) and (d) human tumor cell lines (nMDA-Par = 4, nMDA-BrM = 4, nHCC1954-BrM = 3, nMCF7 = 3, nSKBR3 = 3, nBT474 = 3, nT47D = 3, nH2030-BrM = 3, nPC9 = 4) in comparison to primary bone marrow-derived macrophages (BMDM, n = 13) and the macrophage cell line THP1 (n = 1) as positve control. Data is shown as the mean ± sd. e, Quantification of CSF1R/Csf1r expression levels by RNAseq in FACS-purified MDA-BrM tumor cells, tumor-associated microglia and monocyte-derived macrophages (TAM-MG and TAM-MDM) as well as normal microglia and blood monocytes isolated from tumor-free mice (n = 5 for tumor-bearing mice and n = 3 for tumor-free controls). Data are shown as the mean ± sd. TPM, transcripts per million. Two-sided, Wilcoxon unsigned-rank test. f, Measurement of cell viability in MDA-BrM and 99LN-BrM cells in response to BLZ945 treatment (n = 3 replicates for each condition) using MTS assay.
Extended Data Fig. 2 Lineage tracing- and marker-based delineation of the TAM compartment in mouse BrM.
a, Schematic overview of the Cx3cr1CreERT2-IRES-EYFP:R26-lsl-TdTomato lineage tracing model used to distinguish TAM-MG and TAM-MDM in mouse BrM. b, Representative images of tumor-free brain and 99LN-BrM in the Cx3cr1CreERT2-IRES-EYFP:R26-lsl-TdTomato lineage tracing model stained for the macrophage marker IBA1 and the microglia marker Tmem119. Images are representative for n = 3 samples. Scale bars, 100 µm and 25 µm. c, Schematic overview of the lineage restricted marker combination used for flow cytometry and cell sorting. d, Representative flow cytometry plots of the gating strategy for FACS purification of GFP+ tumor cells and TAM-MG (CD45+ CD11b+ Ly6C-Ly6G-CD49d-) and TAM-MDM (CD45+ CD11b+ Ly6C-Ly6G-CD49d+) from the MDA-BrM model used for RNAseq experiments. Tumor-free mice were used to isolate blood monocytes and normal MG. e, Quantification of the percentage of CD49d- MG and CD49d+ MDM in the CD45+ CD11b+ Ly6C-Ly6G- live cell population (n = 3 for tumor-free controls n = 5 for MDA-BrM and n = 4 for 99LN-BrM mice). Data is shown as the mean ± sd. Two-tailed Student’s t-test. f, Marker gene expression in normal MG and blood monocytes as well as TAM-MG and TAM-MDM to validate the specificity of CD49D and the purity of the FAC-sorted populations. (n = 5; vehicle, n = 4; BLZ945). g, Heatmap depicting the z-standardized normalized enrichment score (NES z-score) of the union of DEG identified as BrM-TAM genes in matched human primary breast cancer and BrM from non-triple negative (Non-TNBC; upper panel) and triple negative breast cancer (TNBC; bottom panel). RNAseq data for patient breast cancer and BrM samples was obtained from31. NES between primary tumors and BrM was assessed using the one-sided, Wilcoxon signed-rank test (P = 0.021).
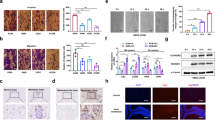
Extended Data Fig. 3 MG and BMDM support tumor cell extravasation.
a, Representative IF images (left) for data shown in (b) and 3D reconstruction (right) of IBA1+ microglia in normal brain parenchyma treated for 7 days with vehicle or BLZ945. Scale bar, 25 µm. Images of 3D reconstruction are representative for n = 3 samples. b, Quantification of IBA1+ cells after vehicle or BLZ945 treatment for 7 days, n = 7 for vehicle and n = 7 for BLZ945 treatment group. c, Experimental design for time-lapse imaging of MG–tumor cell interactions on brain slices isolated from vehicle or BLZ945-treated mice. d, Representative images of MG-tumor cell interactions on brain slices at different time points during time-lapse imaging. (n = 3 independent experiments). e, Quantification of the number of interactions between tumor cells and MG normalized to the total number of MG in each condition (n = 11 for vehicle and n = 12 for BLZ945; results are shown for individual FOVs on brain slices obtained from 3 mice per group) f, Quantification of tumor cells on brain slices after 20 h (n = 11 for vehicle and n = 12 for BLZ945; results are shown for individual FOVs on brain slices obtained from 3 mice per group) g, Quantification of the transmigration of MDA-BrM cells in the presence or absence of primary MG through an artificial blood-brain barrier (BBB) formed by human astrocytes (HA) and human brain microvascular endothelial cells (HBMECs) (n = 20 and n = 13 replicates for vehicle and BLZ945, respectively), or without an artificial BBB (n = 7 and n = 8 replicates for vehicle and BLZ945, respectively). Values are relative to the control condition. h, Quantification of the transmigration of MDA-BrM cells, with or without the addition of the microglia cell line EOC2, in response to BLZ945 or vehicle treatment (n = 4 replicates per condition). Values are relative to the control condition. i, Quantification of the transmigration of 99LN-BrM cells, with or without the addition of the microglia cell line EOC2, in response to BLZ945 or vehicle treatment (n = 10 replicates per condition). Values are relative to the control condition. Boxes represent first, third quartiles and median and whiskers show min and max values in (b,e,f,g,h,i); Two-tailed Student’s t-test in (b, e, f, g,h,i).
