Abstract
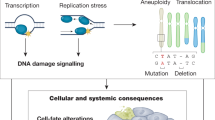
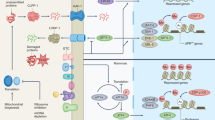
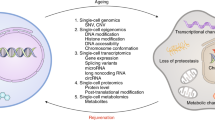
Cellular metabolism and environmental interactions generate molecular damage affecting all levels of biological organization. Accumulation of this damage over time is thought to have a central role in the aging process. Insufficient attention has been paid to the role of molecular damage in aging-related phenotypes, particularly in humans, in part because of the difficulty in measuring its various forms. Recently, omics approaches have been developed that begin to address this challenge, because they can assess a sizable proportion of age-related damage at the level of small molecules, proteins, RNA, DNA, organelles and cells. This Review describes the concept of molecular damage in aging and discusses its diverse aspects from theoretical models to experimental approaches. Measurement of multiple types of damage enables studies of the role of damage in aging and lays a foundation for testing interventions that reduce the burden of molecular damage, thereby targeting aging.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
There are no data.
References
Kirkwood, T. B. & Austad, S. N. Why do we age? Nature 408, 233–238 (2000).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Stadtman, E. R. Protein oxidation and aging. Science 257, 1220–1224 (1992).
Rando, T. A. & Wyss-Coray, T. Asynchronous, contagious and digital aging. Nat. Aging 1, 29–35 (2021).
Kinzina, E. D., Podolskiy, D. I., Dmitriev, S. E. & Gladyshev, V. N. Patterns of aging biomarkers, mortality, and damaging mutations illuminate the beginning of aging and causes of early-life mortality. Cell Rep. 29, 4276–4284 (2019).
Shindyapina, A. V. et al. Germline burden of rare damaging variants negatively affects human healthspan and lifespan. eLife 9, e53449 (2020).
Ogrodnik, M., Salmonowicz, H. & Gladyshev, V. N. Integrating cellular senescence with the concept of damage accumulation in aging: Relevance for clearance of senescent cells. Aging Cell 18, e12841 (2019).
Takasugi, M. Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell 17, e12734 (2018).
Kerepesi, C., Zhang, B., Lee, S. G., Trapp, A. & Gladyshev, V. N. Epigenetic clocks reveal a rejuvenation event during embryogenesis followed by aging. Sci. Adv. 7, eabg6082 (2021).
Golubev, A. G. [The other side of metabolism]. Biokhimiia 61, 2018–2039 (1996).
Golubev, A., Hanson, A. D. & Gladyshev, V. N. A tale of two concepts: harmonizing the free radical and antagonistic pleiotropy theories of aging. Antioxid. Redox Signal. 29, 1003–1017 (2018).
Golubev, A., Hanson, A. D. & Gladyshev, V. N. Non-enzymatic molecular damage as a prototypic driver of aging. J. Biol. Chem. 292, 6029–6038 (2017).
Avanesov, A. S. et al. Age- and diet-associated metabolome remodeling characterizes the aging process driven by damage accumulation. eLife 3, e02077 (2014).
Lee, S. G. et al. Age-associated molecular changes are deleterious and may modulate life span through diet. Sci. Adv. 3, e1601833 (2017).
Hughes, A. L. & Gottschling, D. E. An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature 492, 261–265 (2012).
Sinclair, D. A. & Guarente, L. Extrachromosomal rDNA circles–a cause of aging in yeast. Cell 91, 1033–1042 (1997).
King, G. A. et al. Meiotic cellular rejuvenation is coupled to nuclear remodeling in budding yeast. eLife 8, e47156 (2019).
Kaya, A., Lobanov, A. V. & Gladyshev, V. N. Evidence that mutation accumulation does not cause aging in Saccharomyces cerevisiae. Aging Cell 14, 366–371 (2015).
Harman, D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11, 298–300 (1956).