Extended Data Fig. 4 CSF1R inhibition leads to TAM depletion.
a, Representative immunofluorescence images for data shown in (b). Scale bar, 50 µm. b, Quantification of the number of IBA1+ TAMs in vehicle and BLZ945 treated animals in MDA-BrM (n = 5 for each condition). c, Representative immunofluorescence images for data shown in (d). Scale bar, 50 µm. d, Quantification of the number of IBA1+ TAMs in vehicle and BLZ945 treated animals in 99LN-BrM mice (n = 3 for each condition). e, Quantification of BLI intensity during tumor progression in the MDA-BrM model in vehicle or BLZ945 treated animals in prevention trial setting (n = 16; Vehicle, n = 14; BLZ945). f, Quantification of the tumor volume based on T1 weighted MRI images during tumor progression in the 99LN-BrM model in vehicle or BLZ945 treated animals in the prevention trial setting (n = 10; vehicle, n = 11; BLZ945). For (b,d) data is shown as the mean ± sd. P values were obtained with two-tailed Student’s t-test in (b,d).
Extended Data Fig. 5 CSF1R inhibition results in transient tumor stasis.
a, Representative BLI images of vehicle and BLZ945-treated MDA-BrM mice. b, Representative MRI images of vehicle and BLZ945-treated 99LN-BrM mice. c,d, Waterfall plots of relative BrM growth at d4 after treatment start (MDA-BrM in (c): n = 19, vehicle and n = 20, BLZ945; and 99LN-BrM in (d): n = 12, vehicle and n = 15, BLZ945). Mann-Whitney U log rank test. e,f, Quantification of TAMs in vehicle and BLZ945 treated (e) MDA-BrM (n = 7; vehicle, n = 7; BLZ945) and (f) 99LN-BrM mice (n = 3; vehicle, n = 4; BLZ945). g,h, Quantification of myeloid cell populations in the (g) MDA-BrM (n = 5; vehicle, n = 4; BLZ945) and (h) 99LN-BrM model (n = 3; vehicle, n = 3; BLZ945). Floating bars show min and max values with the line indicating the mean in (e-h). Two-tailed Student’s t-test in (e-h).
Extended Data Fig. 6 CSF1R inhibtion does not lead to sustained tumor control.
a, Representative immunofluorescence images for data shown in (b). Scale bars, 50 µm. b, Ki67:CC3 proliferation:apoptosis index from immunofluorescence staining of vehicle and BLZ945-treated MDA-BrM tumors (n = 5). c, Representative immunofluorescence images for data shown in (d). Scale bar, 50 µm. d, Ki67:CC3 proliferation:apoptosis index from immunofluorescence staining of vehicle and BLZ945-treated 99LN-BrM tumors (n = 3). e, Quantification of BLI intensity of vehicle and BLZ945–treated mice from the MDA-BrM model (n = 10; vehicle and n = 8; BLZ945). f, Quantification of the tumor volume based on T1 weighted MRI images during tumor progression in the 99LN-BrM model in vehicle or BLZ945 treated animals in the intervention trial setting (n = 5; vehicle, n = 7; BLZ945). g,h, Kaplan-Meier survival curves of MDA-BrM and 99LN-BrM mice after treatment as indicated in Fig. 4a (MDA-BrM in (g): n = 10, vehicle and BLZ945; and 99LN-BrM in (h): n = 7, vehicle and n = 9, BLZ945, Mantel-Cox log rank test).
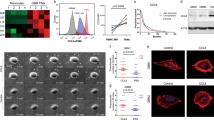
Extended Data Fig. 7 Transcriptional changes of TAMs in response to CSF1R inhibition.
a, Experimental design for the generation of FAC-sorted samples for RNA sequencing. b, Representative in vivo and ex vivo images of vehicle and BLZ945-treated MDA-BrM mice at d7 after treatment start. c, Quantification of BLI intensity in vehicle and BLZ945 treated MDA-BrM tumors subjected to RNAseq analysis (n = 5; vehicle, n = 4; BLZ945). d, tSNE plot on 2000 most variant genes shows clustering of individual samples. e, Euler plot depicting shared and unique DEG (adjusted P < 0.05, log2fc > 1, mean expression > 10) in MG (right panel) and MDM (left panel) in the indicated comparisons. f, Expression heatmap of M1- and M2-like macrophage markers in vehicle and 7d BLZ945 treated TAMs (n = 5; vehicle, n = 4; BLZ945) from the MDA-BrM model and normal MG or control monocytes (n = 3 each). g, Transcription factor (TF) activity for a panel of M1/M2 associated transcription factors (n = 5; vehicle, n = 4; BLZ945 and n = 3; tumor-free controls). Boxes represent first, third quartiles and median and whiskers show 1.5 interquartile range of the 25th and 75th percentile.
Extended Data Fig. 8 Neuroinflammation- pathway overrepresentation in BrM-TAMs upon CSF1R inhibition and CSF2R-complex expression in clinical BrM samples.
a, Gene set ORA (from the MSigDB GO collection) of DEGs that are specifically induced in MDA-BrM TAM-MG and TAM-MDM in response to CSF1R blockade compared to normal MG and blood monocytes. b, Quantification of the cell viability of the microglial cell line EOC2 and bone marrow-derived macrophages (BMDM) in culture in response to CSF2, IL3 or IL5 stimulation in the presence or absence of 67 nM and 670 nM BLZ945 (n = 3 for each condition). Two-tailed Student’s t-test based on area under the curve. c, Western blot analysis of the CSF2R downstream signaling effector STAT5 in response to cytokine stimulation in the presence or absence of 670 nM BLZ945 in the microglial cell line EOC2 and BMDM. Result shown is representative of three independent experiments. d, Gene expression of CSF2RB/Csf2rb, CSF2RA/Csf2ra, IL3RA/Il3ra and IL5RA/Il5ra in FACS purified MDA-BrM tumor cells, TAM-MG and TAM-MDM as well as normal MG and blood monocytes (n = 5; vehicle, n = 4; 7d BLZ945, n = 4; endpoint BLZ945 and n = 3; tumor-free controls). Data are shown as the mean ± sd. e, Expression of CSF2RB, CSF2RA, IL3RA and IL5RA in sorted CD45-, MG and MDM from clinical samples of non-tumor bearing brains (nCD45-=5, nMG = 7), healthy donors (reference, nMDM = 7) and breast to brain metastases (BrM, nCD45-=6, nMG = 7, nMDM = 7). Data from14 are shown as the mean ± sd.
Extended Data Fig. 9 CSF2 protects TAMs from BLZ945-induced cell death.
a, Gene expression of CSF2/Csf2 in FACS purified MDA-BrM tumor cells, TAM-MG and TAM-MDM as well as normal MG and blood monocytes and MG (n = 5; vehicle, n = 4; BLZ945 and n = 3; tumor-free controls). Data is presented as the mean ± sd. b, Quantification of cell viability in EOC2 and BMDM cells in response to increasing concentrations of the STAT5 inhibitor AC4-130 by MTS assay (for EOC2: nCSF2 = 12, nCSF2 + 1μM AC4-130 = 4, nCSF2 + 2μM AC4-130 = 4, nCSF2 + 5μM AC4-130 = 4, nCSF2 + 10μM AC4-130 = 8, ncytokine withdrawal = 8; for BMDM: n = 3 for each condition. Two-tailed Student’s t-test based on area under the curve. c, Western blot analysis of the CSF2R downstream signaling pathway STAT5 in response to CSF2 and increasing concentrations of AC4-130 in the presence or absence of 670 nM BLZ945 in EOC2 and BMDM. Results shown are representative of three independent experiments. d, Quantification of the relative tumor growth rate based on volumetric evaluation of MRI images in the MDA-BrM model (n = 8; vehicle, n = 10; BLZ945, n = 6; AC4-130, n = 8; BLZ945 + AC4-130). Data is represented as individual replicates with lines depicting the mean of each experimental group. Analyzed by Mann-Whitney test based on slopes of individual curves. e, Representative images and 3D reconstruction of IBA1+ cells in the MDA-BrM model depicting the morphology of TAMs in intra- and peri-tumoral areas as well as normal adjacent brain parenchyma. Scale bars, 100 µm and 25 µm. f, Representative images visualizing GFAP+ astrocytes, CD31+ endothelial cells, MAP2+ neurons and CD3+ T cells in 99LN-BrM in response to the indicated treatment. Scale bars, 100 µm and 25 µm. For (e,f) data is representative for n = 3 samples for each condition.