Gladyshev, V. N. The free radical theory of aging is dead. Long live the damage theory! Antioxid. Redox Signal. 20, 727–731 (2014).
Stamatoyannopoulos, J. A. et al. Human mutation rate associated with DNA replication timing. Nat. Genet. 41, 393–395 (2009).
Lodato, M. A. et al. Somatic mutation in single human neurons tracks developmental and transcriptional history. Science 350, 94–98 (2015).
Lodato, M. A. et al. Aging and neurodegeneration are associated with increased mutations in single human neurons. Science 359, 555–559 (2018).
Lodato, M. A. & Walsh, C. A. Genome aging: somatic mutation in the brain links age-related decline with disease and nominates pathogenic mechanisms. Hum. Mol. Genet. 28, R197–R206 (2019).
Podolskiy, D. I., Lobanov, A. V., Kryukov, G. V. & Gladyshev, V. N. Analysis of cancer genomes reveals basic features of human aging and its role in cancer development. Nat. Commun. 7, 12157 (2016).
Brazhnik, K. et al. Single-cell analysis reveals different age-related somatic mutation profiles between stem and differentiated cells in human liver. Sci. Adv. 6, eaax2659 (2020).
Hollstein, M., Alexandrov, L. B., Wild, C. P., Ardin, M. & Zavadil, J. Base changes in tumour DNA have the power to reveal the causes and evolution of cancer. Oncogene 36, 158–167 (2017).
Alexandrov, L. B. & Stratton, M. R. Mutational signatures: the patterns of somatic mutations hidden in cancer genomes. Curr. Opin. Genet. Dev. 24, 52–60 (2014).
Alexandrov, L. B. et al. The repertoire of mutational signatures in human cancer. Nature 578, 94–101 (2020).
Freitas, A. A. & de Magalhaes, J. P. A review and appraisal of the DNA damage theory of ageing. Mutat. Res. 728, 12–22 (2011).
Niedernhofer, L. J. et al. Nuclear genomic instability and aging. Annu. Rev. Biochem. 87, 295–322 (2018).
Baker, D. J. et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016).
Jacob, K. D., Noren Hooten, N., Trzeciak, A. R. & Evans, M. K. Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mech. Ageing Dev. 134, 139–157 (2013).
Wang, J., Clauson, C. L., Robbins, P. D., Niedernhofer, L. J. & Wang, Y. The oxidative DNA lesions 8,5ʹ-cyclopurines accumulate with aging in a tissue-specific manner. Aging Cell 11, 714–716 (2012).
Beerman, I. Accumulation of DNA damage in the aged hematopoietic stem cell compartment. Semin. Hematol. 54, 12–18 (2017).
Robinson, A. R. et al. Spontaneous DNA damage to the nuclear genome promotes senescence, redox imbalance and aging. Redox Biol. 17, 259–273 (2018).
Cui, H., Kong, Y. & Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012, 646354 (2012).
Pfohl-Leszkowicz, A. in Advances in Molecular Toxicology Vol. 2 (ed. Fishbein, J. C.) 183–239 (Elsevier, 2008).
Ioannidou, A., Goulielmaki, E. & Garinis, G. A. DNA damage: from chronic inflammation to age-related deterioration. Front. Genet. 7, 187 (2016).
Roos, W. P. & Kaina, B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 332, 237–248 (2013).
Kang, C. et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 349, aaa5612 (2015).
von Zglinicki, T., Saretzki, G., Ladhoff, J., d’Adda di Fagagna, F. & Jackson, S. P. Human cell senescence as a DNA damage response. Mech. Ageing Dev. 126, 111–117 (2005).
Takahashi, A. et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 8, 15287 (2017).
Teo, Y. V. et al. Cell-free DNA as a biomarker of aging. Aging Cell 18, e12890 (2019).
Kananen, L. et al. Circulating cell-free DNA level predicts all-cause mortality independent of other predictors in the Health 2000 survey. Sci. Rep. 10, 13809 (2020).