Extended Data Fig. 10 Analysis of the effects of CSF1R and STAT5 inhibition in vitro and in vivo.
a, Heatmap depicting mean values of cytokine concentrations in whole tumor lysates prepared from MDA-BrM mice treated with vehicle (n = 7), BLZ945 (n = 8), and BLZ945 + AC4-130 (n = 5), the P values shown were calculated using the Kruskal-Wallis test across all experimental conditions. b, Quantification of the density of VGLUT1 + and VGAT + vesicles in the cortex of tumor-free animals / adjacent normal brain (upper panel) and peri-tumor area (lower panel) of vehicle, BLZ945, BLZ945 + α-CSF2 and BLZ945 + AC4-130 treated mice (for tumor-free brain: n = 22, vehicle; n = 20, BLZ945; n = 14, BLZ945 + α-CSF2; n = 27, BLZ945 + AC4-130; for peri-tumor area: n = 74, vehicle; n = 111, BLZ945; n = 190, BLZ945 + α-CSF2; n = 121, BLZ945 + AC4-130. Results are shown for individual FOVs obtained from 3 mice per group). Two-tailed Student’s t-test. c, Representative transmission electron microscopy images showing axons in the peri-tumor area (upper panel) and adjacent normal brain (lower panel) in the indicated experimental groups. Scale bar, 2 and 5 µm. d, Quantification of the percentage of normal and abnormal axons in the indicated experimental groups in the peri-tumor area and in the adjacent normal brain (for tumor-free brain: n = 10, vehicle; n = 18, BLZ945; n = 10, BLZ945 + AC4-130; for peri-tumor area: n = 22, vehicle; n = 24, BLZ945; n = 10, BLZ945 + AC4-130. Results are shown for individual FOVs obtained from 2 mice per group). Abnormal axons were further classified into axons with irregular morphology (irregular) and those showing inclusions and protrusions or disaggregation of the myelin sheath indicative of degradation based on features depicted in the representative images.
Supplementary information
Supplementary Tables
Supplementary Tables 1–5.
Supplementary Video 1
Brain slice MG vehicle.
Supplementary Video 2
Brain slice MG BLZ945.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 6
Statistical Source Data.
Source Data Fig. 7
Statistical Source Data.
Source Data Fig. 8
Statistical Source Data.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 7
Statistical Source Data.
Source Data Extended Data Fig. 8
Statistical Source Data.
Source Data Extended Data Fig. 8
Unprocessed western blot.
Source Data Extended Data Fig. 9
Statistical Source Data.
Source Data Extended Data Fig 9
Unprocessed western blot.
Source Data Extended Data Fig. 10
Statistical Source Data.
Rights and permissions
About this article
Cite this article
Klemm, F., Möckl, A., Salamero-Boix, A. et al. Compensatory CSF2-driven macrophage activation promotes adaptive resistance to CSF1R inhibition in breast-to-brain metastasis. Nat Cancer 2, 1086–1101 (2021). https://doi.org/10.1038/s43018-021-00254-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-021-00254-0
This article is cited by
-
Cancer cell genetics shaping of the tumor microenvironment reveals myeloid cell-centric exploitable vulnerabilities in hepatocellular carcinoma
Nature Communications (2024)
-
Microenvironmental reorganization in brain tumors following radiotherapy and recurrence revealed by hyperplexed immunofluorescence imaging
Nature Communications (2024)
-
From pre-clinical to translational brain metastasis research: current challenges and emerging opportunities
Clinical & Experimental Metastasis (2024)
-
Harnessing immunotherapy for brain metastases: insights into tumor–brain microenvironment interactions and emerging treatment modalities
Journal of Hematology & Oncology (2023)
-
Therapeutic targeting of tumour myeloid cells
Nature Reviews Cancer (2023)