Farmer, P. B. et al. DNA adducts: mass spectrometry methods and future prospects. Toxicol. Appl. Pharmacol. 207, 293–301 (2005).
Guthrie, O. W. Localization and distribution of neurons that co-express xeroderma pigmentosum-A and epidermal growth factor receptor within Rosenthal’s canal. Acta Histochem. 117, 688–695 (2015).
Carra, A. et al. Targeted high resolution LC/MS(3) adductomics method for the characterization of endogenous DNA damage. Front. Chem. 7, 658 (2019).
Pinto, M. & Moraes, C. T. Mechanisms linking mtDNA damage and aging. Free Radic. Biol. Med. 85, 250–258 (2015).
Carelli, V. & Chan, D. C. Mitochondrial DNA: impacting central and peripheral nervous systems. Neuron 84, 1126–1142 (2014).
Tranah, G. J. et al. Mitochondrial DNA heteroplasmy associations with neurosensory and mobility function in elderly adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 70, 1418–1424 (2015).
Tranah, G. J. et al. Mitochondrial DNA m.3243A > G heteroplasmy affects multiple aging phenotypes and risk of mortality. Sci. Rep. 8, 11887 (2018).
Trifunovic, A. et al. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc. Natl Acad. Sci. USA 102, 17993–17998 (2005).
Booth, L. N. & Brunet, A. The aging epigenome. Mol. Cell 62, 728–744 (2016).
Sinclair, D. A. & Oberdoerffer, P. The ageing epigenome: damaged beyond repair? Ageing Res. Rev. 8, 189–198 (2009).
Gladyshev, V. N. Aging: progressive decline in fitness due to the rising deleteriome adjusted by genetic, environmental, and stochastic processes. Aging Cell 15, 594–602 (2016).
Sziraki, A., Tyshkovskiy, A. & Gladyshev, V. N. Global remodeling of the mouse DNA methylome during aging and in response to calorie restriction. Aging Cell 17, e12738 (2018).
Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 14, R115 (2013).
Johnson, A. A. et al. The role of DNA methylation in aging, rejuvenation, and age-related disease. Rejuvenation Res. 15, 483–494 (2012).
Horvath, S. & Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 19, 371–384 (2018).
Hannum, G. et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 49, 359–367 (2013).
Levine, M. E. et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 10, 573–591 (2018).
Lu, A. T. et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 11, 303–327 (2019).
Belsky, D. W. et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. eLife 9, e54870 (2020).
Bell, C. G. et al. DNA methylation aging clocks: challenges and recommendations. Genome Biol. 20, 249 (2019).
Petkovich, D. A. et al. Using DNA methylation profiling to evaluate biological age and longevity interventions. Cell Metab. 25, 954–960 (2017).
Meer, M. V., Podolskiy, D. I., Tyshkovskiy, A. & Gladyshev, V. N. A whole lifespan mouse multi-tissue DNA methylation clock. eLife 7, e40675 (2018).
Stubbs, T. M. et al. Multi-tissue DNA methylation age predictor in mouse. Genome Biol. 18, 68 (2017).
Thompson, M. J. et al. A multi-tissue full lifespan epigenetic clock for mice. Aging 10, 2832–2854 (2018).
Wang, T. et al. Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol. 18, 57 (2017).
Lu, A. T. et al. Universal DNA methylation age across mammalian tissues. Preprint at https://doi.org/10.1101/2021.01.18.426733 (2021).
Lu, Y. et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 588, 124–129 (2020).
Olova, N., Simpson, D. J., Marioni, R. E. & Chandra, T. Partial reprogramming induces a steady decline in epigenetic age before loss of somatic identity. Aging Cell 18, e12877 (2019).
Fahy, G. M. et al. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell 18, e13028 (2019).
Horvath, S. et al. Reversing age: dual species measurement of epigenetic age with a single clock. Preprint at https://doi.org/10.1101/2020.05.07.082917 (2020).
Bhadra, M., Howell, P., Dutta, S., Heintz, C. & Mair, W. B. Alternative splicing in aging and longevity. Hum. Genet. 139, 357–369 (2020).
Wang, K. et al. Comprehensive map of age-associated splicing changes across human tissues and their contributions to age-associated diseases. Sci. Rep. 8, 10929 (2018).
Heintz, C. et al. Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. elegans. Nature 541, 102–106 (2017).
Kurosaki, T., Popp, M. W. & Maquat, L. E. Quality and quantity control of gene expression by nonsense-mediated mRNA decay. Nat. Rev. Mol. Cell Biol. 20, 406–420 (2019).
Wu, C. C., Peterson, A., Zinshteyn, B., Regot, S. & Green, R. Ribosome collisions trigger general stress responses to regulate cell fate. Cell 182, 404–416 (2020).
Wood, S. H., Craig, T., Li, Y., Merry, B. & de Magalhaes, J. P. Whole transcriptome sequencing of the aging rat brain reveals dynamic RNA changes in the dark matter of the genome. Age 35, 763–776 (2013).
de Magalhaes, J. P., Curado, J. & Church, G. M. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics 25, 875–881 (2009).
Palmer, D., Fabris, F., Doherty, A., Freitas, A. A. & de Magalhaes, J. P. Ageing transcriptome meta-analysis reveals similarities and differences between key mammalian tissues. Aging 13, 3313–3341 (2021).
Consortium, G. T. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 45, 580–585 (2013).
Tabula Muris, C. et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372 (2018).
Grassi, L. & Cabrele, C. Susceptibility of protein therapeutics to spontaneous chemical modifications by oxidation, cyclization, and elimination reactions. Amino Acids 51, 1409–1431 (2019).
Levine, R. L. & Stadtman, E. R. Oxidative modification of proteins during aging. Exp. Gerontol. 36, 1495–1502 (2001).
Lourenco Dos Santos, S., Petropoulos, I. & Friguet, B. The oxidized protein repair enzymes methionine sulfoxide reductases and their roles in protecting against oxidative stress, in ageing and in regulating protein function. Antioxidants 7, 191 (2018).
Mishra, P. K. K. & Mahawar, M. PIMT-mediated protein repair: mechanism and implications. Biochemistry 84, 453–463 (2019).
Weinert, B. T., Moustafa, T., Iesmantavicius, V., Zechner, R. & Choudhary, C. Analysis of acetylation stoichiometry suggests that SIRT3 repairs nonenzymatic acetylation lesions. EMBO J. 34, 2620–2632 (2015).
Van Schaftingen, E., Collard, F., Wiame, E. & Veiga-da-Cunha, M. Enzymatic repair of Amadori products. Amino Acids 42, 1143–1150 (2012).
Gorisse, L. et al. Protein carbamylation is a hallmark of aging. Proc. Natl Acad. Sci. USA 113, 1191–1196 (2016).
Khoury, G. A., Baliban, R. C. & Floudas, C. A. Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database. Sci. Rep. 1, 90 (2011).
Lee, B. C. et al. MsrB1 and MICALs regulate actin assembly and macrophage function via reversible stereoselective methionine oxidation. Mol. Cell 51, 397–404 (2013).
Fedorova, M., Kuleva, N. & Hoffmann, R. Identification of cysteine, methionine and tryptophan residues of actin oxidized in vivo during oxidative stress. J. Proteome Res. 9, 1598–1609 (2010).
Rankin, N. J. et al. High-throughput quantification of carboxymethyl lysine in serum and plasma using high-resolution accurate mass Orbitrap mass spectrometry. Ann. Clin. Biochem. 56, 397–407 (2019).
Fu, M. X. et al. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem. 271, 9982–9986 (1996).
Wang, R. et al. Affinity purification of methyllysine proteome by site-specific covalent conjugation. Anal. Chem. 90, 13876–13881 (2018).
Huseby, C. J. et al. Quantification of tau protein lysine methylation in aging and Alzheimer’s disease. J. Alzheimers Dis. 71, 979–991 (2019).
Lesnefsky, E. J. & Hoppel, C. L. Oxidative phosphorylation and aging. Ageing Res. Rev. 5, 402–433 (2006).
Akimov, V. et al. UbiSite approach for comprehensive mapping of lysine and N-terminal ubiquitination sites. Nat. Struct. Mol. Biol. 25, 631–640 (2018).
Hoopmann, M. R. et al. Kojak: efficient analysis of chemically cross-linked protein complexes. J. Proteome Res. 14, 2190–2198 (2015).
Kim, E., Lowenson, J. D., MacLaren, D. C., Clarke, S. & Young, S. G. Deficiency of a protein-repair enzyme results in the accumulation of altered proteins, retardation of growth, and fatal seizures in mice. Proc. Natl Acad. Sci. USA 94, 6132–6137 (1997).
Kaushik, S. & Cuervo, A. M. Proteostasis and aging. Nat. Med. 21, 1406–1415 (2015).
Hohn, A. & Grune, T. Lipofuscin: formation, effects and role of macroautophagy. Redox Biol. 1, 140–144 (2013).
Sardiello, M. et al. A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 (2009).
Gan, J., Leestemaker, Y., Sapmaz, A. & Ovaa, H. Highlighting the proteasome: using fluorescence to visualize proteasome activity and distribution. Front. Mol. Biosci. 6, 14 (2019).
Juste, Y. R. & Cuervo, A. M. Analysis of chaperone-mediated autophagy. Methods Mol. Biol. 1880, 703–727 (2019).
Raz, Y. et al. Activation-induced autophagy is preserved in CD4+ T-cells in familial longevity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 72, 1201–1206 (2017).
Lerma-Ortiz, C. et al. ‘Nothing of chemistry disappears in biology’: the top 30 damage-prone endogenous metabolites. Biochem. Soc. Trans. 44, 961–971 (2016).
Chen, L., Ducker, G. S., Lu, W., Teng, X. & Rabinowitz, J. D. An LC–MS chemical derivatization method for the measurement of five different one-carbon states of cellular tetrahydrofolate. Anal. Bioanal. Chem. 409, 5955–5964 (2017).
Zhang, J. et al. Determination of the cytosolic NADPH/NADP ratio in Saccharomyces cerevisiae using shikimate dehydrogenase as sensor reaction. Sci. Rep. 5, 12846 (2015).
Niehaus, T. D. et al. Plants utilize a highly conserved system for repair of NADH and NADPH hydrates. Plant Physiol. 165, 52–61 (2014).
Tawfik, D. S. Enzyme promiscuity and evolution in light of cellular metabolism. FEBS J. 287, 1260–1261 (2020).
Linster, C. L., Van Schaftingen, E. & Hanson, A. D. Metabolite damage and its repair or pre-emption. Nat. Chem. Biol. 9, 72–80 (2013).
Bommer, G. T., Van Schaftingen, E. & Veiga-da-Cunha, M. Metabolite repair enzymes control metabolic damage in glycolysis. Trends Biochem. Sci. 45, 228–243 (2020).
Piedrafita, G., Keller, M. A. & Ralser, M. The impact of non-enzymatic reactions and enzyme promiscuity on cellular metabolism during (oxidative) stress conditions. Biomolecules 5, 2101–2122 (2015).
Kuiper, H. C., Miranda, C. L., Sowell, J. D. & Stevens, J. F. Mercapturic acid conjugates of 4-hydroxy-2-nonenal and 4-oxo-2-nonenal metabolites are in vivo markers of oxidative stress. J. Biol. Chem. 283, 17131–17138 (2008).
Marnett, L. J. & Plastaras, J. P. Endogenous DNA damage and mutation. Trends Genet. 17, 214–221 (2001).
Rockwood, K. & Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 62, 722–727 (2007).
Longo, V. D., Mitteldorf, J. & Skulachev, V. P. Programmed and altruistic ageing. Nat. Rev. Genet. 6, 866–872 (2005).
Williams, G. C. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 (1957).
Medawar, P. B. An Unsolved Problem of Biology (University College London, 1952).
Hamilton, W. D. The moulding of senescence by natural selection. J. Theor. Biol. 12, 12–45 (1966).
Ronce, O. & Promislow, D. Kin competition, natal dispersal and the moulding of senescence by natural selection. Proc. Biol. Sci. 277, 3659–3667 (2010).
de Magalhaes, J. P. Programmatic features of aging originating in development: aging mechanisms beyond molecular damage? FASEB J. 26, 4821–4826 (2012).
Kirkwood, T. B. Evolution of ageing. Nature 270, 301–304 (1977).
Blagosklonny, M. V. Aging: ROS or TOR. Cell Cycle 7, 3344–3354 (2008).
Blagosklonny, M. V. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle 5, 2087–2102 (2006).
Blagosklonny, M. V. Paradoxes of aging. Cell Cycle 6, 2997–3003 (2007).
Vina, J., Borras, C., Abdelaziz, K. M., Garcia-Valles, R. & Gomez-Cabrera, M. C. The free radical theory of aging revisited: the cell signaling disruption theory of aging. Antioxid. Redox Signal. 19, 779–787 (2013).
Miquel, J., Economos, A. C., Fleming, J. & Johnson, J. E. Jr. Mitochondrial role in cell aging. Exp. Gerontol. 15, 575–591 (1980).
Gladyshev, V. N. The origin of aging: imperfectness-driven non-random damage defines the aging process and control of lifespan. Trends Genet. 29, 506–512 (2013).
Acknowledgements
The review is based in part on a workshop, Biological Damage and Human Aging held in December 2019, sponsored by the Longevity Consortium with support from the National Institute of Aging. This work was supported by the National Institutes of Health (NIH) AG021332 (S.K.), NSF MCB-1714569, Life Extension Foundation and the Elizabeth and Thomas Plott Chair in Gerontology (S.G.C.), NIH AG064223, AG067782, AG065403 and AG047200 (V.N.G.), NIH HL121023 and AG032498 (G.T.) and NIA U19AG023122 (the Longevity Consortium).
Author information
Authors and Affiliations
Contributions
V.N.G. and S.B.K. were responsible for conception and design, drafting and substantial revisions. S.G.C., B.Z. and T.M. were responsible for drafting, creation of figures and substantial revision. A.M.C., O.F., J.P.M., M.M., R.M., E.V.S., G.T., K.W. and Y.Y. were responsible for drafting and substantial revisions. L.J.N. was responsible for drafting, creation of tables and substantial revisions. S.R.C. was responsible for conception, design, oversight, drafting and substantial revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Aging thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gladyshev, V.N., Kritchevsky, S.B., Clarke, S.G. et al. Molecular damage in aging. Nat Aging 1, 1096–1106 (2021). https://doi.org/10.1038/s43587-021-00150-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-021-00150-3
This article is cited by
-
Glyoxal-derived advanced glycation end products (GO-AGEs) with UVB critically induce skin inflammaging: in vitro and in silico approaches
Scientific Reports (2024)
-
Causality-enriched epigenetic age uncouples damage and adaptation
Nature Aging (2024)
-
Cellular reprogramming as a tool to model human aging in a dish
Nature Communications (2024)
-
Epigenetic clock work ticks forward
Nature Aging (2024)
-
The meaning of adaptation in aging: insights from cellular senescence, epigenetic clocks and stem cell alterations
Nature Aging (2023)